Combining Neuropsychological Assessment with Neuroimaging to Distinguish Early-Stage Alzheimer’s Disease from Frontotemporal Lobar Degeneration in Non-Western Tonal Native Language-Speaking Individuals Living in Taiwan: A Case Series
Abstract
1. Introduction
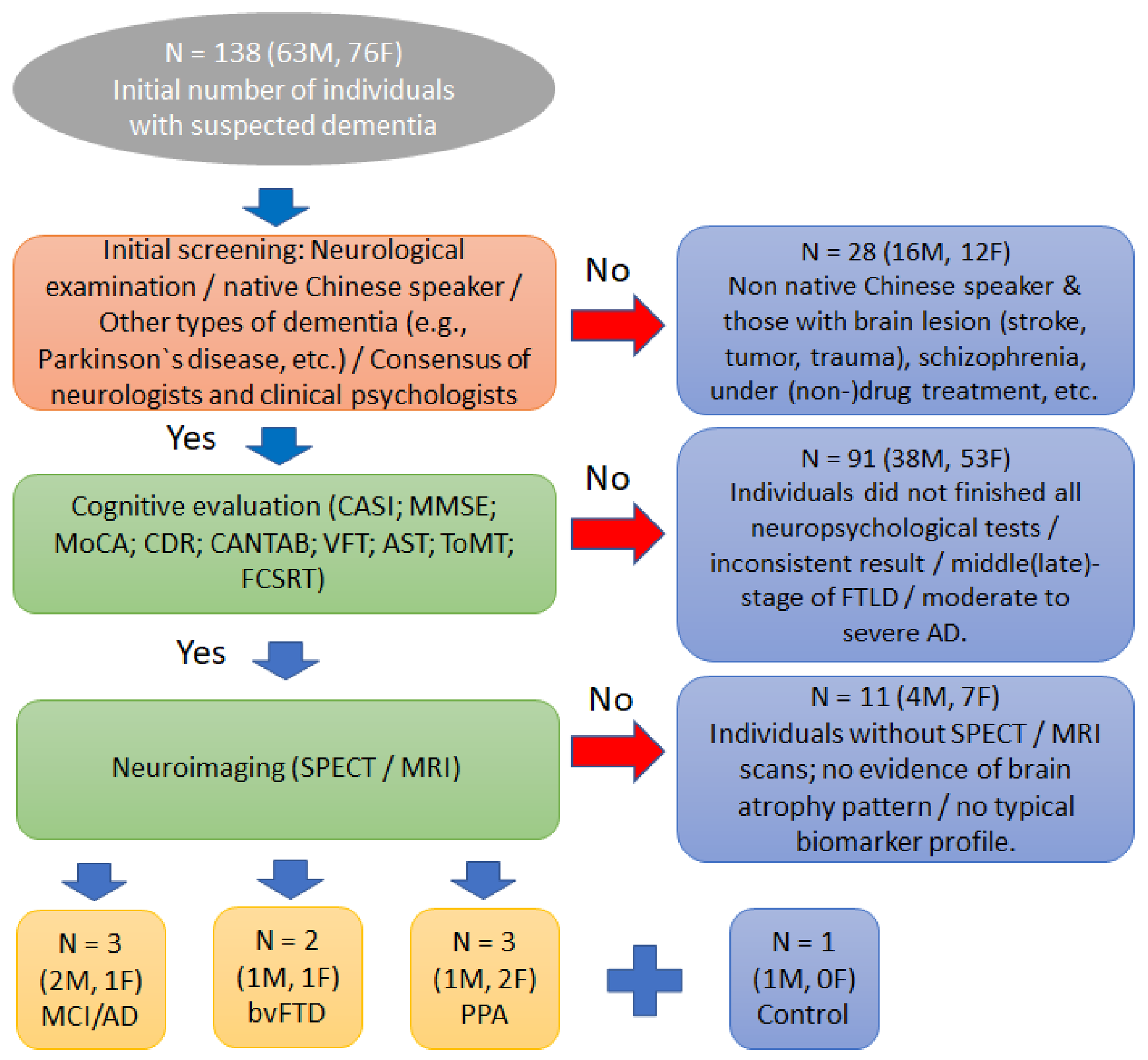
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Tests
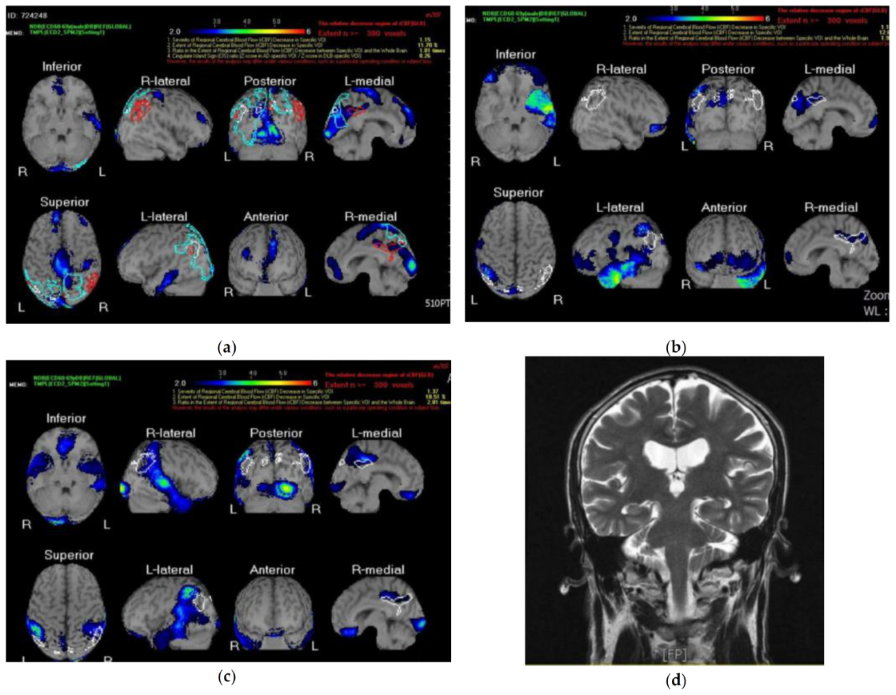
2.3. Neuroimagining
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2021; Available online: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf (accessed on 5 February 2021).
- Kuo, C.Y.; Hsiao, H.T.; Lo, I.H.; Nikolai, T. Association between obstructive sleep apnea, its treatment, and Alzheimer’s disease: Systematic mini-review. Front. Aging Neurosci. 2021, 12, 591737. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.L.; Karikari, T.; Suárez-Calvet, M.; Troakes, C.; King, A.; Emersic, A.; Aarsland, D.; Hye, A.; Zetterberg, H.; Blennow, K.; et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterization of cognitive decline. Acta Neuropathol. 2020, 140, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimaki, M.; Sabia, S. Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 2017, 74, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Villain, N.; Dubois, B. Alzheimer’s disease including focal presentations. Semin. Neurol. 2019, 39, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer’s disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Stachiv, I.; Nikolai, T. Association of Late Life Depression, (Non-) Modifiable Risk and Protective Factors with Dementia and Alzheimer’s Disease: Literature Review on Current Evidences, Preventive Interventions and Possible Future Trends in Prevention and Treatment of Dementia. Int. J. Environ. Res. Public Health 2020, 17, 7475. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Frozza, R.L.; Lourenco, M.V.; De Felice, F.G. Challenges for Alzheimer’s disease therapy: Insight from novel mechanisms beyond memory defects. Front. Neorosci. 2018, 12, 37. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuromuscul Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Paris, D.; Melck, D.; Angiolillo, A.; Corso, G.; Maniscalco, M.; Motta, A. Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Sci. Rep. 2020, 10, 15612. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef]
