The Importance of Optical Coherence Tomography in the Diagnosis of Atypical or Subclinical Optic Neuritis: A Case Series Study
Abstract
:1. Introduction
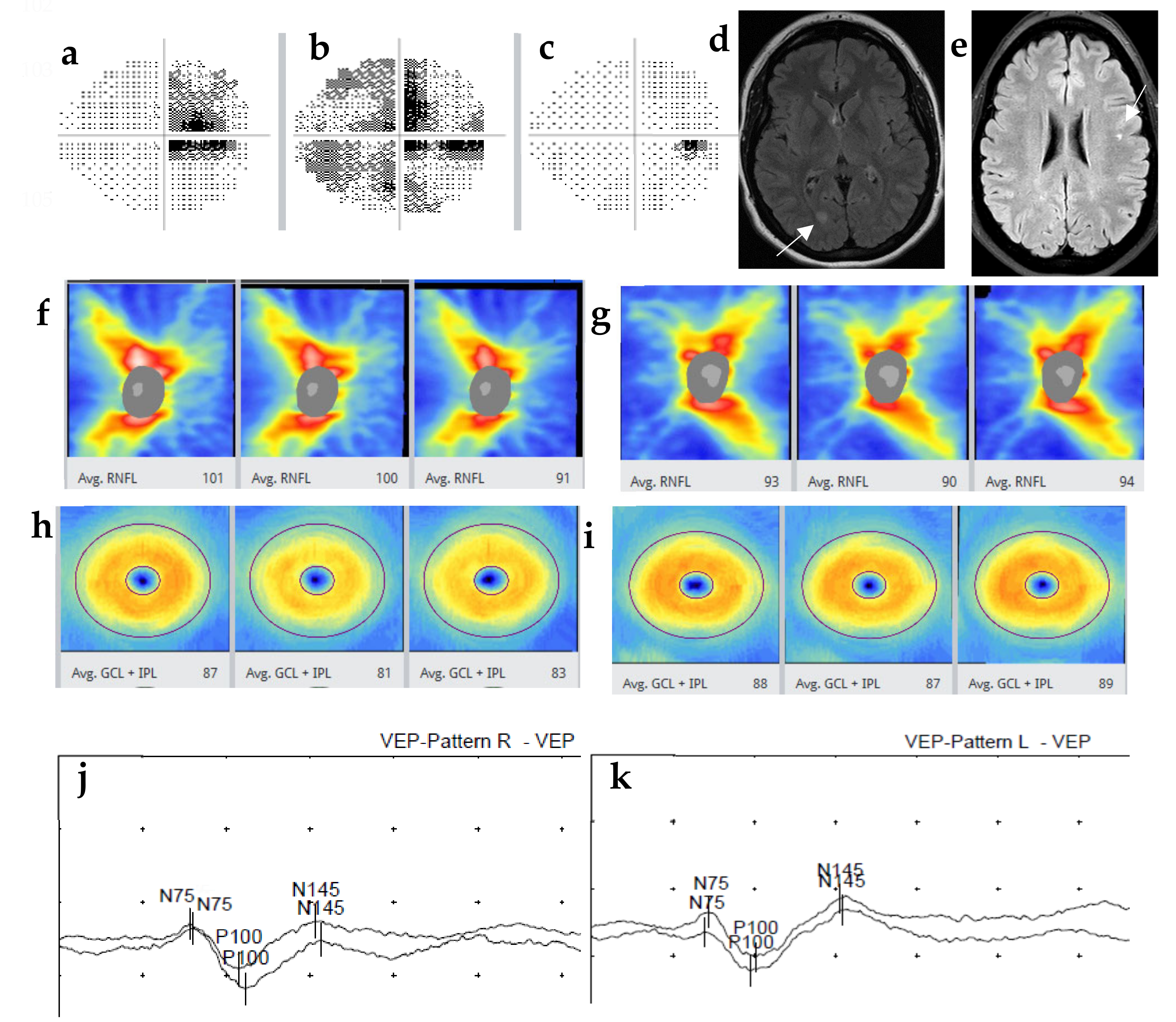
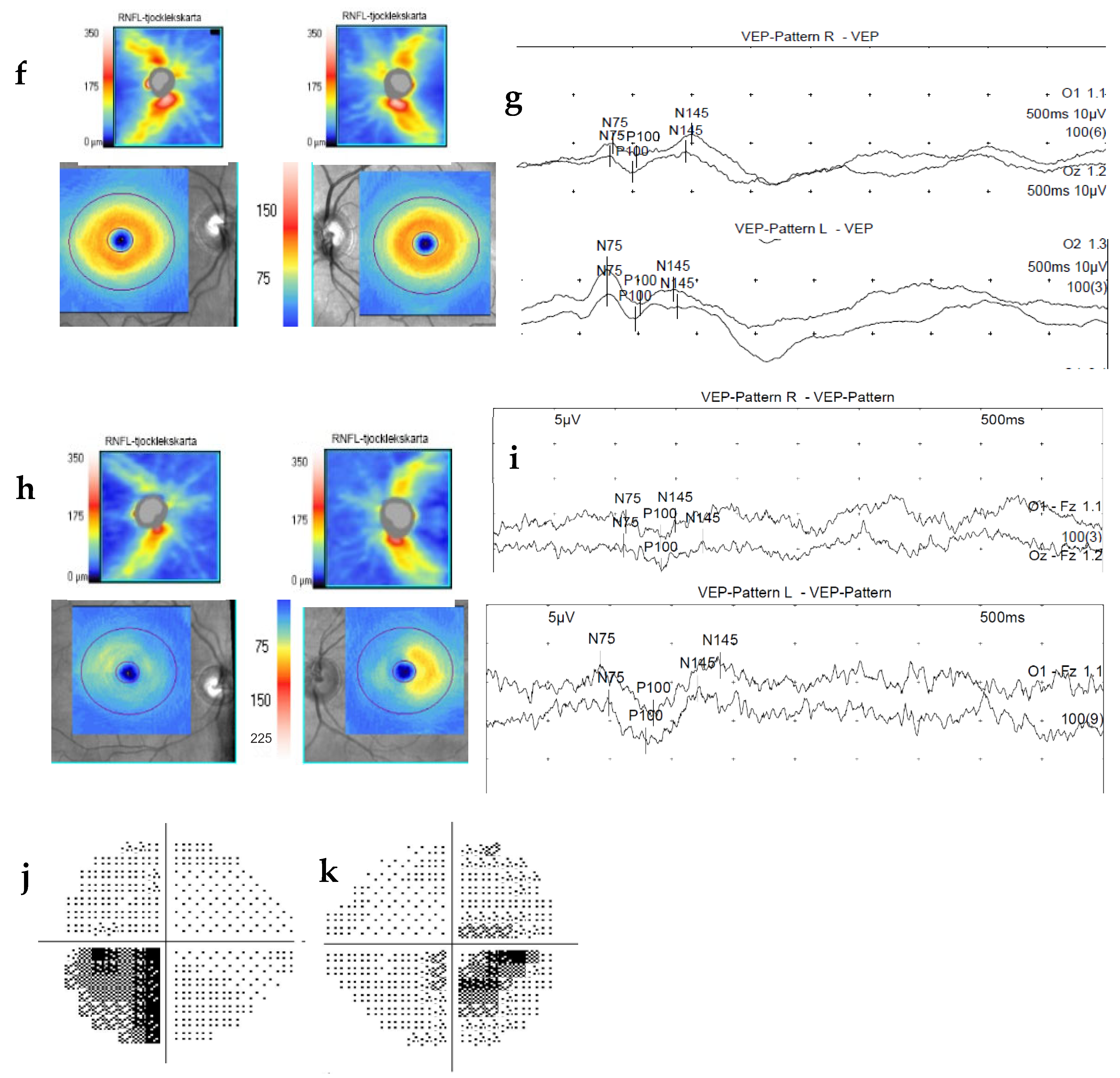
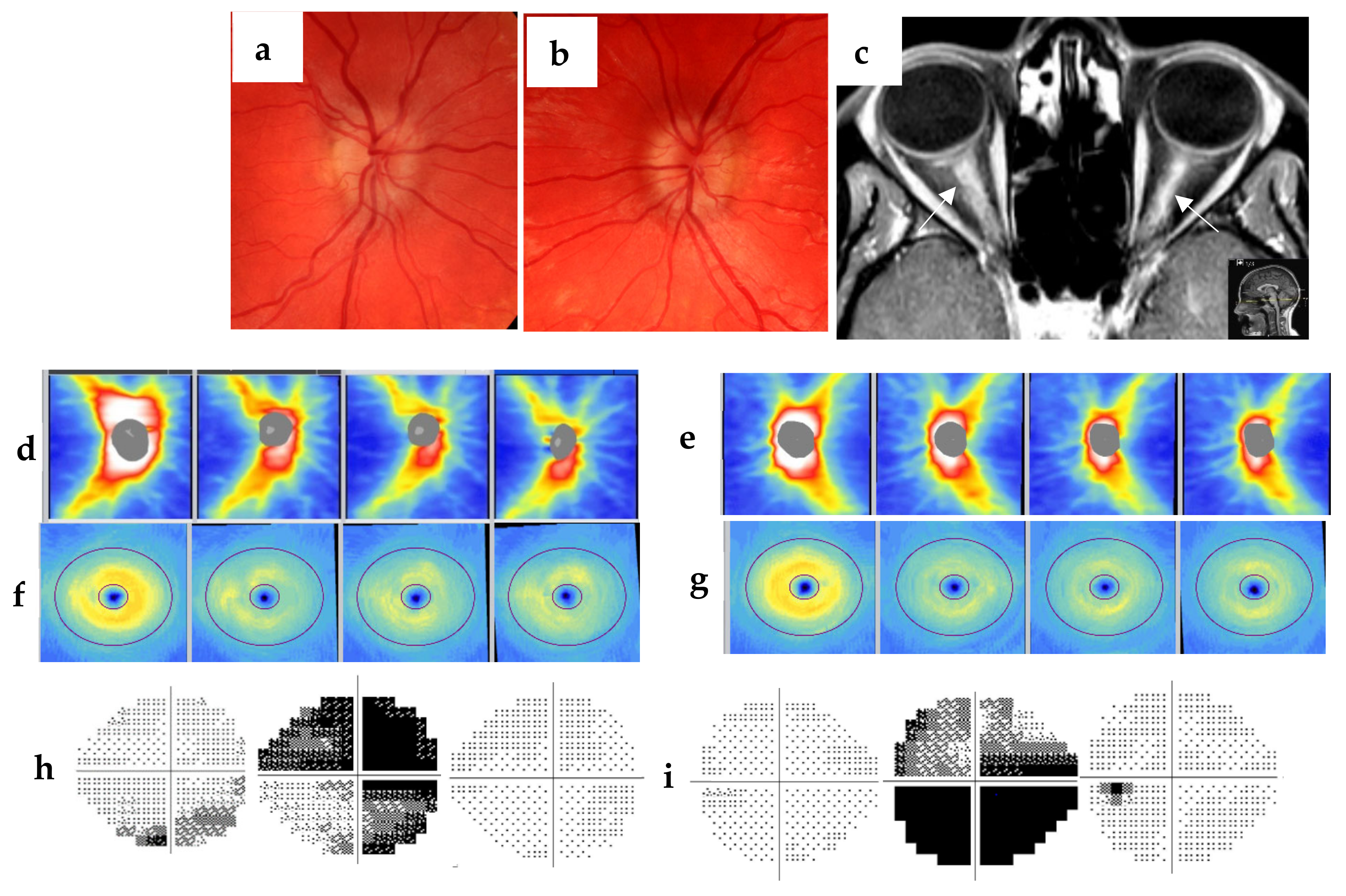
2. Methods and Materials
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vries-Knoppert, W.A.; Baaijen, J.C.; Petzold, A. Patterns of retrograde axonal degeneration in the visual system. Brain 2019, 142, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Galetta, S. Anatomy and physiology of the afferent visual system. Handb. Clin. Neurol. 2012, 102, 3–19. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Pittock, S.J.; Flanagan, E.P.; Lennon, V.A.; Bhatti, M.T. Optic neuritis in the era of biomarkers. Surv. Ophthalmol. 2020, 65, 12–17. [Google Scholar] [CrossRef]
- Lu, A.; Zimmermann, H.G.; Specovius, S.; Motamedi, S.; Chien, C.; Bereuter, C.; Lana-Peixoto, M.A.; Fontenelle, M.A.; Ashtari, F.; Kafieh, R.; et al. Astrocytic outer retinal layer thinning is not a feature in AQP4-IgG seropositive neuromyelitis optica spectrum disorders. J. Neurol. Neurosurg. Psychiatry 2022, 93, 188–195. [Google Scholar] [CrossRef]
- Bennett, J.; de Seze, J.; Lana-Peixoto, M.; Palace, J.; Waldman, A.; Schippling, S.; Tenembaum, S.; Banwell, B.; Greenberg, B.; Levy, M.; et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult. Scler. J. 2015, 21, 678–688. [Google Scholar] [CrossRef]
- Gaier, E.D.; Boudreault, K.; Rizzo, J.F.; Falardeau, J.; Cestari, D.M. Atypical Optic Neuritis. Curr. Neurol. Neurosci. Rep. 2015, 15, 76. [Google Scholar] [CrossRef]
