Sex Differences in the Feasibility of Aerobic Exercise Training for Improving Cardiometabolic Health Outcomes in Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment and Eligibility
2.3. Interventions
2.3.1. Aerobic-Exercise
2.3.2. Placebo Exercise Control
2.4. Data Collection
2.5. Primary Outcome
Feasibility
2.6. Exploratory Outcomes
2.6.1. Pulse Wave Analysis and Central Hemodynamic Responses
2.6.2. Pulse Wave Velocity
2.6.3. Anthropometry
2.6.4. Cardiorespiratory Fitness
2.6.5. Blood Sampling and Analysis
2.6.6. Resting Heart Rate and Blood Pressure
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Feasibility Outcomes
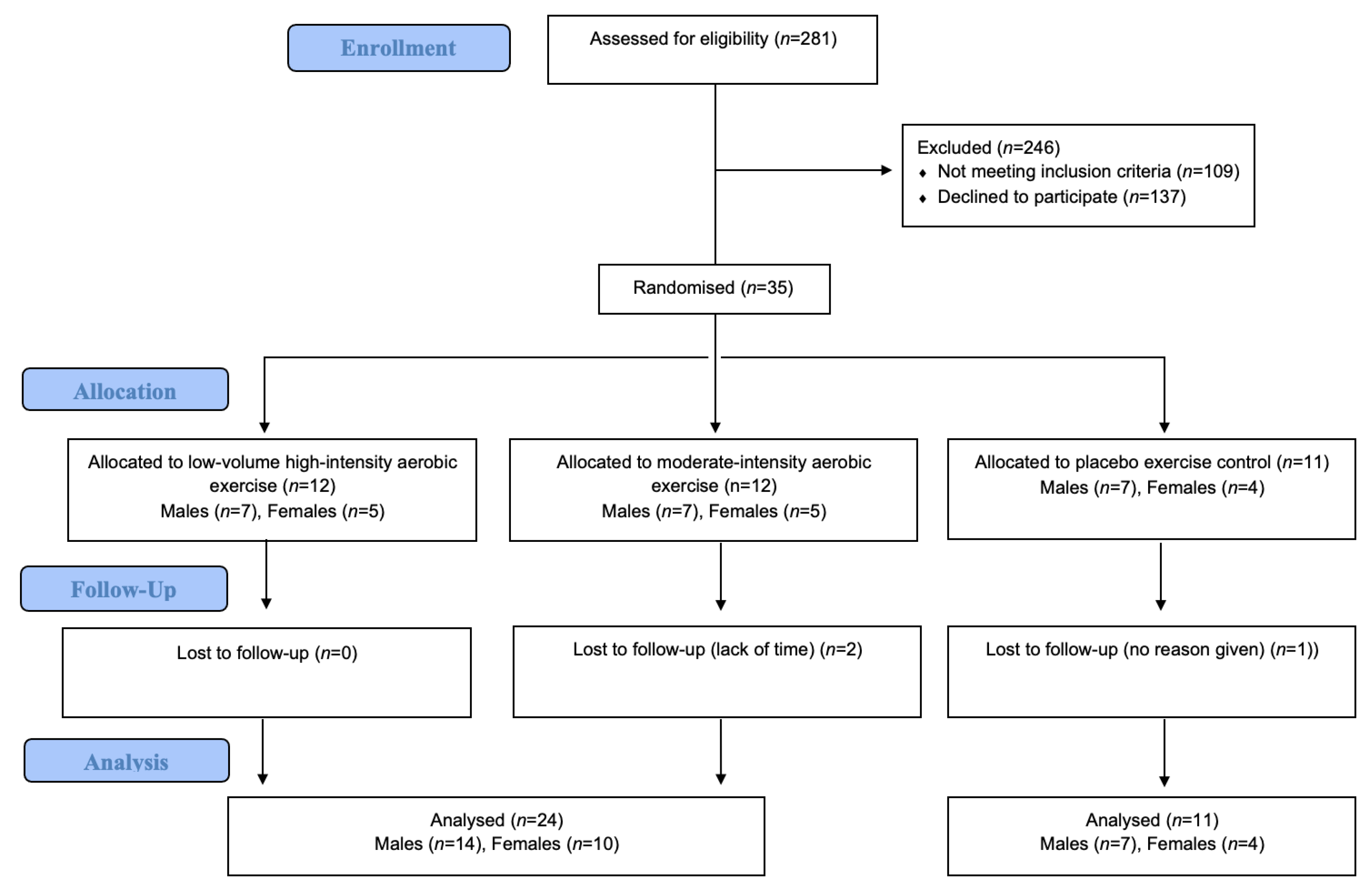
3.2.1. Recruitment and Retention
3.2.2. Treatment Fidelity
3.2.3. Safety
3.3. Exploratory Sex-Specific Outcomes
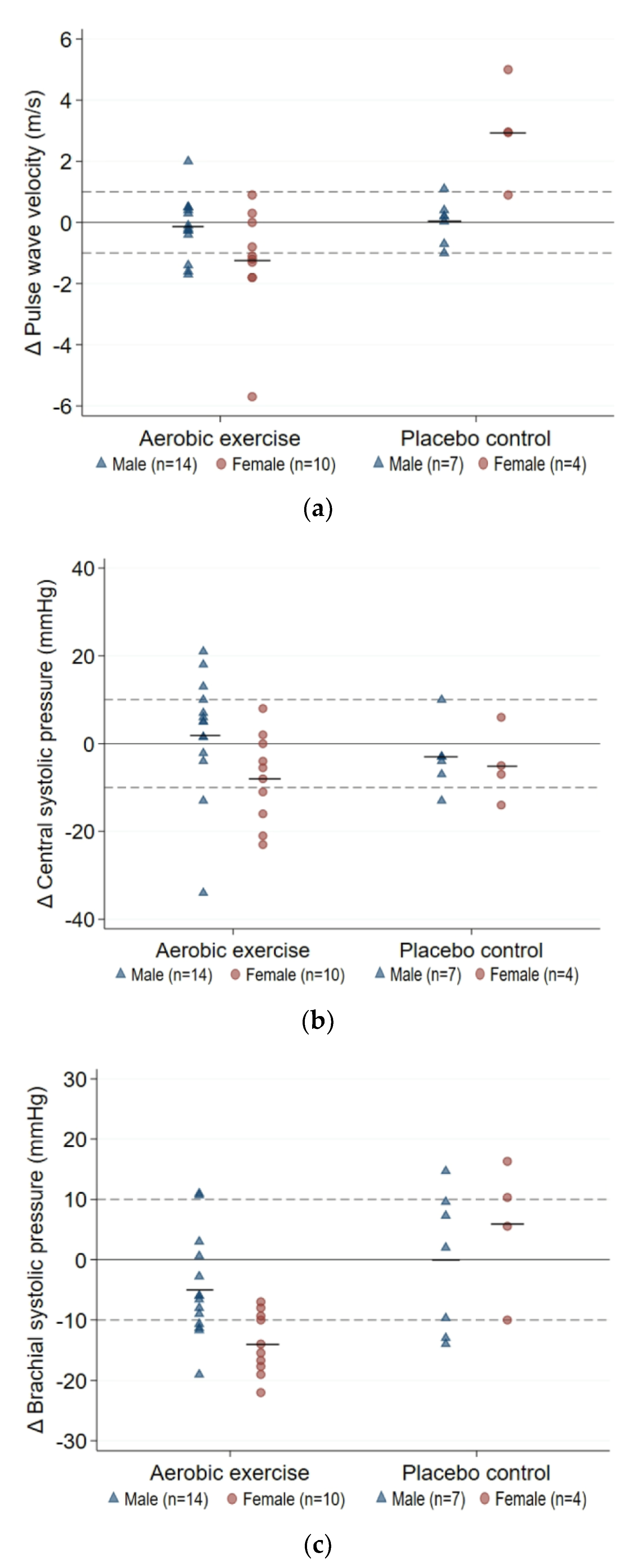
3.3.1. Arterial Health
3.3.2. Cardiometabolic Health
4. Discussion
4.1. Feasibility Outcomes
4.2. Sex-specific Responses to Aerobic Training
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabet. 2015, 6, 1246. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabet. 2014, 5, 444. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Lazo, M.; Ouyang, P.; Turkbey, E.; Chevalier, K.; Brancati, F.; Becker, D.; Vaidya, D. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 2014, 37, 830–838. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex differences in the cardiovascular consequences of diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2015, 132, 2424–2447. [Google Scholar] [CrossRef]
- Williams, J.S.; Bishu, K.; Dismuke, C.E.; Egede, L.E. Sex differences in healthcare expenditures among adults with diabetes: Evidence from the medical expenditure panel survey, 2002–2011. BMC 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Steinberg, J.R.; Turner, B.E.; Weeks, B.T.; Magnani, C.J.; Wong, B.O.; Rodriguez, F.; Yee, L.M.; Cullen, M.R. Analysis of female enrollment and participant sex by burden of disease in US clinical trials between 2000 and 2020. JAMA 2021, 4, e21137499. [Google Scholar] [CrossRef]
