Abstract
Background: The aims of this study were to compare the survival outcomes of salvage vs. primary total laryngectomy (TL) in patients with locally advanced laryngeal or hypopharyngeal carcinoma and to determine their predictive factors. Methods: Overall (OS), cause-specific (CSS) and recurrence-free survival (RFS) of primary vs. salvage TL were compared in univariate and multivariate analysis taking into account other potential predictive factors (tumor site, tumor stage, comorbidity level etc.). Results: A total of 234 patients were included in this study. Five-year OS was 53% and 25% for the primary and salvage TL groups, respectively. Multivariate analysis confirmed the independent negative impact of salvage TL on OS (p = 0.0008), CSS (p < 0.0001) and RFS (p < 0.0001). Hypopharyngeal tumor site, ASA score ≥ 3, N-stage ≥ 2a and positive surgical margins were the main other predictors of oncologic outcomes. Conclusions: Salvage TL is associated with significantly worse survival rates than primary TL highlighting the need for careful selection of patients who are candidates for larynx preservation. The predictive factors of survival outcomes identified here should be considered in the therapeutic decision-making, especially in the setting of salvage TL, given the poor prognosis of these patients.
1. Introduction
There are different validated therapeutic options to preserve laryngeal functions in patients with laryngeal or hypopharyngeal carcinoma [1,2,3]. Patients with early stage disease are offered definitive radiation therapy (RT) or partial surgery (i.e., endoscopic laser CO2 surgery, transoral robotic surgery or open partial pharyngo-laryngectomy) [1,2,3]. Since the 1990s and the development of laryngeal preservation (LP) protocols, most patients with locally advanced laryngeal and hypopharyngeal carcinoma are included in these complex therapeutic programs, combining either induction or concurrent chemotherapy with RT [3,4,5,6,7]. Gradually, radical surgery with total laryngectomy (TL) was therefore relegated to the salvage treatment of recurrent tumors [8,9].
The first LP studies showed that induction chemotherapy (ICT) with cisplatin and 5-fluorouracil (PF) followed by RT in good responders could preserve nearly 60% of larynx without deleterious impact on survival compared with the gold standard TL followed by RT [3,4,5]. Thereafter, other LP protocols have been developed investigating the role of definitive concurrent chemoradiation therapy (CRT) or of the combination of docetaxel (T) with the standard PF ICT regimen for LP [6,7]. Indeed, to date, there are two main validated modalities of LP in patients with locally advanced laryngeal or hypopharyngeal carcinoma: TPF ICT followed by RT in responders (preferred option in France and Europe) or definitive concurrent CRT (preferred option in North America) [3].
Twenty years after the development of LP, an epidemiological study in the USA covering 158,426 cases of laryngeal carcinoma from the “National Cancer Data Base” observed a decrease in the survival rate of patients since the mid-1990s, potentially in relationship with changes in therapeutic strategies and, in particular, the increase in the initial use of non-surgical treatments [10]. In this context, several studies have recently focused on the results of salvage TL but included a significant proportion of patients with initial early stage tumors and/or treated outside of a LP program [11,12,13,14,15,16,17,18,19]. As a result, it is largely unknown whether TL performed as a salvage procedure after failure of a LP program is associated with worse survival compared with primary TL for patients with locally advanced laryngeal or hypopharyngeal carcinoma.
The aims of this study were therefore to compare the survival outcomes of salvage TL with those of primary TL in patients with locally advanced laryngeal or hypopharyngeal carcinoma and to determine the predictive factors of oncologic results.
2. Materials and Methods
2.1. Ethical Considerations
The study protocol was reviewed and approved by the institutional ethics committee prior to the start of the study (approval code: F20220422105156). Informed consent was obtained from each of the participants.
2.2. Subjects
In this retrospective study, we included all patients who underwent TL between 2000 and 2020, at our institution, for a locally advanced (T3 or T4) squamous cell carcinoma of the larynx or hypopharynx, either as a primary therapeutic option or as a salvage procedure after failure of an LP program. The therapeutic strategy was elaborated for each specific patient during a multidisciplinary tumor board (MTB) discussion. The exclusion criteria were as follows: histology other than squamous cell carcinoma, tumor stage T1 or T2 at diagnosis, TL performed for an indication other than progressive cancer, salvage TL performed after a treatment other than a LP protocol (open or transoral partial laryngectomy, radiotherapy alone). LP protocols consisted of either cisplatin-based concurrent CRT or ICT (with PF before 2005 or TPF since 2005) followed by RT in good responders.
