Prognostic Value of the Radiographic Assessment of Lung Edema Score in Mechanically Ventilated ICU Patients
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Population
2.3. ARDS
2.4. RALE Scoring
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
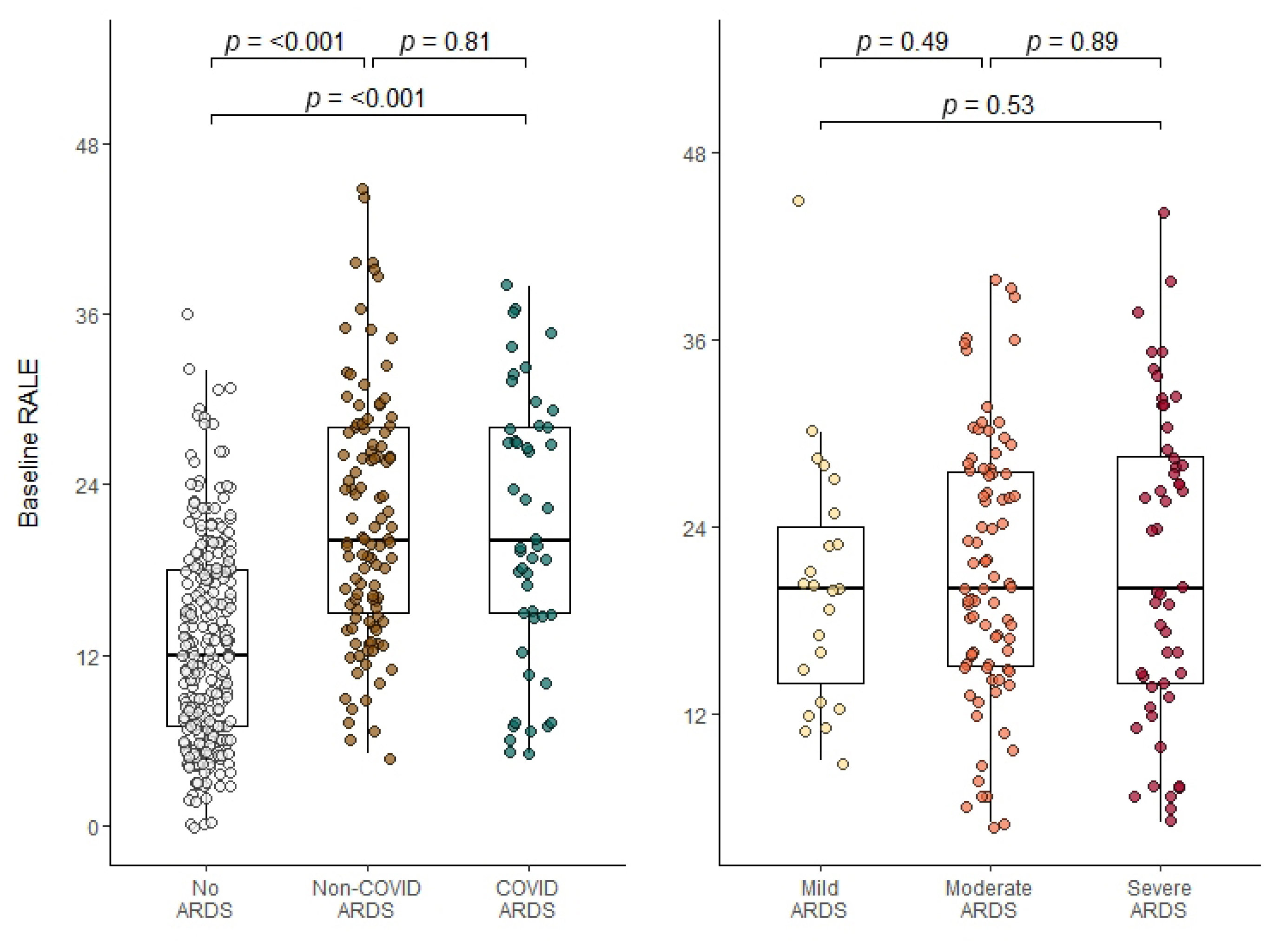
3.2. RALE Scoring
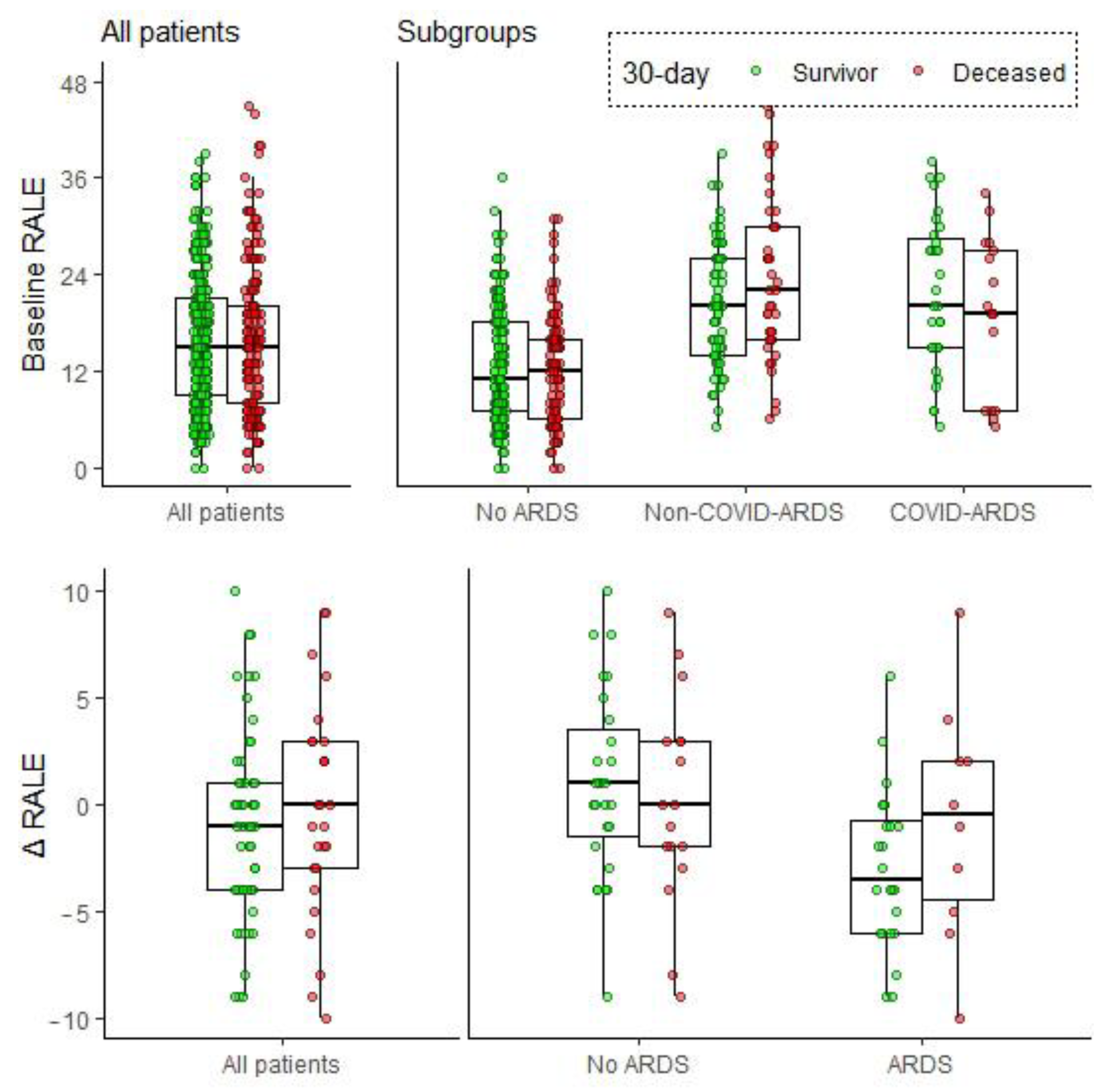
3.3. Association between Baseline RALE and Mortality
3.4. Early Changes in RALE Score and Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A. ARDS Scoring Method
Appendix B. Characteristics of CXR Scorers
| Name | Title | Current Role |
| D.F.L.F. | Medical Doctor | PhD-student |
| L.N.A. | Medical Doctor | PhD-student |
| M.R.S. | PhD, Technical Physician | Postdoctoral researcher |
| C.Z. | Anaesthesiologist, Intensivist | Intensivist, PhD-student |
References
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. Acute Pulmonary Edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Staub, N.C. Pulmonary edema. Physiol. Rev. 1974, 54, 678–811. [Google Scholar] [CrossRef]
- Warren, M.A.; Zhao, Z.; Koyama, T.; Bastarache, J.A.; Shaver, C.M.; Semler, M.W.; Rice, T.W.; Matthay, M.A.; Calfee, C.S.; Ware, L.B. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018, 73, 840–846. [Google Scholar] [CrossRef]