- Whipple, M.O.; Pinto, A.J.; Abushamat, L.A.; Bergouignan, A.; Chapman, K.; Huebschmann, A.G.; Masters, K.S.; Nadeau, K.J.; Scalzo, R.L.; Schauer, I.E.; et al. Sex Differences in Physical Activity Among Individuals With Type 2 Diabetes Across the Life Span: A Systematic Review and Meta-analysis. Diabetes Care 2022, 45, 2163–2177. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Crisafulli, A. Gender differences in hemodynamic regulation and cardiovascular adaptations to dynamic exercise. Curr. Cardiol. Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Snyder, E.M.; Johnson, B.D.; Olson, T.P. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus 2014, 3, 1–13. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Sabag, A.; Way, K.L.; Sultana, R.N.; Keating, S.E.; Gerofi, J.A.; Chuter, V.H.; Byrne, N.M.; Baker, M.K.; George, J.; Caterson, I.D.; et al. The effect of a novel low-volume aerobic exercise intervention on liver fat in type 2 diabetes: A randomized controlled trial. Diabetes Care 2020, 43, 2371–2378. [Google Scholar] [CrossRef]
- Way, K.L.; Sabag, A.; Sultana, R.N.; Baker, M.K.; Keating, S.E.; Lanting, S.; Gerofi, J.; Chuter, V.H.; Caterson, I.D.; Twigg, S.M.; et al. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: A randomised controlled trial. Int. J. Cardiol. 2020, 320, 148–154. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- National Health and Medical Research Council. Guidance: Safety Monitoring and Reporting in Clinical Trials Involving Therapeutic Goods; National Health and Medical Research Council: Canberra, Australia, 2016.
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials–a practical guide with flowcharts. BMC 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Johnston, M.G.; Faulkner, C. A bootstrap approach is a superior statistical method for the comparison of non-normal data with differing variances. New Phytol. 2021, 230, 23–26. [Google Scholar] [CrossRef]
- Medicine, A.C.o.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Li, W.-F.; Huang, Y.-Q.; Feng, Y.-Q. Association between central haemodynamics and risk of all-cause mortality and cardiovascular disease: A systematic review and meta-analysis. J. Hum. Hypertens. 2019, 33, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, P.J.; Rodgers, P.A.; Rahimi, P.K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Niles, B.L.; Mori, D.L. Targeted recruitment of adults with type 2 diabetes for a physical activity intervention. Diabetes Spectrum. 2015, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.; Daly, R.M.; Sharman, J.; Bruce, D.; Davis, T.; Greenaway, T.; Nolan, M.; Beare, R.; Schultz, M.G.; Phan, T.; et al. Feasibility of a multi-modal exercise program on cognition in older adults with type 2 diabetes–a pilot randomised controlled trial. BMC 2017, 17, 1–10. [Google Scholar] [CrossRef]