2.3. Follow-Up
Post-therapeutic follow-up was conducted in accordance with national guidelines. Patient clinical examination was scheduled every two months during the first two years, then every four months. A head and neck and thoracic CT-scan was performed 3 to 4 months after the initial treatment and once a year for the next 5 years, or in cases of clinical suspicion of tumor recurrence.
2.4. Main Outcome Measures
Patients’ general health status and comorbidity level were assessed using the American Society of Anesthesiologists (ASA) score. Tumor stage was determined according to the 8th edition (2017) of the Tumor Node Metastasis (TNM) classification of the Union for International Cancer Control and the American Joint Committee on Cancer.
Overall survival (OS), cause-specific survival (CSS) and recurrence-free survival (RFS) were determined by Kaplan–Meier analysis.
2.5. Statistical Analyses
Patients were divided into two groups based on the type of surgery: primary TL group and salvage TL group. Each patient’s clinical characteristics (gender, age, tumor site, T- and N-stage, etc.) of these two groups of patients were compared using Chi-square tests.
We analyzed the impact on OS, CSS and RFS of the type of surgery (primary vs. salvage TL) and of the following factors: age (< vs. >70 years), gender (male vs. female), ASA score (< vs. ≥3), primary tumor site (larynx vs. hypopharynx), preoperative tracheostomy, surgical margins (negative vs. positive), T-stage (3 vs. 4) and N-stage (< vs. ≥2A). Univariate analyses were performed using Log Rank tests. For multivariate analysis (conducted only when more than one factor was significant in univariate analyses), all variables associated with p < 0.05 in univariate analysis were included in Cox regression models with forward stepwise selection. The predictive ability of each Cox regression model was determined using the area under the receiver operating characteristic (ROC) curve (AUC) with a clinical endpoint fixed at 5 years.
All statistical analyses were performed at 5% alpha risk or 95% confidence interval by the biostatistician using R.3.0.1 software on Windows.
3. Results
3.1. Sample Description
Between 2000 and 2020, 353 patients underwent TL at our institution. After applying exclusion criteria (Figure 1), a total of 234 patients were included in the study (209 men and 25 women, mean age = 67.1 ± 10.5 years), 184 (79%) in the primary TL group and 50 (21%) in the salvage TL group. In the salvage TL group, 8 patients had received cisplatin-based concurrent CRT and 42 patients ICT with PF (n = 5) or TPF (n = 37) followed by RT.
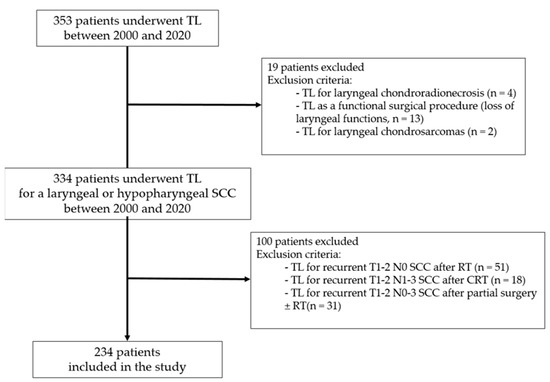
Figure 1.
Flowchart of patient selection. TL: total laryngectomy; SCC: squamous cell carcinoma; RT: radiation therapy; CRT: chemoradiation therapy.
Patients’ clinical characteristics for the whole cohort and according to the type of surgery (primary vs. salvage TL) are presented in Table 1. Compared with the primary TL group, patients of the salvage TL group displayed a significantly lower T-stage, a higher N-stage and higher rates of hypopharyngeal tumor, TL with circular pharyngectomy, pedicled or free flap pharyngeal reconstruction and involved surgical margins.

Table 1.
Patients’ clinical characteristics according to the type of surgery.
3.2. Oncologic Outcomes in the Primary vs. Salvage TL Groups
Median follow-up was 46.5 months (95% CI (95% confidence interval): 33.1–87.5 months). As shown in Figure 2a–c, OS, CSS and RFS were significantly lower (p < 0.001) for patients of the salvage TL group than for those of the primary TL group. Five-year OS, CSS and RFS were 25 vs. 53%, 34 vs. 64% and 16 vs. 45% for the salvage and primary TL groups, respectively.