- Yuksel, B.; Dogan, B.; Koctekin, B.; Atis, N.; Erdal, A.; Kurtulus, F.; Erol, M.K.; Gomceli, Y.B. Color vision testing versus pattern visual evoked potentials and optical coherence tomography parameters in subclinical optic nerve involvement in multiple sclerosis. J. Clin. Neurosci. 2019, 61, 48–53. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Thouvenot, É. Update on clinically isolated syndrome. Press Med. 2015, 44, e121–e136. [Google Scholar] [CrossRef]
- Ikuta, F.; Zimmerman, H.M. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 1976, 26, 26–28. [Google Scholar] [CrossRef]
- Davion, J.-B.; Lopes, R.; Drumez, É.; Labreuche, J.; Hadhoum, N.; Lannoy, J.; Vermersch, P.; Pruvo, J.-P.; Leclerc, X.; Zéphir, H.; et al. Asymptomatic optic nerve lesions An underestimated cause of silent retinal atrophy in MS. Neurology 2020, 94, e2468–e2478. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Fraser, C.L.; Abegg, M.; Alroughani, R.; Alshowaeir, D.; Alvarenga, R.; Andris, C.; Asgari, N.; Barnett, Y.; Battistella, R.; et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022, 21, 1120–1134. [Google Scholar] [CrossRef]
- Soelberg, K.; Specovius, S.; Zimmermann, H.G.; Grauslund, J.; Mehlsen, J.J.; Olesen, C.; Neve, A.S.B.; Paul, F.; Brandt, A.U.; Asgari, N. Optical coherence tomography in acute optic neuritis: A population-based study. Acta Neurol. Scand. 2018, 138, 566–573. [Google Scholar] [CrossRef]