- De Groot, M.; Shubrook, J.H.; Hornsby, W.G., Jr.; Pillay, Y.; Mather, K.J.; Fitzpatrick, K.; Yang, Z.; Saha, C. Program ACTIVE II: Outcomes from a randomized, multistate community-based depression treatment for rural and urban adults with type 2 diabetes. Diabetes Care 2019, 42, 1185–1193. [Google Scholar] [CrossRef]
- Miller, E.G.; Nowson, C.A.; Dunstan, D.W.; Kerr, D.A.; Solah, V.; Menzies, D.; Daly, R.M. Recruitment of older adults with type 2 diabetes into a community-based exercise and nutrition randomised controlled trial. Trials 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Schneider, K.L.; Panza, E.; Handschin, B.; Ma, Y.; Busch, A.M.; Waring, M.E.; Appelhans, B.M.; Whited, M.C.; Keeney, J.; Kern, D.; et al. Feasibility of pairing behavioral activation with exercise for women with type 2 diabetes and depression: The get it study pilot randomized controlled trial. Behav. Ther. 2016, 47, 198–212. [Google Scholar] [CrossRef]
- Whitaker, C.; Stevelink, S.; Fear, N. The use of Facebook in recruiting participants for health research purposes: A systematic review. J. Med. Int. Res. 2017, 19, e7071. [Google Scholar] [CrossRef]
- Markanday, S.; Brennan, S.L.; Gould, H.; Pasco, J.A. Sex-differences in reasons for non-participation at recruitment: Geelong Osteoporosis Study. BMC 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Dunn, K.M.; Jordan, K.; Lacey, R.J.; Shapley, M.; Jinks, C. Patterns of consent in epidemiologic research: Evidence from over 25,000 responders. Am. J. Epidemiol. 2004, 159, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Galea, S.; Tracy, M. Participation rates in epidemiologic studies. Ann. Epidemiol. 2007, 17, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Morgan, A.R.; Patel, D.; Dhatariya, K.; Greenwood, S.; Newland-Jones, P.; Hicks, D.; Yousef, Z.; Moore, J.; Kelly, B.; et al. Risk prediction of the diabetes missing million: Identifying individuals at high risk of diabetes and related complications. Diabetes Ther. 2021, 12, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.; Mahmood, W.; Fenton, R.; Redziniak, N.; Kyaw Tun, T.; Sreenan, S.; McDermott, J.H. Barriers to exercise in obese patients with type 2 diabetes. QJM 2013, 106, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Korkiakangas, E.E.; Alahuhta, M.A.; Laitinen, J.H. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: A systematic review. Health Promot Int. 2009, 24, 416–427. [Google Scholar] [CrossRef]
- Harris, L.K.; Skou, S.T.; Juhl, C.B.; Jäger, M.; Bricca, A. Recruitment and retention rates in randomised controlled trials of exercise therapy in people with multimorbidity: A systematic review and meta-analysis. Trials 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: Cancer, cardiovascular disease, and diabetes. BMC 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Colbert, J.D.; Martin, B.-J.; Haykowsky, M.J.; Hauer, T.L.; Austford, L.D.; Arena, R.A.; Knudtson, M.L.; Meldrum, D.A.N.; Aggarwal, S.G.; Stone, J.A. Cardiac rehabilitation referral, attendance and mortality in women. Eur. J. Prev. Cardiol. 2015, 22, 979–986. [Google Scholar] [CrossRef]
- Oosenbrug, E.; Marinho, R.P.; Zhang, J.; Marzolini, S.; Colella, T.J.; Pakosh, M.; Grace, S.L. Sex differences in cardiac rehabilitation adherence: A meta-analysis. Can J. Cardiol. 2016, 32, 1316–1324. [Google Scholar] [CrossRef]
- Grace, S.L.; Gravely-Witte, S.; Kayaniyil, S.; Brual, J.; Suskin, N.; Stewart, D.E. A multisite examination of sex differences in cardiac rehabilitation barriers by participation status. J. Women’s Health 2009, 18, 209–216. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39. [Google Scholar] [CrossRef]
- Madden, K.M.; Lockhart, C.; Cuff, D.; Potter, T.F.; Meneilly, G.S. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 2009, 32, 1531–1535. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.-H. Arterial stiffness: A brief review. Acta Pharmacol. Sinica. 2010, 31, 1267–1276. [Google Scholar] [CrossRef]
- Nabeel, P.; Chandran, D.S.; Kaur, P.; Thanikachalam, S.; Sivaprakasam, M.; Joseph, J. Association of incremental pulse wave velocity with cardiometabolic risk factors. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Mikkelsen, E.M.; Cronin-Fenton, D.; Kristensen, N.R.; Pham, T.M.; Pedersen, L.; Petersen, I. Missing data and multiple imputation in clinical epidemiological research. Clin. Epidemiol. 2017, 9, 157. [Google Scholar] [CrossRef]