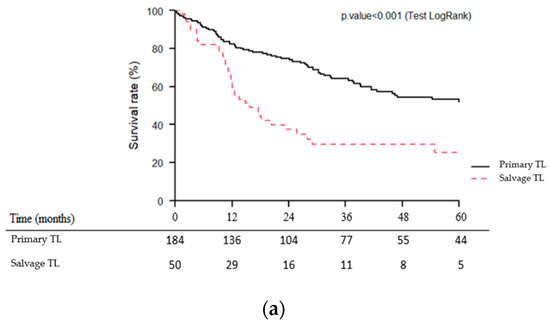
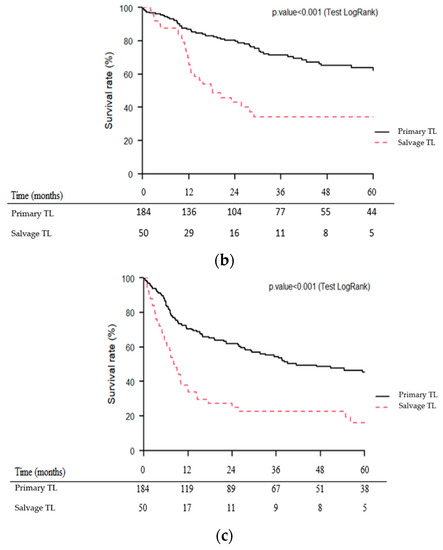
Figure 2.
Kaplan–Meier overall (a), cause-specific (b) and recurrence-free (c) survival curves for patients undergoing primary vs. salvage total laryngectomy (TL).
3.3. Impact of Tumor Site on Oncologic Outcomes
Considering the observed differences in baseline patients’ clinical characteristics between the two treatment groups (primary vs. salvage TL) regarding the distribution of primary tumor site (larynx vs. hypopharynx), we compared the oncologic outcomes of primary vs. salvage TL separately, for patients with laryngeal carcinoma and for those with hypopharyngeal carcinoma. These results are shown in Table 2.

Table 2.
Oncologic outcomes according to type of surgery and tumor site.
For patients with laryngeal carcinoma, primary TL was significantly associated with better OS (p = 0.002), CSS (p < 0.001) and RFS (p = 0.02) than salvage TL. For patients with hypopharyngeal carcinoma, primary TL tended to be associated with better OS (p = 0.06) and CSS (p = 0.1) than salvage TL. No significant difference was observed for RFS (p = 0.2).
Kaplan–Meier survival curves of the four subgroups of patients (1: larynx—primary TL, 2: larynx—salvage TL, 3: hypopharynx—primary TL, 4: hypopharynx—salvage TL) for OS, CSS and RFS are presented in Figure 3a–c.
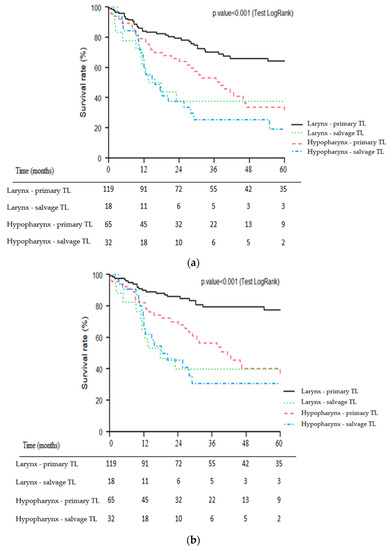
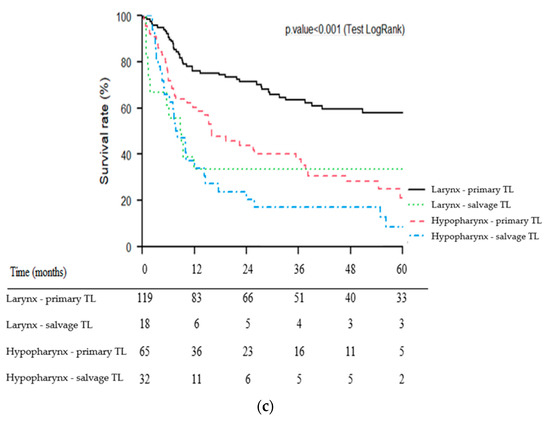
Figure 3.
Kaplan–Meier overall (a), cause-specific (b) and recurrence-free (c) survival curves for patients undergoing primary vs. salvage total laryngectomy (TL) according to primary tumor site (larynx vs. hypopharynx).
3.4. Multivariate Analysis of the Predictive Factors of Oncologic Outcomes
The impact of all the factors considered on the oncologic outcomes in uni- and multivariate analyses are presented in Table 3.