- Paul, F.; Calabresi, P.A.; Barkhof, F.; Green, A.J.; Kardon, R.; Sastre-Garriga, J.; Schippling, S.; Vermersch, P.; Saidha, S.; Gerendas, B.S.; et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Ann. Clin. Transl. Neurol. 2021, 8, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Naismith, R.T.; Cross, A.H. Misdiagnosis of multiple sclerosis. Neurology 2019, 92, 26–33. [Google Scholar] [CrossRef]
- Fujihara, K. Neuromyelitis optica spectrum disorders: Still evolving and broadening. Curr. Opin. Neurol. 2019, 32, 385–394. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Yeo, T.; Probert, F.; Jurynczyk, M.; Sealey, M.; Cavey, A.; Claridge, T.D.; Woodhall, M.; Waters, P.; Leite, M.I.; Anthony, D.C.; et al. Classifying the antibody-negative NMO syndromes. Neurol.-Neuroimmunol. Neuroinflamm. 2019, 6, e626. [Google Scholar] [CrossRef]
- Sato, D.K.; Callegaro, D.; Lana-Peixoto, M.A.; Waters, P.J.; de Haidar Jorge, F.M.; Takahashi, T.; Nakashima, I.; Apostolos-Pereira, S.L.; Talim, N.; Simm, R.F.; et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014, 82, 474–481. [Google Scholar] [CrossRef]
- Toosy, A.T.; Mason, D.F.; Miller, D.H. Optic neuritis. Lancet Neurol. 2014, 13, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.; Meier, U.-C.; Marta, M.; Costa, G.D.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. J. 2015, 21, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Nolan-Kenney, R.C.; Liu, M.; Akhand, O.; Calabresi, P.A.; Paul, F.; Petzold, A.; Balk, L.; Brandt, A.U.; Martínez-Lapiscina, E.H.; Saidha, S.; et al. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: An international study. Ann. Neurol. 2019, 85, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Huang-Link, Y.