| Outcome | Measurements |
|---|---|
| Recruitment | Number of enrolled-participants versus total-screened participants |
| Reasons for non-participation | |
| Retention | Number of participants available at follow-up |
| Reasons for loss to follow-up | |
| Treatment Fidelity | Attendance: proportion of prescribed sessions attended Compliance: proportion of aerobic training sessions attended with completion of full prescribed duration |
| Global adherence: proportion of participants with ≥70% attendance and ≥70% compliance Perceived exertion: mean RPE across attended sessions | |
| Safety | Number and severity of adverse events across attended sessions |
| Demographic | Aerobic Exercise (n = 24) | Placebo Control (n = 11) | ||
|---|---|---|---|---|
| Males (n = 14) | Females (n = 10) | Males (n = 7) | Females (n = 4) | |
| Age (years) | 57 ± 2 | 56 ± 2 | 51 ± 4 | 53 ± 2 |
| T2D duration (years) | 7 ± 2 | 11 ± 2 | 6 ± 2 | 9 ± 4 |
| HbA1c (%) | 6.8 ± 0.3 | 7.5 ± 0.6 | 7.8 ± 0.7 | 7.2 ± 0.7 |
| BMI (m/kg2) | 36.0 ± 1.5 | 35.8 ± 1.3 | 36.6 ± 1.9 | 34.4 ± 3.8 |
| Weight (kg) | 110.1 ± 4.3 | 94.3 ± 4.6 | 114.0 ± 6.0 | 92.1 ± 6.0 |
| Waist circumference (cm) | 118.4 ± 3.5 | 114.3 ± 3.4 | 122.5 ± 4.2 | 105.1 ± 5.6 |
| VO2peak (ml/kg/min) | 23.8 ± 1.1 | 17.7 ± 0.7 | 21.4 ± 1.9 | 18.1 ± 5.0 |
| SBP (mmHg) | 132 ± 4 | 130 ± 4 | 135 ± 9 | 111 ± 6 |
| DBP (mmHg) | 81 ± 1 | 78 ± 2 | 86 ± 6 | 79 ± 4 |
| HR (bpm) | 66 ± 2 | 71 ± 3 | 77 ± 6 | 78 ± 4 |
| Medications, n (%) | ||||
| Anti-hyperglycemic | 13 (37) | 10 (29) | 7 (20) | 3 (9) |
| Anti-hypertensive | 10 (29) | 4 (11) | 5 (14) | 1 (3) |
| Lipid lowering | 10 (29) | 5 (14) | 6 (17) | 1 (3) |
| Aerobic Exercise | Placebo Control | Interaction Effect (Group × Sex) | |||||
|---|---|---|---|---|---|---|---|
| Males | Females | Net Sex Difference | Males | Females | Net Sex Difference | ||
| PWV (m/s) | |||||||
| Baseline | 8.89 ± 0.43 | 8.28 ± 0.28 | 9.09 ± 0.60 | 6.55 ± 3.05 | |||
| Δ12-week | −0.12 (−0.69, 0.44) | −1.25 (−2.54, 0.04) | 1.13 (−0.06, 2.31) | 0.03 (−0.61, 0.68) | 2.95 (0.29, 5.61) | −2.92 (−4.51, −1.33) | p < 0.0001 |
| AIx (%) | |||||||
| Baseline | 22.4 ± 1.9 | 32.6 ± 5.2 | 25.9 ± 4.2 | 31.3 ± 5.7 | |||