- Draper, B.; Cations, M.; White, F.; Trollor, J.; Loy, C.; Brodaty, H.; Sachdev, P.; Gonski, P.; Demirkol, A.; Cumming, R.G.; et al. Time of diagnosis in young-onset dementia and its determinants: The INSPIRED study. Int. J. Geriatr. Psychiatry. 2016, 31, 1217–1224. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Katisko, K.; Cajanus, A.; Korhonen, T.; Remes, A.M.; Haapasalo, A.; Solje, E. Prodromal and Early bvFTD: Evaluating Clinical Features and Current Biomarkers. Front. Neurosci. 2019, 13, 658. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: S systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Reul, S.; Lohmann, H.; Weindl, H.; Duning, T.; Johnen, A. Can cognitive assessment really discriminate early stages of Alzheimer’s disease and behavioral variant frontotemporal dementia at initial clinical presentation? Alzheimers Res. Ther. 2017, 9, 61. [Google Scholar] [CrossRef]
- Krudop, W.A.; Dols, A.; Kerssens, C.J.; Eikelenboom, P.; Prins, N.D.; Möller, C.; Schouws, S.; Rhebergen, D.; van Exel, E.; van der Flier, W.M.; et al. The pitfall of behavioral variant frontotemporal dementia mimics despite multidisciplinary application of the FTDC criteria. J. Alzheimer’s Dis. 2017, 60, 959–975. [Google Scholar] [CrossRef]
- Musa, G.; Slachevsky, A.; Muñoz-Neira, C.; Méndez-Orellana, C.; Villagra, R.; González-Billault, C.; Ibáñez, A.; Hornberger, M.; Lillo, P. Alzheimer’s Disease or Behavioral Variant Frontotemporal Dementia? Review of Key Points Toward an Accurate Clinical and Neuropsychological Diagnosis. J. Alzheimer’s Dis. 2020, 73, 833–843. [Google Scholar] [CrossRef]
- Mendez, M.F.; Shapira, J.S.; McMurtray, A.; Licht, E.; Miller, B.L. Accuracy of the Clinical Evaluation for Frontotemporal Dementia. Arch. Neurol. 2007, 64, 830–835. [Google Scholar] [CrossRef]
- Leroy, M.; Bertoux, M.; Skrobala, E.; Mode, E.; Adnet-Bonte, C.; Le Ber, I.; Bombois, S.; Cassagnaud, P.; Chen, Y.; Deramecourt, V.; et al. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alz. Res. Therapy 2021, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, S.; Dols, A.; LaForce, R.; Devenney, E.; Kumfor, F.; Stock, J.V.D.; Dallaire-Théroux, C.; Seelaar, H.; Gossink, F.; Vijverberg, E.; et al. Recommendations to distinguish behavioral variant frontotemporal dementia from psychiatric disorders. Brain 2020, 143, 1632–1650. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lu, D.; Popuri, K.; Wang, L.; Beg, M.F.; Alzheimer’s Disease Neuroimaging Initiative. Differential Diagnosis of Frontotemporal Dementia, Alzheimer’s Disease, and Normal Aging Using a Multi-Scale Multi-Type Feature Generative Adversarial Deep Neural Network on Structural Magnetic Resonance Images. Front. Neurosci. 2020, 14, 853. [Google Scholar] [CrossRef]
- Johnen, A.; Bertoux, M. Psychological and cognitive markers of behavioral variant frontotemporal dementia- A clinical neuropsychologist’s view on diagnostic criteria and beyond. Front. Neurol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Ng, K.P.; Chiew, H.J.; Lim, L.; Rosa-Neto, P.; Kandiah, N.; Gauthier, S. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Rev. Neurother. 2018, 18, 859–869. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wang, A.; Qi, Y.; Feng, W.; Hou, C.; Tao, L.; Liu, X.; Li, X.; Wang, W.; et al. Associations between social and intellectual activities with cognitive trajectories in Chinese middle-aged and older adults: A nationally representative cohort study. Alz. Res. Therapy 2020, 12, 115. [Google Scholar] [CrossRef]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; p. 607. [Google Scholar]
- Daugherty, J.C.; Puente, A.E.; Fasfous, A.F.; Hidalgo-Ruzzante, N.; Perez-Garcia, M. Diagnostic mistakes of culturally diverse individuals when using North American neuropsychological tests. Appl. Neuropsychol. Adult. 2017, 24, 16–22. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Brayne, C.; Matthews, F.E. Prevalence of dementia in East Asia: A systematic review of time trends. Int. J. Geriatr. Psychiatry 2015, 30, 793–801. [Google Scholar] [CrossRef]
- Kucukguc, O.; Soylemez, B.A.; Yener, G.; Barutcu, C.D.; Akyol, M.A. Examining factors affecting caregiver burden: A comparison of frontotemporal dementia and Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2017, 32, 200–206. [Google Scholar] [CrossRef]