Table 3.
Predictors of oncologic outcomes in univariate and multivariate analyses.
The negative independent impact of salvage TL on oncologic outcomes (OS, CSS and RFS) was confirmed in multivariate analysis. Hypopharyngeal tumor site, ASA score ≥ 3, N-stage ≥ 2a and positive surgical margins were the main other predictors of poor prognosis.
ROC curves with their AUC for the three Cox regression models (OS, CSS and RFS) are presented in Figure 4. Of note, an AUC > 0.80 were obtained for the three ROC curves indicating a good predictive ability for the three Cox regression models.
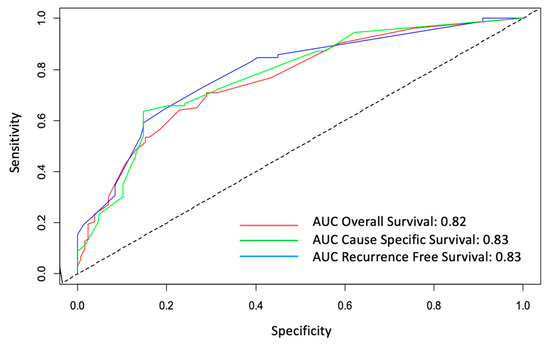
Figure 4.
ROC curves for overall (OS), cause-specific (CSS) and recurrence-free survivals (RFS). AUC: area under the curve.
4. Discussion
To our knowledge, the present study is the first to demonstrate the prognostic role of salvage TL compared to primary TL for patients with a locally advanced laryngeal or hypopharyngeal carcinoma (T3 or T4) at initial presentation. Indeed, previous studies on this subject had included in the salvage TL group a variety of tumors, especially second primary carcinomas developed in a previously irradiated neck and recurrences of early stage tumors (T1 or T2) initially treated by partial surgery (endoscopic or external) or by RT [16,17]. Obviously, this inclusion bias leads to an overestimation of patient survival rates in the salvage TL group. However, in terms of oncologic results, it is the comparison of primary vs. salvage TL for patients who are all initially eligible for TL that is meaningful and raises the question of the proper selection of patients for LP. This negative impact of salvage surgery was independently confirmed in multivariate analysis on the three survival parameters studied (OS, CSS and RFS).
This multivariate analysis was even more necessary as the two groups compared (primary vs. salvage TL) were not similar in terms of baseline clinical characteristics and, particularly, in terms of tumor site distribution (larynx vs. hypopharynx). Indeed, hypopharyngeal carcinomas are known to be more aggressive than laryngeal carcinomas, with notably higher submucosal, lymphatic and metastatic spread [20,21]. Thus, it could be argued that the difference in survival between primary and salvage TL was related to the over-representation of hypopharyngeal carcinomas in the salvage TL group compared to the primary TL group. However, the multivariate analysis confirmed the independent negative impact on survival outcomes of both salvage surgery and hypopharyngeal tumor site.
Moreover, the subgroup analysis according to the tumor site clearly validated the negative impact of salvage surgery on oncologic outcomes in the subgroup of patients with laryngeal carcinomas. For hypopharyngeal carcinomas, the negative impact of salvage surgery on patient survival also appeared to be present with worse crude survival rates than the primary TL group but with a statistical significance threshold that was not reached due to the limited number of patients. The differences observed in terms of survival were indeed major with survival rates almost halved between primary and salvage TL. Thus, the 5-year OS rates were 64% vs. 38% for laryngeal carcinomas and 34% vs. 19% for hypopharyngeal carcinomas in the primary and salvage TL groups, respectively.
The large LP studies performed in the 1990s and early 2000s were based on very strict patient selection [1,2,3,4,5,6,7]. For studies involving ICT, the assessment of tumor response to ICT based on both objective tumor response (tumor volume) and laryngeal remobilization, was also a very strict condition for continuing the LP protocol [3,4,5,7]. In addition, several patients included in these LP studies had tumors of T-stage ≤ 2, without laryngeal fixation, but with lymph node extension (explaining the overall stage III–IV), for which a conservative surgery was an option [1]. Thus, in the VA study, 10% of patients had a T stage ≤ 2 tumor and 43% of patients did not have laryngeal fixation (a major criterion contraindicating partial surgery) [4]. The Radiation Therapy Oncology Group (RTOG) study conducted by Forastiere et al., 2003 [6] included 20% T2 tumors and 44% mobile larynx [6].
