Role of Autologous Fat Grafting in the Conservative Treatment of Fecal Incontinence in Children
Abstract
1. Introduction
2. Materials & Methods
2.1. Anal-Lipofilling: Surgical Procedure
2.1.1. Fat Tissue Harvesting
2.1.2. Fat Tissue Processing
2.1.3. Purified Tissue Injection
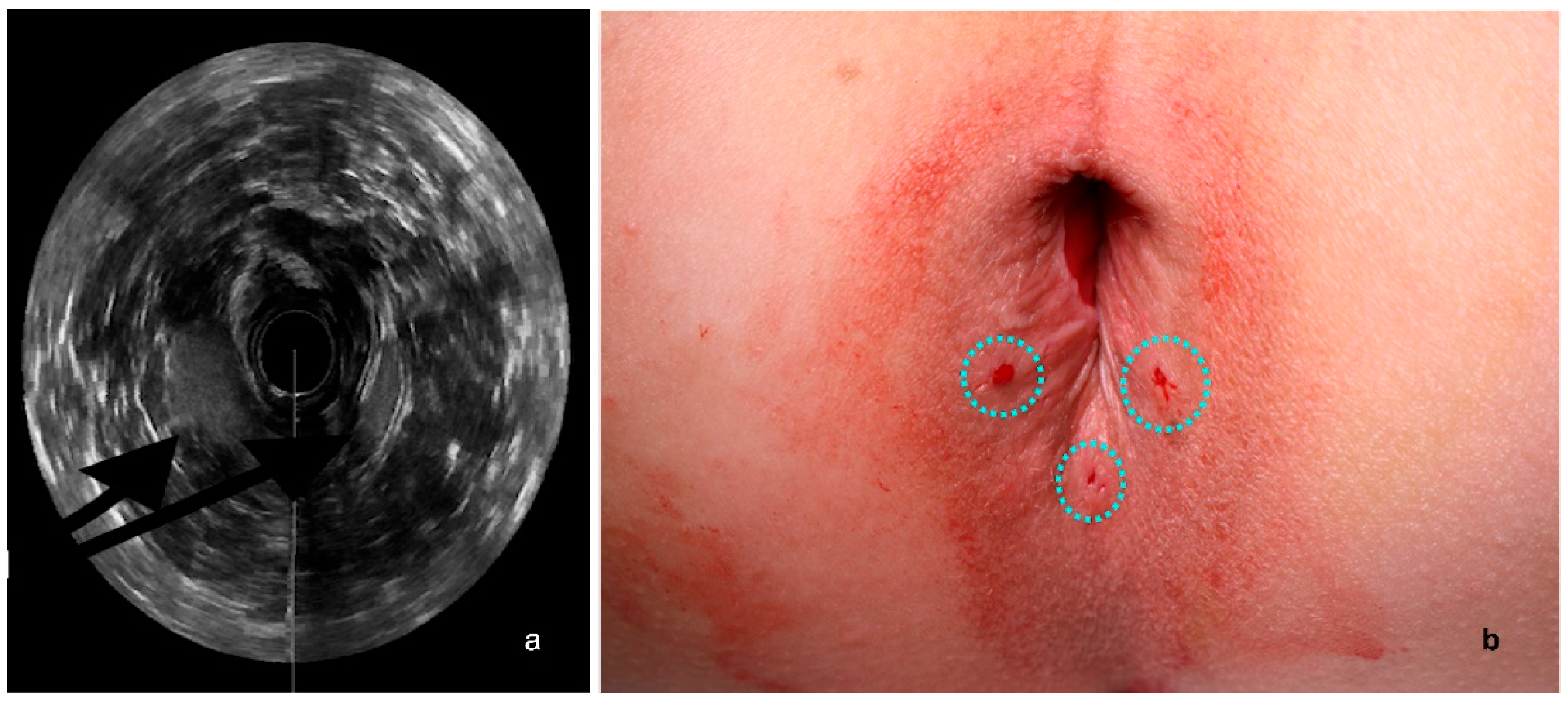
2.2. US Assessment
2.3. Manometry
2.4. Parents’ QoL Evaluation
3. Results
3.1. US Assessment
3.2. Manometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSC | adipose-derived stem cell |
| ARMs | Ano-Rectal Malformations |
| ARP | average resting pressure |
| ASP | anal squeeze pressure |
| KS | Krickenbeck scale |
| MCV | maximum voluntary contraction |
| PSARP | posterior sagittal anorectoplasty |
| QoL | quality of life |
| SVF | stromal vascular fraction |
References
- Rajindrajith, S.; Devanarayana, N.M.; Benninga, M.A. Review article: Faecal incontinence in children: Epidemiology, pathophysiology, clinical evaluation and management. Aliment. Pharmacol. Ther. 2013, 37, 37–48. [Google Scholar] [CrossRef]
- Kyrklund, K.; Neuvonen, M.I.; Pakarinen, M.P.; Rintala, R.J. Social Morbidity in Relation to Bowel Functional Outcomes and Quality of Life in Anorectal Malformations and Hirschsprung’s Disease. Eur. J. Pediatr. Surg. 2018, 28, 522–528. [Google Scholar]
- Molina, M.E.; Lema, A.; Palacios, M.G.; Somoza, I.; Gómez, J.V.; Tellado, M.G.; Pais, E.; Dargallo, T.; Vela, D. Quality of life in children operated on for anal atresia. Cir. Pediatr. 2010, 23, 19–23. [Google Scholar]
- Grasshoff-Derr, S.; Backhaus, K.; Hubert, D.; Meyer, T. A successful treatment strategy in infants and adolescents with anorectal malformation and incontinence with combined hydrocolonic ultrasound and bowel management. Pediatr. Surg. Int. 2011, 27, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Hwang, S.H.; Kim, H.R.; Ryu, K.O.; Lim, J.; Yu, H.M.; Yoon, J.; Kim, C.Y.; Jeong, K.Y.; Jung, Y.J.; et al. Effectiveness of Autologous Fat Graft in Treating Fecal Incontinence. Ann. Coloproctol. 2019, 35, 144–151. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A. Perianal injection of autologous fat for treatment of sphincteric incontinence. Dis. Colon. Rectum. 1995, 38, 583–587. [Google Scholar] [CrossRef]
- Bernardi, C.; Favetta, U.; Pescatori, M. Autologous fat injection for treatment of fecal incontinence: Manometric and echographic assessment. Plast. Reconstr. Surg. 1998, 102, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Sarveazad, A.; Newstead, G.L.; Mirzaei, R.; Joghataei, M.T.; Bakhtiari, M.; Babahajian, A.; Mahjoubi, B. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: Preliminary findings of a randomized double-blind clinical trial. Stem Cell Res. Ther. 2017, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Parente, G.; Pinto, V.; Di Salvo, N.; D’Antonio, S.; Libri, M.; Gargano, T.; Catania, V.D.; Ruggeri, G.; Lima, M. Preliminary Study on the Echo-Assisted Intersphincteric Autologous Microfragmented Adipose Tissue Injection to Control Fecal Incontinence in Children Operated for Anorectal Malformations. Children 2020, 7, 181. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Ceserani, V.; Ferri, A.; Berenzi, A.; Benetti, A.; Ciusani, E.; Pascucci, L.; Bazzucchi, C.; Coccè, V.; Bonomi, A.; Pessina, A.; et al. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc. Cell. 2016, 8, 3. [Google Scholar] [CrossRef]