| Δ12-week | 5.7 (−1.1, 12.5) | 0.3 (−8.9, 9.5) | 5.4 (−5.1, 15.9) | 0.9 (−7.2, 8.9) | 9.7 (1.4, 17.9) | −8.8 (−19.8, 2.1) | p = 0.098 |
| AIx@75 (%) | |||||||
| Baseline | 18.0 ± 1.9 | 26.8 ± 4.0 | 27.0 ± 3.9 | 32.8 ± 7.0 | |||
| Δ12-week | 8.6 (2.2, 15.0) | 0.67 (−7.9, 9.2) | 7.9 (−1.9, 17.8) | 0.43 (−7.1, 8.0) | 1.33 (−9.5, 12.2) | 8.8 (−7.3, 24.9) | p = 0.272 |
| AP (mmHg) | |||||||
| Baseline | 9 ± 1 | 15 ± 3 | 11 ± 2 | 15 ± 5 | |||
| Δ12-week | 3 (−1, 12) | −1 (−5, 4) | 4 (−2, 9) | 1 (−4, 7) | 0 (−11.30, 10.80) | 0 (−9, 9) | p = 0.999 |
| PP (mmHg) | |||||||
| Baseline | 39 ± 2 | 44 ± 3 | 41 ± 3 | 36 ± 6 | |||
| Δ12-week | 1 (−3, 6) | −4 (−10, 2) | 5 (−2, 12) | 0 (−7, 7) | −4 (−18, 9) | 4 (−7, 16) | p = 0.862 |
| MAP (mmHg) | |||||||
| Baseline | 97 ± 3 | 98 ± 4 | 109 ± 7 | 95 ± 8 | |||
| Δ12-week | 2 (−4, 9) | −3 (−10, 4) | 5 (−4, 14) | −3 (−10, 3) | −2 (−8, 4) | −1 (−10, 8) | p = 0.253 |
| CSP (mmHg) | |||||||
| Baseline | 121 ± 3 | 123 ± 5 | 132 ± 8 | 118 ± 12 | |||
| Δ12-week | 2 (−5, 10) | −8 (−15, −1) | 10 (0, 21) | −3 (−10, 3) | −5 (−19, 8) | 2 (−9, 12) | p = 0.310 |
| CDP (mmHg) | |||||||
| Baseline | 82 ± 2 | 81 ± 4 | 92 ± 6 | 82 ± 6 | |||
| Δ12-week | 1 (−4, 7) | −2 (−9, 9) | 3 (−5, 12) | −3 (−11, 5) | −1 (−3, 2) | −2 (−13, 8) | p = 0.256 |
| SBP (mmHg) | |||||||
| Baseline | 132 ± 4 | 130 ± 4 | 135 ± 9 | 111 ± 6 | |||
| Δ12-week | −5 (−10, 0) | −14 (−18, −10) | 9 (3, 15) | 0 (−11, 10) | 6 (−12, 23) | −6 (−22, 10) | p = 0.004 |
| DBP (mmHg) | |||||||
| Baseline | 81 ± 1 | 78 ± 2 | 86 ± 6 | 79 ± 4 | |||
| Δ12-week | −3 (−6, 1) | −1 (−7, 4) | −1 (−7, 4) | −4 (−14, 5) | −3 (−8, 3) | −2 (−14, 11) | p = 0.939 |
| HRrest (bpm) | |||||||
| Baseline | 66 ± 2 | 71 ± 3 | 77 ± 6 | 78 ± 4 | |||
| Can removeΔ12-week | 2 (−3, 6) | 0 (−7, 7) | 2 (−6, 9) | 4 (−3, 11) | −9 (−20, 3) | 13 (2, 23) | p = 0.068 |
| Aerobic Exercise | Placebo Control | Interaction Effect (Group × Sex) | |||||
|---|---|---|---|---|---|---|---|
| Males | Females | Net Sex Difference | Males | Females | Net Sex Difference | ||
| VO2peak (mL/kg/min) | |||||||
| Baseline | 23.82 ± 3.92 | 17.67 ± 2.34 | 21.35 ± 5.14 | 18.07 ± 4.96 | |||
| Δ12-week | 0.76 (−1.00, 2.53) | 2.72 (0.11, 5.34) | −1.96 (−4.81, 0.80) | −1.76 (−5.21, 1.70) | −0.63 (−1.49, 0.23) | −1.13 (−5.47, 3.22) | p = 0.734 |
| BMI (kg/m2) | |||||||