At the opposite, patients with a T4-stage tumor invading the thyroid cartilage are not good candidates for LP and should theoretically be referred to radical surgery [3,8]. However, for more than 20 years, in daily practice, the selection of patients included in LP protocols has not been as rigorous and a certain number of patients have not been redirected to TL or were even refused this procedure despite the absence of significant response to ICT [1,3,8]. This explains why the results of LP protocols outside of clinical trials are not as promising as those reported in the original studies. At the same time, the possibilities for surgical salvage of recurrent tumor after a LP program are not as good in clinical practice as they were in the early studies. An update of the RTOG 91-11 study focusing on the quarter of patients who required a salvage TL showed a very favorable two-year OS rate of 72% (compared to 38% in our study for the larynx subgroup) [11]. This result may appear very similar to what can be achieved after a primary TL, leading to the erroneous, yet widespread consideration that there is always time to perform salvage TL after an LP failure and that it does not compromise survival. However, a critical analysis of patient selection shows that many of the patients who underwent salvage TL in the RTOG 91-11 study did not correspond to the indications for primary TL at initial diagnosis [6,11]. Although overall tumor stage is usually well correlated with patient prognosis, it is important to accurately describe T- and N-stage separately, to better define patient selection criteria. For this reason, in the present study, we chose to independently analyze the impact of T- and N-stage on survival outcomes.
Several retrospective studies have more recently analyzed a large series of salvage TL to assess the results of this surgery without comparison to a primary TL group [12,13,14,15]. Sandulache et al. reported a 5-year OS rate of 57% in 218 patients undergoing salvage surgery for laryngeal carcinoma, but most of them presented a stage I-II tumor at diagnosis [12]. There is considerable variability in the oncological results of salvage TL in the literature, the populations of the different studies being rarely superimposable [11,12,13,14,15,16,17,18,19]. To increase the volume of patients for statistical analysis, many authors included patients with T1-T2 tumors relapsing after RT or conservative surgery [11,12,13,14,15,16,17,18,19]. In the present study, we chose to exclude these patients, to keep only those presenting with a stage T ≥ 3 tumor at initial diagnosis for whom a primary TL could be discussed.
In another study of 244 patients undergoing salvage TL for laryngeal carcinoma, Birkeland et al. found a 5-year OS of 49% [18]. In this study, which did not yet include only patients with an initial stage T ≥ 3, the authors also showed that a high comorbidity index was the main factor of poor prognosis [18]. This negative role of the level of comorbidity on OS is usually found in surgical oncology [22]. In this regard, in multivariate analysis, we also showed the negative prognostic role of an ASA score ≥ 3 on both OS and DFS. This highlights the importance of selecting patients who are candidates for salvage surgery, whose prognosis is already poor and could also be threatened by uncontrolled comorbidities.
Few studies on salvage TL have focused on hypopharyngeal carcinomas. It is likely that in the setting of recurrent locally advanced tumors, it can sometimes be challenging to distinguish hypopharyngeal carcinomas from those arising in the supraglottis. In our series, the hypopharyngeal tumor site was an independent factor of poor prognosis which has also been reported in several studies [19,22,23]. Detailed analysis of the survival curves (Figure 3a–c) of the present study also showed that the impact of tumor site appeared to be greater in the primary TL group (well-separated larynx vs. hypopharynx curves) than in the salvage group. The salvage TL subgroup for hypopharyngeal carcinoma had the poorest prognosis with particularly poor survival rates (3- and 5-year OS of 25% and 19%, respectively). Even in LP trials, long-term survival rates for patients with hypopharyngeal carcinoma are particularly poor [5,7]. For example, in the EORTC LP study (trial 24891), patients in the ICT group had 5- and 10-year OS rates of 21.9% and 8.7%, respectively [5]. In a multicenter study of 21 patients who underwent salvage circular TL for recurrent hypopharyngeal carcinoma, Fakhry et al. showed a 5-year OS rate of 16% [24]. For hypopharyngeal carcinoma patients, the indication of a salvage TL must therefore be carefully weighed, especially since it is accompanied by a high morbidity and mortality [19,24]. Rigorous patient information is crucial here, as well as considering the patient’s overall situation (tumor extension, previous treatment, general health status, comorbidities, psychosocial status, etc.) [25]. The appropriate selection of patients for salvage surgery is indeed one of the most difficult decisions in head and neck oncology and can only be made in the context of a multidisciplinary discussion integrating the patient’s preferences.