-M.; Al-Hawasi, A.; Lindehammar, H. Acute optic neuritis: Retinal ganglion cell loss precedes retinal nerve fiber thinning. Neurol. Sci. 2015, 36, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Grecescu, M. Optical Coherence Tomography versus Visual Evoked Potentials in detecting subclinical visual impairment in multiple sclerosis. J. Med. Life 2014, 7, 538–541. [Google Scholar]
- Janáky, M.; Jánossy, Á.; Horváth, G.; Benedek, G.; Braunitzer, G. VEP and PERG in patients with multiple sclerosis, with and without a history of optic neuritis. Doc. Ophthalmol. 2017, 134, 185–193. [Google Scholar] [CrossRef]
- Hardmeier, M.; Leocani, L.; Fuhr, P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. Mult. Scler. J. 2017, 23, 1309–1319. [Google Scholar] [CrossRef]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef]
- Friedman, D.I.; Quiros, P.A.; Subramanian, P.S.; Mejico, L.J.; Gao, S.; McDermott, M.; Wall, M.; the NORDIC IIHTT Study Group. Headache in Idiopathic Intracranial Hypertension: Findings From the Idiopathic Intracranial Hypertension Treatment Trial. Headache 2017, 57, 1195–1205. [Google Scholar] [CrossRef]
- Ungureanu, A.; De Seze, J.; Ahle, G.; Sellal, F. Myelin oligodendrocyte glycoprotein antibodies in neuromyelitis optica spectrum disorder. Rev. Neurol. 2018, 174, 675–679. [Google Scholar] [CrossRef]
- Albrecht, P.; Blasberg, C.; Ringelstein, M.; Müller, A.-K.; Finis, D.; Guthoff, R.; Kadas, E.-M.; Lagreze, W.; Aktas, O.; Hartung, H.-P.; et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J. Neurol. 2017, 264, 1370–1380. [Google Scholar] [CrossRef]