- Wong, H.Y.; Zhong, H.; Zhong, M.; Zhou, X.; Chan, P.Y.C.; Kwok, T.C.Y.; Mok, K.; Hardy, J.; Ip, F.C.; Fu, A.K.; et al. Demographics and medication use of patients with late-onset Alzheimer’s disease in Hong Kong. J. Alzheimers Dis. 2022, 87, 1205–1213. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, C.; Wang, M.; Chen, W.; Wu, Y.; Tang, W. A clinical memory battery for screening for mild cognitive impairment in elderly Chinese population. J. Clin. Neurosci. 2011, 18, 774–779. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, D.W.; Cho, S.-J.; Na, D.L.; Jeon, H.J.; Kim, S.-K.; Lee, Y.R.; Youn, J.-H.; Kwon, M.; Lee, J.-H.; et al. Brief Screening for Mild Cognitive Impairment in Elderly Outpatient Clinic: Validation of the Korean Version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Chen, K.-L.; Xu, Y.; Chu, A.-Q.; Ding, D.; Liang, X.-N.; Nasreddine, Z.S.; Dong, Q.; Hong, Z.; Zhao, Q.-H.; Guo, Q.-H. Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 2016, 64, e285–e290. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, A.; Wei, C.; Zuo, X.; Ma, X.; Zhao, L.; Jin, H.; Li, Y.; Guo, D.; Jia, J. Good performance of the Chinese Version of Mini social cognition and emotional assessment in the early diagnosis of behavioral variant frontotemporal dementia. Front. Neurol. 2022, 13, 827945. [Google Scholar] [CrossRef]
- Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Synofzik, M.; Otto, M.; Graff, C.; Galimberti, D.; Todd, E.; Heslegrave, A.J.; van der Ende, E.L.; et al. Fluid biomarkers in frontotemporal dementia: Past, present and future. J. Neurol. Neurosurg. Psychiatry 2021, 92, 204–215. [Google Scholar] [CrossRef]
- Stachiv, I.; Kuo, C.-Y.; Li, W. Protein adsorption by nanomechanical mass spectrometry: Beyond the real-time molecular weighting. Front. Mol. Biosci. 2023, 9, 1058441. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Cohen, A.D.; Landau, S.M.; Snitz, B.E.; Klunk, W.E.; Blennow, K.; Zetterberg, H. Fluid and PET biomarkers for amyloid pathology in Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 97, 3–17. [Google Scholar] [CrossRef]
- Albert, M.; DeCarli, C.; DeKosky, S.; de Leon, M.; Foster, N.L.; Fox, N.; Frank, R.; Frackowiak, R.; Jack, C.; Jagus, W.J.; et al. The use of MRI and PET for clinical diagnosis of dementia and investigation of cognitive impairment: A consensus report. Alzheimer’s Assoc. Neuroimaging Work Gr. Consens. Rep. 2005, 1, 1–15. Available online: http://www.alz.org/national/documents/imaging_consensus_report.pdf (accessed on 15 September 2022).