Among the other poor prognostic factors identified in the present study, the role of N stage ≥ 2a should be noted, with a high statistically significant negative impact on the three survival end-points (OS, CSS and RFS). These results are consistent with the literature [26,27,28,29]. In a study of 316 patients with all stages of laryngeal carcinoma, Haapaniemi et al. reported a recurrence rate of 22% and showed that the initial presence of lymph node metastases was an independent predictor of recurrence [26]. In another study of 105 patients with recurrent head and neck carcinoma, Agrawal et al. showed that in patients who underwent salvage surgery, early initial tumor stage and isolated local recurrence without lymph node involvement were associated with a better prognosis [27]. Lymphatic spread is therefore a major factor to consider in therapeutic decision making, particularly before salvage TL, given the poor prognosis of the patients. The probability of achieving free surgical margins is also an important element to consider since our study showed, as is usual in oncologic surgery, the independent prognostic impact of the surgical margin status [22,23,24].
The increase in mortality of laryngeal carcinomas observed in the USA over the last 30 years could be interpreted in the light of the results of our study [10]. A policy of LP “at all cost” could have led to a decrease in patient survival [1,2,3,30]. Obviously, the present study is not intended to discuss the interest of LP and the benefits it has brought to patients for many years, particularly in terms of quality of life. Results of LP studies have indeed allowed many patients to preserve a functional larynx without compromising their survival [3]. However, the present study underlines the importance of an accurate selection of patients who are candidates for LP, knowing that the negative prognostic impact of tumor recurrence after a LP program will not be reversed by salvage surgery.
The main limitations of our study are its retrospective and single-center study design. The integration of data from other referral centers would increase the number of patients in the different subgroups, especially for salvage TL, and thus increase the power of our study. Analyzing together patients with laryngeal and hypopharyngeal carcinoma who have a very different prognosis may represent a weakness of our study. Nevertheless, this enabled us to assess the impact of the primary tumor site (hypopharynx vs. larynx) on patient oncologic outcomes and to evaluate separately the prognostic effect of the type of surgery (salvage vs. primary TL) in these two subgroups of patients. Moreover, it should be noted that the salvage TL group did not include all the failures of LP since some patients with recurrent tumors after an LP program are not candidates for salvage surgery (unresectable tumor, metastatic disease, poor general health status, etc.). The prognosis of this type of patients is certainly even worse than that of patients selected for salvage TL. Furthermore, our study did not address the impact of salvage surgery on postoperative complications, functional results and patient quality of life in comparison with primary TL [31].
5. Conclusions
Salvage TL is associated with significantly worse survival rates than primary TL. This adverse impact of salvage TL on patient survival was confirmed in multivariate analysis taking into account other relevant clinical factors such as patient comorbidity level, tumor site, N-stage and surgical margin status that were identified as the main other independent prognostic factors. These factors should be considered in the therapeutic decision-making, especially in the setting of salvage TL, given the poor prognosis of these patients.
The decision of enrolling a patient in an LP program must be taken by an MTB. There is great variability between centers regarding the applicability of clinical trial results on LP approaches. To translate the results of these trials into clinical practice, it is essential to strictly follow the study protocols particularly regarding eligibility criteria. Patients with T4a laryngeal or hypopharyngeal carcinoma, as well as those with poor pretreatment laryngoesophageal function should be referred to immediate TL.
Comparison of salvage vs. primary TL in terms of functional results (swallowing and voice outcomes) has to be explored further in future research. There is also a need to compare the two validated approaches for LP (i.e., TPF ICT followed by RT in responders vs. concurrent cisplatin-based CRT) using a modern composite primary end-point such as laryngoesophageal dysfunction-free survival. This is the objective of the ongoing French phase III trial (GORTEC 2014-03-SALTORL, clinicaltrials.gov NCT03340896).