| Baseline | 35.99 ± 1.45 | 35.78 ± 1.31 | 36.57 ± 1.86 | 34.40 ± 3.75 | |||
| Δ12-week | 0.36 (−0.32, 1.04) | −0.17 (−0.76, 0.41) | 0.53 (−0.36, 1.43) | 0.61 (−0.61, 1.82) | 0.05 (−0.49, 0.60) | 0.55 (−0.99, 2.10) | p = 0.981 |
| Weight (kg) | |||||||
| Baseline | 110.13 ± 4.27 | 94.33 ± 4.56 | 113.98 ± 5.97 | 92.13 ± 5.98 | |||
| Δ12-week | 1.13 (−0.93, 3.18) | −0.45 (−1.88, 0.99) | 1.58 (−1.02, 4.17) | 1.92 (−1.88, 5.71) | 0.07 (−1.36, 1.49) | 1.85 (−2.96, 6.66) | p = 0.890 |
| Waist circumference (cm) | |||||||
| Baseline | 118.4 ± 3.5 | 114.3 ± 3.4 | 122.5 ± 4.2 | 105.1 ± 5.6 | |||
| Δ12-week | −1.9 (−3.3, −0.5) | −5.8 (−7.8, −3.7) | 3.8 (1.6, 6.1) | −1.5 (−3.8, 0.9) | 1.3 (−3.4, 6.0) | −2.8 (−6.5, 1.0) | p = 0.002 |
| HbA1c (%) | |||||||
| Baseline | 6.83 ± 0.2 | 7.79 ± 1.89 | 7.52 ± 1.74 | 7.23 ± 0.74 | |||
| Δ12-week | 0.04 (−0.35, 0.44) | −0.64 (−1.49, 0.22) | 0.68 (−0.12, 1.48) | 0.30 (−0.41, 1.01) | 0.20 (−0.27, 0.67) | 0.10 (−0.82, 1.02) | p = 0.373 |
| FBG (mmol/L) | |||||||
| Baseline | 7.36 ± 0.49 | 8.20 ± 0.97 | 10.26 ± 1.83 | 6.95 ± 0.45 | |||
| Δ12-week | 0.44 (−0.45, 1.34) | −1.15 (−2.42, 0.12) | 1.59 (0.18, 3.01) | −0.24 (−4.93, 4.45) | 0.17 (−0.84, 1.18) | −0.41 (−6.30, 5.48) | p = 0.355 |
| Fasting insulin (mU/L) | |||||||
| Baseline | 11.07 ± 1.88 | 25.60 ± 10.66 | 14.00 ± 1.20 | 13.25 ± 5.36 | |||
| Δ12-week | 2.56 (0.25, 4.88) | −0.45 (−5.52, 4.62) | 3.01 (−1.70, 7.72) | −1.33 (−5.46, 2.79) | 3.33 (−3.08, 9.74) | −4.67 (−10.79, 1.46) | p = 0.057 |
| Total cholesterol (mmol/L) | |||||||
| Baseline | 4.17 ± 0.17 | 4.76 ± 0.24 | 4.23 ± 0.32 | 4.95 ± 0.49 | |||
| Δ12-week | 0.15 (−0.32, 0.61) | −0.35 (−1.08, 0.39) | 0.49 (−0.29, 1.27) | 0.06 (−0.45, 0.56) | 0.03 (−0.24, 0.30) | 0.02 (−0.62, 0.67) | p = 0.444 |
| LDL (mmol/L) | |||||||
| Baseline | 2.36 ± 0.20 | 2.77 ± 0.20 | 2.03 ± 0.24 | 2.88 ± 0.40 | |||
| Δ12-week | 0.04 (−0.36, 0.43) | −0.32 (−0.94, 0.30) | 0.35 (−0.30, 1.01) | −0.08 (−0.47, 0.30) | −0.13 (−0.53, 0.26) | 0.05 (−0.48, 0.58) | p = 0.434 |
| HDL (mmol/L) | |||||||
| Baseline | 1.02 ± 0.05 | 1.28 ± 0.08 | 1.06 ± 0.10 | 1.43 ± 0.13 | |||
| Δ12-week | −0.01 (−0.08, 0.06) | 0.07 (−0.07, 0.20) | −0.07 (−0.20, 0.06) | −0.01 (−0.08, 0.05) | −0.07 (−0.27, 0.13) | 0.05 (−0.08, 0.18) | p = 0.229 |
| Triglycerides (mmol/L) | |||||||