- De la Portilla, F.; López-Alonso, M. Endosonography of the anal canal: Findings in children. Dis. Colon. Rectum. 2009, 52, 711–714. [Google Scholar] [CrossRef]
- Holschneider, A.; Hutson, J.; Peña, A.; Beket, E.; Chatterjee, S.; Coran, A.; Davies, M.; Georgeson, K.; Grosfeld, J.; Gupta, D.; et al. Preliminary report on the International Conference for the Development of Standards for the Treatment of Anorectal Malformations. J. Pediatr. Surg. 2005, 40, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Van den Hondel, D.; Sloots, C.E.; Gischler, S.J.; Meeussen, C.J.; Wijnen, R.M.; IJsselstijn, H. Prospective long-term follow up of children with anorectal malformation: Growth and development until 5 years of age. J. Pediatr. Surg. 2013, 48, 818–825. [Google Scholar] [CrossRef]
- Hussain, Z.I.; Lim, M.; Stojkovic, S.G. Systematic review of perianal implants in the treatment of faecal incontinence. Br. J. Surg. 2011, 98, 1526–1536. [Google Scholar] [CrossRef]
- Vaizey, C.J.; Kamm, M.A. Injectable bulking agents for treating fecal incontinence. Br. J. Surg. 2005, 92, 521–527. [Google Scholar] [CrossRef]
- Malizia, A.A.; Rushton, H.G.; Woodard, J.R.; Newton, N.E.; Reiman, H.M.; Lopez, O.F. Migration and granulomatous reaction after intravesical subureteric injection of polytef. J. Urol. 1987, 137, A122. [Google Scholar] [CrossRef]
- Tjandra, J.J.; Chan, M.K.; Yeh, H.C. Injectable silicone biomaterial (PTQ) is more effective than carbon-coated beads (Durasphere) in treating passive faecal incontinence--a randomized trial. Color. Dis. 2009, 11, 382–389. [Google Scholar] [CrossRef]
- Maeda, Y.; Laurberg, S.; Norton, C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst. Rev. 2013, CD007959. [Google Scholar] [CrossRef]
- Quiroz, L.H.; Galliano DEJr da Silva, G.; Carmichael, J.C.; Pan, L.C.; Bromley, E.R.; Hinahara, J.; Goss, T.F. Efficacy and Safety of a Non-animal Stabilized Hyaluronic Acid/Dextranomer in Improving Fecal Incontinence: A Prospective, Single-Arm, Multicenter, Clinical Study with 36-Month Follow-up. Dis. Colon. Rectum. 2022; ahead of print. [Google Scholar] [CrossRef]
- Hong, K.D.; Kim, J.S.; Ji, W.B.; Um, J.W. Midterm outcomes of injectable bulking agents for fecal incontinence: A systematic review and meta-analysis. Technol. Coloproctol. 2017, 21, 203–210. [Google Scholar] [CrossRef]
- Cantarella, G.; Mazzola, R.F.; Mantovani, M.; Mazzola, I.C.; Baracca, G.; Pignataro, L. Fat injections for the treatment of velopharyngeal insufficiency. J. Craniofac. Surg. 2012, 23, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Koonce, S.L.; Grant, D.G.; Cook, J.; Stelnicki, E.J. Autologous Fat Grafting in the Treatment of Cleft Lip Volume Asymmetry. Ann. Plast. Surg. 2018, 80, S352–S355. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Abu-Ghname, A.; Davis, M.J.; Xue, A.S.; Dempsey, R.F.; Buchanan, E.P. Applications of Fat Grafting in Pediatric Patients. Semin. Plast. Surg. 2020, 34, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Krastev, T.K.; Schop, S.J.; Hommes, J.; Piatkowski, A.; van der Hulst, R.R.W.J. Autologous fat transfer to treat fibrosis and scar-related conditions: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 2033–2048. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafts: The ideal filler? Clin. Plast. Surg. 2001, 28, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Hand rejuvenation with structural fat grafting. Plast. Reconstr. Surg. 2002, 110, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Long-term survival of fat transplants: Controlled demonstrations. Aesthetic Plast. Surg. 1995, 19, 421–425. [Google Scholar] [CrossRef]
- Galiè, M.; Pignatti, M.; Scambi, I.; Sbarbati, A.; Rigotti, G. Comparison of different centrifugation protocols for the best yield of adipose-derived stromal cells from lipoaspirates. Plast. Reconstr. Surg. 2008, 122, 233e–234e. [Google Scholar] [CrossRef]
- Kosowski, T.R.; Rigotti, G.; Khouri, R.K. Tissue-Engineered Autologous Breast Regeneration with Brava®-Assisted Fat Grafting. Clin. Plast. Surg. 2015, 42, 325–337, VIII. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, M.S.; Veronesi, E.; Loschi, P.; Pignatti, M.; Murgia, A.; Grisendi, G.; Castelli, I.; Bernabei, D.; Candini, O.; Conte, P.; et al. Adipose stromal/stem cells assist fat transplantation reducing necrosis and increasing graft performance. Apoptosis 2013, 18, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 2006, 118, 108S–120S. [Google Scholar] [CrossRef]
- Pignatti, M.; Spinella, A.; Cocchiara, E.; Boscaini, G.; Lusetti, I.L.; Citriniti, G.; Lumetti, F.; Setti, G.; Dominici, M.; Salvarani, C.; et al. Autologous Fat Grafting for the Oral and Digital Complications of Systemic Sclerosis: Results of a Prospective Study. Aesthetic Plast Surg. 2020, 44, 1820–1832. [Google Scholar] [CrossRef]
- Mazzola, R.F.; Cantarella, G.; Torretta, S.; Sbarbati, A.; Lazzari, L.; Pignataro, L. Autologous fat injection to face and neck: From soft tissue augmentation to regenerative medicine. Acta Otorhinolaryngol. Ital. 2011, 31, 59–69. [Google Scholar] [PubMed]
- Mazzola, R.F.; Cantarella, G.; Mazzola, I.C. Regenerative Approach to Velopharyngeal Incompetence with Fat Grafting. Clin. Plast. Surg. 2015, 42, 365–374, IX. [Google Scholar] [CrossRef]
- Lahav, Y.; Malka-Yosef, L.; Shapira-Galitz, Y.; Cohen, O.; Halperin, D.; Shoffel-Havakuk, H. Vocal Fold Fat Augmentation for Atrophy, Scarring, and Unilateral Paralysis: Long-term Functional Outcomes. Otolaryngol. Head Neck Surg. 2021, 164, 631–638. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Trans. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Laureti, S.; Gionchetti, P.; Cappelli, A.; Vittori, L.; Contedini, F.; Rizzello, F.; Golfieri, R.; Campieri, M.; Poggioli, G. Refractory Complex Crohn’s Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm. Bowel Dis. 2020, 26, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Van der Steeg, H.J.; Schmiedeke, E.; Bagolan, P.; Broens, P.; Demirogullari, B.; Garcia-Vazquez, A.; Grasshoff-Derr, S.; Lacher, M.; Leva, E.; Makedonsky, I.; et al. European consensus meeting of ARM-Net members concerning diagnosis and early management of newborns with anorectal malformations. Technol. Coloproctol. 2015, 19, 181–185. [Google Scholar] [CrossRef] [PubMed]