- Staffaroni, A.M.; Elahi, F.M.; McDermott, D.; Marton, K.; Karageorgiou, E.; Sacco, S. Neuroimiging in Dementia. Semin. Neurol. 2017, 37, 510–537. [Google Scholar] [CrossRef]
- Qi, W.; Sun, X.; Hong, Y. Normative Data for Adult Mandarin-Speaking Populations: A Systematic Review of Performance-Based Neuropsychological Instruments. J. Int. Neuropsychol. Soc. 2022, 28, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Walterfang, M.; Velakoulis, D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Bell, B.; Erbetta, A.; Paterlini, C.; Bugiani, O. Analyzing theory of mind impairment in patients with behavioral variant frontotemporal dementia. Neurol. Sci. 2019, 40, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.R.; Adams, W.; Lloyd, J.J.; Carson, K.J.; Snowden, J.S.; Testa, H.J.; Jackson, A.; Neary, D. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia. Acta Neurol. Scand. 2002, 105, 261–269. [Google Scholar] [CrossRef]
- Valotassiou, V.; Malamitsi, J.; Papatriantafyllou, J.; Dardiotis, E.; Tsougos, I.; Psimadis, D.; Alexiou, S.; Hadjigeorgiou, G.; Georgoulias, P. SPECT and PET imaging in Alzheimer’s disease. Ann. Nucl. Med. 2018, 32, 583–593. [Google Scholar] [CrossRef]
- Rivas-Fernández, M.A.; Lindín, M.; Zurrón, M.; Diaz, F.; Aldrey-Vázquez, J.M.; Pías-Peleteiro, J.M.; Vázquez-Vázquez, L.; Pereiro, A.X.; Lojo-Seoane, C.; Nieto-Vieites, A.; et al. Brain Atrophy and Clinical Characterization of Adults With Mild Cognitive Impairment and Different Cerebrospinal Fluid Biomarker Profiles According to the AT(N) Research Framework of Alzheimer’s Disease. Front. Hum. Neurosci. 2022, 16, 799347. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evaluation. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Chare, L.; Hodges, J.R.; Leyton, C.E.; McGinley, C.; Tan, R.H.; Kril, J.J.; Halliday, G.M. New criteria for frontotemporal dementia syndromes: Clinical and pathological diagnostic implications. J. Neurol. Neurosurg. Psychiatry 2014, 85, 866–871. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997, 24, 29–36. [Google Scholar] [CrossRef]
- Mesulam, M.M. Primary progressive aphasia. Ann. Neurol. 2001, 49, 425–432. [Google Scholar] [CrossRef]
- Marshall, C.R.; Hardy, C.J.D.; Volkmer, A.; Russell, L.L.; Bond, R.L.; Fletcher, P.D.; Clark, C.N.; Mummery, C.J.; Schott, J.M.; Rossor, M.N.; et al. Primary progressive aphasia: A clinical approach. J. Neurol. 2018, 265, 1474–1490. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Wang, H.; Sun, Y. Sentence comprehension in patients with dementia of the Alzheimer’s type. PeerJ 2019, 7, e8181. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Waring, S.C.; Cullum, C.M.; Hall, J.; Lacritz, L.; Massman, P.J.; Lupo, P.J.; Reisch, J.S.; Doody, R.; Texas Alzheimer’s Research Consortium. Stating dementia using clinical dementia rating scale sum of boxes scores. Arch. Neurol. 2008, 65, 1091–1095. [Google Scholar] [CrossRef]