Author Contributions
Conceptualization, S.T.S., D.C. and A.B.; methodology, A.B., J.G., R.S. and E.C.; software, J.G.; validation, S.T.S., R.S., E.C. and A.B.; formal analysis, J.G.; investigation, S.T.S.; resources, S.T.S.; data curation, A.B.; writing—original draft preparation, S.T.S. and A.B.; writing—review and editing, S.T.S., A.B., O.D. and G.P.; visualization, D.A., M.B., I.S. and E.S.; supervision, A.B.; project administration, A.B. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Antoine Lacassagne Centre (approval code: F20220422105156).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steuer, C.E.; El-Deiry, M.; Parks, R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Giger, R. Comparison of Current Surgical and Non-Surgical Treatment Strategies for Early and Locally Advanced Stage Glottic Laryngeal Cancer and Their Outcome. Cancers 2020, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Lefebvre, J.L. Laryngeal preservation strategies in locally advanced laryngeal and hypopharyngeal cancers. Front. Oncol. 2019, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef]
- Lefebvre, J.-L.; Andry, G.; Chevalier, D.; Luboinski, B.; Collette, L.; Traissac, L.; de Raucourt, D.; Langendijk, J. Laryngeal preservation with induction chemotherapy for hypophayngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann. Oncol. 2012, 23, 2708–2714. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef]
- Janoray, G.; Pointreau, Y.; Garaud, P.; Chapet, S.; Alfonsi, M.; Sire, C.; Jadaud, E.; Calais, G. Long-Term Results of a Multicenter Randomized Phase III Trial of Induction Chemotherapy With Cisplatin, 5-fluorouracil, ± Docetaxel for Larynx Preservation. JNCI J. Natl. Cancer Inst. 2015, 108, djv368. [Google Scholar] [CrossRef]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current Role of Total Laryngectomy in the Era of Organ Preservation. Cancers 2020, 12, 584. [Google Scholar] [CrossRef]
- Poorten, V.V.; Meulemans, J.; Beitler, J.J.; Piazza, C.; Kowalski, L.P.; Mäkitie, A.A.; Paleri, V.; Rinaldo, A.; Robbins, K.T.; Rodrigo, J.P.; et al. Salvage surgery for residual or recurrent laryngeal squamous cell carcinoma after (Chemo)radiotherapy: Oncological outcomes and prognostic factors. Eur. J. Surg. Oncol. 2021, 47, 2711–2721. [Google Scholar] [CrossRef]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Cooper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.-K.; Gay, G.; Stewart, A.; Robinson, R.A. Laryngeal Cancer in the United States: Changes in Demographics, Patterns of Care, and Survival. Laryngoscope 2006, 116, 1–13. [Google Scholar] [CrossRef]
- Weber, R.S.; Berkey, B.A.; Forastiere, A.; Cooper, J.; Maor, M.; Goepfert, H.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Outcome of Salvage Total Laryngectomy Following Organ Preservation Therapy: The Radiation Therapy Oncology Group Trial 91-11. Arch. Otolaryngol. Neck Surg. 2003, 129, 44. [Google Scholar] [CrossRef] [PubMed]
- Sandulache, V.C.; Bs, L.J.V.; Skinner, H.D.; Cata, J.; Hutcheson, K.; Fuller, C.D.; Phan, J.; Ms, Z.S.; Lai, S.Y.; Weber, R.S.; et al. Salvage total laryngectomy after external-beam radiotherapy: A 20-year experience. Head Neck 2016, 38 (Suppl. S1), E1962–E1968. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, L.; de Bree, R.; Kuik, D.J.; Rietveld, D.H.F.; Buter, J.; Eerenstein, S.E.J.; Leemans, C. Salvage laryngectomy: Oncological and functional outcome. Oral Oncol. 2011, 47, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Esteller, E.; Vega, M.C.; López, M.; Quer, M.; León, X. Salvage surgery after locoregional failure in head and neck carcinoma patients treated with chemoradiotherapy. Eur. Arch. Otorhinolaryngol. 2011, 268, 295–301. [Google Scholar] [CrossRef]
- Tan, H.K.; Giger, R.; Auperin, A.; Bourhis, J.; Janot, F.; Temam, S. Salvage surgery after concomitant chemoradiation in head and neck squamous cell carcinomas—Stratification for postsalvage survival. Head Neck 2010, 32, 139–147. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Ostedgaard, K.L.; Al-Qurayshi, Z.; Pagedar, N.A.; Sperry, S.M. Primary Laryngectomy Versus Salvage Laryngectomy: A Comparison of Outcomes in the Chemoradiation Era. Laryngoscope 2020, 130, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, A.; Lionello, M.; Zanotti, C.; Franz, L.; Bd, L.G.; Rizzotto, G.; Marioni, G. Oncological and Functional Outcomes of Primary and Salvage Total Laryngectomy. Laryngoscope 2021, 131, E569–E575. [Google Scholar] [CrossRef]