|
Feeling of urge, capacity to verbalize and hold the bowel movements
|
|
|
|
|
| Questions | Answers | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| A | Scale 1: Lifestyle | ||||||
| 1 | I cannot do many of things I want to do with my family and with other families |
| |||||
| 2 | I am afraid to go out like going to a movie or to church | ||||||
| 3 | I avoid traveling by plane or train | ||||||
| 4 | I avoid visiting friends | ||||||
| 5 | I avoid going out to eat | ||||||
| 6 | I avoid staying overnight away from home with friends and their families | ||||||
| B | Scale 2: Depression/Self Perception | ||||||
| 1 | I feel different from other mothers/parents |
| |||||
| 2 | I enjoy my family less | ||||||
| 3 | I feel depressed | ||||||
| 4 | The relationship with my husband and other children suffers | ||||||
| 5 | I’m worried that my son is distracted at school during lessons and miss school days for visits and operations | ||||||
| C | Scale 3: Embarrassment | ||||||
| 1 | I worry about my son leaks stool without even knowing it and others smelling stool on him/her I worry children make fun of him/her |
| |||||
| 2 | It makes me depressed to think that teachers or other mothers might believe that I did not wash my child properly or I did not educate him | ||||||
| Data Collection | ||
| DEMOGRAPHIC AND CLINICAL DATA | N° of patients treated | 6 |
| Gender | 100% M | |
| Age at surgery | 10.7 ± 4.4 years (range: 6–17 years) | |
| Causes of fecal incontinence | anorectal malformation 83% (5) n = 4 recto-urethral fistulas n = 1 recto-perineal fistula tethered cord 17% (1) | |
| CLINICAL ASSESSMENT | Preoperative KS score | 100% (6): 1.0, 2.3, 3.0 |
| Postoperative KS score | 83% (5): 1.0, 2.1, 3.0 17% (1): 1.0, 2.3, 3.0 | |
| SURGICAL DATA | N° of procedures | 12 |
| N° of procedures per patients | 2.0 ± 1.3 (range: 1–4) | |
| Interval time between the procedures | 343.8 days ± 220.1 days (range 203–733 days) | |
| Pre-op IAS thickness (mean) | 0.9 mm in ARM patients (5); 2.0 mm tethered cord patient (1) | |
| Post-op IAS thickness (mean) | 1.3 mm in ARM patients (5); 2.2 mm tethered cord patient (1) | |
| Patient | Pre-Existing Condition | Pre-Operative AS Thickness (mm) | Post-Operative IAS Thickness (mm) |
|---|---|---|---|
| ARM with recto-urethral fistula | 0.6 | 1.00 |
| ARM with recto-urethral fistula | 1.0 | 1.2 |
| ARM with recto-urethral fistula | 0.9 | 1.4 |
| ARM with recto-urethral fistula | 0.8 | 1.2 |
| ARM with recto-perineal fistula | 1.1 | 1.5 |
| Normal ano-rectal anatomy and tethered cord | 2.0 | 2.2 |
| Questions | Pre-Surgical Answers | Post-Surgical Answers | ||||||
|---|---|---|---|---|---|---|---|---|
| Always | Often | Sometimes | Never | Always | Often | Sometimes | Never | |
| A—Scale 1: Lifestyle | ||||||||