- Sahakian, B.J.; Morris, R.G.; Evenden, J.L.; Heald, A.; Levy, R.; Philpot, M.; Robbins, T.W. A Comparative Study of Visuospatial Memory and Learning in Alzheimer-Type Dementia and Parkinson’s Disease. Brain 1988, 111, 695–718. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Hua, M.-S.; Hsuech, H.-C.; Chang, Y.-S.; Chiu, C.-F.; Chen, M.-C. The Performance Pattern of Normal Illiterate and Patients with Early Alzheimer’s Disease on the Semantic Association of Verbal Fluency Test. Chin. J. Psychol. 2007, 49, 73–86. [Google Scholar] [CrossRef]
- Zarino, B.; Crespi, M.; Launi, M.; Casarotti, A. A new standardization of semantic verbal fluency test. Neurol. Sci. 2014, 35, 1405–1411. [Google Scholar] [CrossRef]
- Johnen, A.; Frommever, J.; Modes, F.; Wiendl, H.; Duning, T.; Lohmann, H. Dementia Apraxia Test (DATE): A Brief Tool to Differentiate Behavioral Variant Frontotemporal Dementia from Alzheimer’s Dementia Based on Apraxia Profiles. J. Alzheimer’s Dis. 2016, 49, 593–605. [Google Scholar] [CrossRef]
- Gregory, C.; Lough, S.; Stone, V.; Erzinclioglu, S.; Martin, L.; Baron-Cohen, S.; Hodges, J.R. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain 2002, 125, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Grober, E.; Sanders, A.E.; Hall, C.; Lipton, R.B. Free and Cued Selective Reminding Identifies Very Mild Dementia in Primary Care. Alzheimer Dis. Assoc. Disord. 2010, 24, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.; Howieson, D.; Bigler, E.; and Tranel, D. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Montembeault, M.; Brambati, S.M.; Gorno-Tempini, M.L.; Migliaccio, R. Clinical, anatomical, and pathological features in the three variants of primary progressive Aphasia: A review. Front. Neurol. 2018, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ono, M.; Kimura, H.; Ueda, M.; Saji, H. Technetium-99m Labeled Pyridyl Benzofuran Derivatives as Single Photon Emission Computed Tomography Imaging Probes for β-Amyloid Plaques in Alzheimer’s Brains. J. Med. Chem. 2012, 55, 2279–2286. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Vemuri, V.L.P.; Mielke, M.M.; Jones, D.T.; Senjem, M.L.; Gunter, J.L.; Gregg, B.E. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 2013, 12, 1732–1740. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Valotassiou, V.; Angelidis, G.; Psimadas, D.; Tsougos, I.; Georgoulias, P. In the era of FDG PET, is it time for brain perfusion SPECT to gain a place in Alzheimer’s disease imaging biomarkers? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 969–971. [Google Scholar] [CrossRef]
- Hashimoto, H.; Nakanishi, R.; Mizumura, S.; Hashimoto, Y.; Okamura, Y.; Yamanaka, K.; Ikeda, T. Prognostic value of 99mTc-ECD brain perfusion SPECT in patients with atrial fibrillation and dementia. EJNMMI Res. 2020, 10, 3. [Google Scholar] [CrossRef]
- Davison, C.M.; O’Brien, J.T. A comparison of FDG-PET and blood flow SPECT in the diagnosis of neurodegenerative dementias: A systematic review. Int. J. Geriatr. Psychiatry 2014, 29, 551–561. [Google Scholar] [CrossRef]
- Baker, J.G.; Williams, A.J.; Wack, D.S.; Miletich, R.S. Correlation of Cognition and SPECT Perfusion: Easy Z Score and SPM Analysis of a Pilot Sample with Cerebral Small Vessel Disease. Dement. Gediatr.Cogn. Dis. 2013, 36, 290–299. [Google Scholar] [CrossRef]