| Baseline | 1.69 ± 0.19 | 1.54 ± 0.19 | 1.87 ± 0.28 | 1.40 ± 0.15 | |||
| Δ12-week | 0.32 (0.03, 0.61) | −0.21 (−0.59, 0.18) | 0.53 (0.08, 0.97) | 0.38 (−0.38, 1.15) | 0.47 (−0.47, 1.41) | −0.08 (−1.16, 0.99) | p = 0.185 |
| hsCRP (mg/L) | |||||||
| Baseline | 2.47 ± 0.50 | 8.03 ± 2.53 | 2.81 ± 0.68 | 6.85 ± 2.71 | |||
| Δ12-week | 0.21 (−0.46, 0.89) | −3.05 (−6.33, 0.24) | 3.26 (0.62, 5.89) | 2.34 (−2.19, 6.87) | −1.53 (−5.33, 2.26) | 3.88 (−2.12, 9.88) | p = 0.820 |
| Male, n (%) | Female, n (%) | Total, n (%) | |
|---|---|---|---|
| Screened | 120 (43) | 161 (57) | 281 (100) |
| Unable to be contacted | 21 (7) | 31 (11) | 50 (18) |
| Declined participation | 35 (13) | 50 (18) | 87 (31) |
| Ineligible | 43 (15) | 66 (24) | 109 (39) |
| Total randomized | 21 (7) | 14 (5) | 35 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Gorman, S.A.; Miller, C.T.; Rawstorn, J.C.; Sabag, A.; Sultana, R.N.; Lanting, S.M.; Keating, S.E.; Johnson, N.A.; Way, K.L. Sex Differences in the Feasibility of Aerobic Exercise Training for Improving Cardiometabolic Health Outcomes in Adults with Type 2 Diabetes. J. Clin. Med. 2023, 12, 1255. https://doi.org/10.3390/jcm12041255
O’Gorman SA, Miller CT, Rawstorn JC, Sabag A, Sultana RN, Lanting SM, Keating SE, Johnson NA, Way KL. Sex Differences in the Feasibility of Aerobic Exercise Training for Improving Cardiometabolic Health Outcomes in Adults with Type 2 Diabetes. Journal of Clinical Medicine. 2023; 12(4):1255. https://doi.org/10.3390/jcm12041255
Chicago/Turabian StyleO’Gorman, Sian Alice, Clint Thomas Miller, Jonathan Charles Rawstorn, Angelo Sabag, Rachelle Noelle Sultana, Sean Michael Lanting, Shelley Elizabeth Keating, Nathan Anthony Johnson, and Kimberley Larisa Way. 2023. "Sex Differences in the Feasibility of Aerobic Exercise Training for Improving Cardiometabolic Health Outcomes in Adults with Type 2 Diabetes" Journal of Clinical Medicine 12, no. 4: 1255. https://doi.org/10.3390/jcm12041255
APA StyleO’Gorman, S. A., Miller, C. T., Rawstorn, J. C., Sabag, A., Sultana, R. N., Lanting, S. M., Keating, S. E., Johnson, N. A., & Way, K. L. (2023). Sex Differences in the Feasibility of Aerobic Exercise Training for Improving Cardiometabolic Health Outcomes in Adults with Type 2 Diabetes. Journal of Clinical Medicine, 12(4), 1255. https://doi.org/10.3390/jcm12041255