| A1 | 0% (N = 0) | 100% (N = 6) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) |
| A2 | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) |
| A3 | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) |
| A4 | 16.7% (N = 1) | 33.3% (N = 2) | 33.3% (N = 2) | 16.7% (N = 1) | 0% (N = 0) | 0% (N = 0) | 83.3% (N = 5) | 16.7% (N = 1) |
| A5 | 16.7% (N = 1) | 33.3% (N = 2) | 33.3% (N = 2) | 16.7% (N = 1) | 0% (N = 0) | 0% (N = 0) | 83.3% (N = 5) | 16.7% (N = 1) |
| A6 | 16.7% (N = 1) | 33.3% (N = 2) | 33.3% (N = 2) | 16.7% (N = 1) | 0% (N = 0) | 0% (N = 0) | 83.3% (N = 5) | 16.7% (N = 1) |
| AGGREGATED A | 13.8% (N = 5) | 60.1% (N = 22) | 16.6% (N = 6) | 8.31 (N = 3) | 0% (N = 0) | 8.31% (N = 3) | 83.1% (N = 30) | 8.31% (N = 3) |
| B—Scale 2: Depression/ Self-Perception | ||||||||
| B1 | 0% (N = 0) | 16.7% (N = 1) | 33.3% (N = 2) | 50% (N = 3) | 0% (N = 0) | 0% (N = 0) | 33.3% (N = 2) | 66.7% (N = 4) |
| B2 | 16.7% (N = 1) | 33.3% (N = 2) | 16.7% (N = 1) | 33.3% (N = 2) | 0% (N = 0) | 0% (N = 0) | 66.7% (N = 4) | 33.3% (N = 2) |
| B3 | 33.3% (N = 2) | 33.3% (N = 2) | 16.7% (N = 1) | 16.7% (N = 1) | 16.7% (N = 1) | 0% (N = 0) | 66.7% (N = 4) | 16.7% (N = 1) |
| B4 | 0% (N = 0) | 16.7% (N = 1) | 50% (N = 3) | 33.3% (N = 2) | 0% (N = 0) | 0% (N = 0) | 66.7% (N = 4) | 33.3% (N = 2) |
| B5 | 16.7% (N = 1) | 16.7% (N = 1) | 66.7% (N = 4) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | 100% (N = 6) | 0% (N = 0) |
| AGGREGATED B | 13.3% (N = 4) | 23.3% (N = 7) | 36.6% (N = 11) | 26.6% (N = 8) | 3.33% (N = 1) | 0% (N = 0) | 66.6% (N = 20) | 29.97% (N = 9) |
| C—Scale 3: Embarrassment | ||||||||
| C1 | 0% (N = 0) | 83.3% (N = 5) | 16.7% (N = 1) | 0% (N = 0) | 0% (N = 0) | 16.7% (N = 1) | 83.3% (N = 5) | 0% (N = 0) |
| C2 | 0% (N = 0) | 16.7% (N = 1) | 16.7% (N = 1) | 66.7% (N = 4) | 0% (N = 0) | 0% (N = 0) | 33.3% (N = 2) | 66.7% (N = 4) |
| AGGREGATED C | 0% (N = 0) | 49.8% (N = 6) | 16.6% (N = 2) | 33.2% (N = 4) | 0% (N = 0) | 8.3% (N = 1) | 58.1% (N = 7) | 33.2% (N = 4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, V.; Pignatti, M.; Parente, G.; Di Salvo, N.; Contu, L.; Lima, M. Role of Autologous Fat Grafting in the Conservative Treatment of Fecal Incontinence in Children. J. Clin. Med. 2023, 12, 1258. https://doi.org/10.3390/jcm12041258
Pinto V, Pignatti M, Parente G, Di Salvo N, Contu L, Lima M. Role of Autologous Fat Grafting in the Conservative Treatment of Fecal Incontinence in Children. Journal of Clinical Medicine. 2023; 12(4):1258. https://doi.org/10.3390/jcm12041258
Chicago/Turabian StylePinto, Valentina, Marco Pignatti, Giovanni Parente, Neil Di Salvo, Luca Contu, and Mario Lima. 2023. "Role of Autologous Fat Grafting in the Conservative Treatment of Fecal Incontinence in Children" Journal of Clinical Medicine 12, no. 4: 1258. https://doi.org/10.3390/jcm12041258
APA StylePinto, V., Pignatti, M., Parente, G., Di Salvo, N., Contu, L., & Lima, M. (2023). Role of Autologous Fat Grafting in the Conservative Treatment of Fecal Incontinence in Children. Journal of Clinical Medicine, 12(4), 1258. https://doi.org/10.3390/jcm12041258