- Jack, C.; Petersen, R.C.; Xu, Y.C.; O’Brien, P.C.; Smith, G.; Ivnik, R.J.; Boeve, B.F.; Waring, S.C.; Tangalos, E.G.; Kokmen, E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999, 52, 1397–1403. [Google Scholar] [CrossRef]
- Darcourt, J.; Booij, J.; Tatsch, K.; Varrone, A.; Borght, T.V.; Kapucu, L.; Någren, K.; Nobili, F.; Walker, Z.; Van Laere, K. EANM procedure guidelines for brain neurotransmission SPECT using 123I-labelled dopamine transporter ligands, version 2. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 443–450. [Google Scholar] [CrossRef]
- Brown, W.R.; Moody, D.M.; Thore, C.R.; Challa, V.R. Cerebrovascular pathology in Alzheimer’s disease and leukoaraiosis. Ann. N. Y. Acad. Sci. 2000, 903, 39–45. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Abe, J. Bias in cross-cultural neuropsychological testing: Problems and possible solutions. Cult. Brain 2018, 6, 1–35. [Google Scholar] [CrossRef]
- Te, M.; Zhao, E.; Xingyue, Z.; Qinjian, S.; Chuanqiang, Q. Leukoaraiosis with mild cognitive impairment. Neurol. Res. 2014, 37, 410–414. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Stachiv, I. Biological mechanisms and possible primary prevention of depression. World J. Psychiatry 2022, 12, 770–772. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Duclos, H.; Desgranges, B.; Eustache, F.; Laisney, M. Impairment of social cognition in neurological diseases. Rev. Neurol. 2018, 174, 190–198. [Google Scholar] [CrossRef]
- Baez, S.; Manes, F.; Huepe, D.; Torralva, T.; Fiorentino, N.; Richter, F.; Huepe-Artigas, D.; Ferrari, J.; Montaã±Es, P.; Reyes, P.A.; et al. Primary empathy deficits in frontotemporal dementia. Front. Aging Neurosci. 2014, 6, 262. [Google Scholar] [CrossRef]
- Koelkebeck, K.; Uwatoko, T.; Tanaka, J.; Kret, M.E. How culture shapes social cognition deficits in mental disorders: A review. Soc. Neurosci. 2017, 12, 102–112. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Wormley, A.S.; Vaarnum, E.W. Changing cultures, changing brains: A framework for integrating cultural neuroscience and cultural change research. Biol. Psychol. 2021, 162, 108087. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.Z.; Rosen, H.J.; Azor, V.; Ong, H.; Tse, M.M.; Lai, N.B.; Hou, C.E.; Seeley, W.W.; Miller, B.L.; Matthews, B.R. Frontotemporal dementia in eight Chinese individuals. Neurocase 2013, 19, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Weekes, B.S.H. Aphasia in Alzheimer’s disease and other dementias (ADOD): Evidence from Chinese. Am. J. Alzheimer’s Dis. Other Dement. 2020, 35, 1–12. [Google Scholar] [CrossRef]
- Tee, B.L.; Tempini, M.L.G.; Chen, T.; Lo, R.Y.; Wang, P.; Chan, A.L.; Chen, L.L.K.; Wong, A.; Yan, C.T.; Tsoh, J.; et al. Another side of the coin: Primary progressive aphasia in Chinese language. Alzheimers Dement. 2021, 17, e052482. [Google Scholar] [CrossRef]
- Grube, M.; Bruffaerts, R.; Schaeverbeke, J.; Neyens, V.; De Weer, A.-S.; Seghers, A.; Bergmans, B.; Dries, E.; Griffiths, T.D.; Vandenberghe, R. Core auditory processing deficits in primary progressive aphasia. Brain 2016, 139, 1817–1829. [Google Scholar] [CrossRef]