- Huang-Link, Y.; Eleftheriou, A.; Yang, G.; Johansson, J.M.; Apostolou, A.; Link, H.; Jin, Y.-P. Optical coherence tomography represents a sensitive and reliable tool for routine monitoring of idiopathic intracranial hypertension with and without papilledema. Eur. J. Neurol. 2019, 26, 808-e57. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang-Link, Y.; Yang, G.; Gustafsson, G.; Gauffin, H.; Landtblom, A.-M.; Mirabelli, P.; Link, H. The Importance of Optical Coherence Tomography in the Diagnosis of Atypical or Subclinical Optic Neuritis: A Case Series Study. J. Clin. Med. 2023, 12, 1309. https://doi.org/10.3390/jcm12041309
Huang-Link Y, Yang G, Gustafsson G, Gauffin H, Landtblom A-M, Mirabelli P, Link H. The Importance of Optical Coherence Tomography in the Diagnosis of Atypical or Subclinical Optic Neuritis: A Case Series Study. Journal of Clinical Medicine. 2023; 12(4):1309. https://doi.org/10.3390/jcm12041309
Chicago/Turabian StyleHuang-Link, Yumin, Ge Yang, Greta Gustafsson, Helena Gauffin, Anne-Marie Landtblom, Pierfrancesco Mirabelli, and Hans Link. 2023. "The Importance of Optical Coherence Tomography in the Diagnosis of Atypical or Subclinical Optic Neuritis: A Case Series Study" Journal of Clinical Medicine 12, no. 4: 1309. https://doi.org/10.3390/jcm12041309
APA StyleHuang-Link, Y., Yang, G., Gustafsson, G., Gauffin, H., Landtblom, A.-M., Mirabelli, P., & Link, H. (2023). The Importance of Optical Coherence Tomography in the Diagnosis of Atypical or Subclinical Optic Neuritis: A Case Series Study. Journal of Clinical Medicine, 12(4), 1309. https://doi.org/10.3390/jcm12041309