- Birkeland, A.C.; Beesley, L.; Bellile, E.; Rosko, A.J.; Hoesli, R.; Chinn, S.; Shuman, A.G.; Prince, M.E.; Wolf, G.T.; Bradford, C.R.; et al. Predictors of survival after total laryngectomy for recurrent/persistent laryngeal squamous cell carcinoma. Head Neck 2017, 39, 2512–2518. [Google Scholar] [CrossRef]
- Stoeckli, S.J.; Pawlik, A.B.; Lipp, M.; Huber, A.; Schmid, S. Salvage surgery after failure of nonsurgical therapy for carcinoma of the larynx and hypopharynx. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 1473–1477. [Google Scholar] [CrossRef]
- Takes, R.P.; Strojan, P.; Silver, C.E.; Bradley, P.J.; Haigentz, M.; Wolf, G.T.; Shaha, A.R.; Hartl, D.M.; Olofsson, J.; Langendijk, J.A.; et al. Current trends in initial management of hypopharyngeal cancer: The declining use of open surgery. Head Neck 2012, 34, 270–281. [Google Scholar] [CrossRef]
- Spector, J.G.; Sessions, D.G.; Haughey, B.H.; Chao, K.S.C.; Simpson, J.; El Mofty, S.; Perez, C.A. Delayed Regional Metastases, Distant Metastases, and Second Primary Malignancies in Squamous Cell Carcinomas of the Larynx and Hypopharynx. Laryngoscope 2001, 111, 1079–1087. [Google Scholar] [CrossRef]
- Milliet, F.; Gal, J.; Chamorey, E.; Dassonville, O.; Poissonnet, G.; Peyrade, F.; Benezery, K.; Hechema, R.; Sudaka, A.; Sanchez-Luini, M.; et al. Total pharyngolaryngectomy in the elderly: The impact of age on postoperative complications and oncologic and functional outcomes. Surg. Oncol. 2018, 27, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Matoscevic, K.; Graf, N.; Pezier, T.F.; Huber, G.F. Success of Salvage Treatment: A Critical Appraisal of Salvage Rates for Different Subsites of HNSCC. Otolaryngol. Neck Surg. 2014, 151, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, N.; Chamorey, E.; Michel, J.; Collet, C.; Santini, L.; Poissonnet, G.; Santini, J.; Dessi, P.; Giovanni, A.; Dassonville, O.; et al. Salvage circular laryngopharyngectomy and radial forearm free flap for recurrent hypopharyngeal cancer. Laryngoscope 2013, 123, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, W.J. Salvage Surgery for Patients with Recurrent Squamous Cell Carcinoma of the Upper Aerodigestive Tract: When Do the Ends Justify the Means? Laryngoscope 2000, 110, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, A.; Väisänen, J.; Atula, T.; Alho, O.P.; Mäkitie, A.; Koivunen, P. Predictive factors and treatment outcome of laryngeal carcinoma recurrence. Head Neck 2017, 39, 555–563. [Google Scholar] [CrossRef]
- Agrawal, A.; Hammond, T.H.; Young, G.S.; Avon, A.L.; Ozer, E.; Schuller, D.E. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope 2009, 119, 2135–2140. [Google Scholar] [CrossRef]
- Gross, J.H.; Patel, M.R.; Switchenko, J.M.; Chan, T.G.; Baddour, H.M.; Kaka, A.; Boyce, B.J.; Saba, N.F.; Beitler, J.J.; El-Deiry, M. Oncologic Outcomes After Clinically Node-Negative Salvage Laryngectomy. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 24–33. [Google Scholar] [CrossRef]
- Dassé, R.; Dupin, C.; Gorphe, P.; Temam, S.; Dupret-Bories, A.; Vergez, S.; Dufour, X.; Aubry, K.; de Monès, E. Proposal for an algorithm to avoid neck dissection during salvage total laryngectomy. A GETTEC multicentric study. Oral Oncol. 2022, 33, 106026. [Google Scholar] [CrossRef]
- Grover, S.; Swisher-McClure, S.; Mitra, N.; Li, J.; Cohen, R.B.; Ahn, P.H.; Lukens, J.N.; Chalian, A.A.; Weinstein, G.S.; O’Malley, B.W.; et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int. J. Radiat. Oncol. 2015, 92, 594–601. [Google Scholar] [CrossRef]
- Grasl, S.; Schmid, E.; Heiduschka, G.; Brunner, M.; Marijić, B.; Grasl, M.; Faisal, M.; Erovic, B.; Janik, S. A New Classification System to Predict Functional Outcome after Laryngectomy and Laryngopharyngectomy. Cancers 2021, 13, 1474. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).