- Harris, J.M.; Gall, C.; Thompson, J.C.; Richardson, A.M.; Neary, D.; du Plessis, D.; Pal, P.; Mann, D.M.; Snowden, J.S.; Jones, M. Classification and pathology of primary progressive aphasia. Neurology 2013, 81, 1832–1839. [Google Scholar] [CrossRef]
| Participant No. (Age)/Sex/Years of Education | Type of Dementia (bvFTD; nfvPPA; MCI/AD; Control) | NPTs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CASI | MMSE | MoCA | CDR | VFT | AST (L/R) | ToMT | CANTAB (PAL/IED/IED∑/RVP) | FCSRT (FR/TR/CE/DFR/DTR/DCE) | ||
| 1 (66)/M/9 | bvFTD | 74 * | 25 | 18 * | 1 | 11 * | 7 */9 | 7 * | −4.01/3 */−5.86/0.82 * | 18/48/1/13/16/1 |
| 2 (65)/M/16 | MCI/AD | 87 | 28 | 25 * | 0.5 | 27 | 12/12 | 13 | 0.24/7 */−1.36/0.84 * | 18/48/1/10/16/1 |
| 3 (71)/M/6 | MCI/AD | 88 | 27 | 26 | 0.5 | 43 | 12/12 | 15 | 0.61/8/0.02/0.72 * | 31/48/1/14/16/1 |
| 4 (62)/M/12 | nfvPPA | 73 * | 26 | 18 * | 0.5 | 5 * | 12/12 | 4 * | 0.63/9/−0.43/0.91 * | 10 */27/0.5/4 */8 */0.3 * |
| 5 (54)/F/16 | bvFTD | 66 * | 25 | 18 * | 0.5 | 16 * | 12/12 | 8 * | −5.67/2 */−4.43/0.90 * | 36/48/1/12/16/1 |
| 6 (66)/F/9 | MCI/AD | 98 | 27 | 29 | 0.5 | 30 | 12/12 | 17 | −0.86/9/−0.34/0.92 | 29/48/1/10/14/0.7 |
| 7 (62)/F/16 | nfvPPA | 68 * | 23 * | 16 * | 0.5 | 8 * | 12/12 | 9 * | −2.25/7/−0.71/0.89 * | 5 */48/1/0 */6 */0.3 * |
| 8 (67)/F/16 | nfvPPA | 52 * | 13 * | 13 * | 0.5 | 10 * | 8 */8 * | 4 * | −1.18/9/0.15/0.86 * | 9 */37/0.9/0 */4 */0.3 * |
| 9 (64)/M/12 | Control | 96 | 30 | 26 | 0 | 34 | 12/12 | 18 | 0.82/9/0.82/0.95 | 36/48/1/11/15/0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-Y.; Tseng, H.-Y.; Stachiv, I.; Tsai, C.-H.; Lai, Y.-C.; Nikolai, T. Combining Neuropsychological Assessment with Neuroimaging to Distinguish Early-Stage Alzheimer’s Disease from Frontotemporal Lobar Degeneration in Non-Western Tonal Native Language-Speaking Individuals Living in Taiwan: A Case Series. J. Clin. Med. 2023, 12, 1322. https://doi.org/10.3390/jcm12041322
Kuo C-Y, Tseng H-Y, Stachiv I, Tsai C-H, Lai Y-C, Nikolai T. Combining Neuropsychological Assessment with Neuroimaging to Distinguish Early-Stage Alzheimer’s Disease from Frontotemporal Lobar Degeneration in Non-Western Tonal Native Language-Speaking Individuals Living in Taiwan: A Case Series. Journal of Clinical Medicine. 2023; 12(4):1322. https://doi.org/10.3390/jcm12041322
Chicago/Turabian StyleKuo, Chih-Yun, Hsin-Yi Tseng, Ivo Stachiv, Chon-Haw Tsai, Yi-Chun Lai, and Tomas Nikolai. 2023. "Combining Neuropsychological Assessment with Neuroimaging to Distinguish Early-Stage Alzheimer’s Disease from Frontotemporal Lobar Degeneration in Non-Western Tonal Native Language-Speaking Individuals Living in Taiwan: A Case Series" Journal of Clinical Medicine 12, no. 4: 1322. https://doi.org/10.3390/jcm12041322
APA StyleKuo, C.-Y., Tseng, H.-Y., Stachiv, I., Tsai, C.-H., Lai, Y.-C., & Nikolai, T. (2023). Combining Neuropsychological Assessment with Neuroimaging to Distinguish Early-Stage Alzheimer’s Disease from Frontotemporal Lobar Degeneration in Non-Western Tonal Native Language-Speaking Individuals Living in Taiwan: A Case Series. Journal of Clinical Medicine, 12(4), 1322. https://doi.org/10.3390/jcm12041322