- Zimatore, C.; Pisani, L.; Lippolis, V.; Warren, M.A.; Calfee, C.S.; Ware, L.B.; Algera, A.G.; Smit, M.R.; Grasso, S.; Schultz, M.J. Accuracy of the Radiographic Assessment of Lung Edema Score for the Diagnosis of ARDS. Front. Physiol. 2021, 12, 672823. [Google Scholar] [CrossRef]
- Jabaudon, M.; Audard, J.; Pereira, B.; Jaber, S.; Lefrant, J.Y.; Blondonnet, R.; Godet, T.; Futier, E.; Lambert, C.; Bazin, J.E.; et al. Early Changes Over Time in the Radiographic Assessment of Lung Edema Score Are Associated With Survival in ARDS. Chest 2020, 158, 2394–2403. [Google Scholar] [CrossRef]
- Kotok, D.; Yang, L.; Evankovich, J.W.; Bain, W.; Dunlap, D.G.; Shah, F.; Zhang, Y.; Manatakis, D.V.; Benos, P.V.; Barbash, I.J.; et al. The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients. J. Crit. Care 2020, 56, 222–228. [Google Scholar] [CrossRef]
- Valk, C.M.A.; Zimatore, C.; Mazzinari, G.; Pierrakos, C.; Sivakorn, C.; Dechsanga, J.; Grasso, S.; Beenen, L.; Bos, L.D.J.; Paulus, F.; et al. The Prognostic Capacity of the Radiographic Assessment for Lung Edema Score in Patients With COVID-19 Acute Respiratory Distress Syndrome-An International Multicenter Observational Study. Front. Med. 2021, 8, 772056. [Google Scholar] [CrossRef]
- Herrmann, J.; Adam, E.H.; Notz, Q.; Helmer, P.; Sonntagbauer, M.; Ungemach-Papenberg, P.; Sanns, A.; Zausig, Y.; Steinfeldt, T.; Torje, I.; et al. COVID-19 Induced Acute Respiratory Distress Syndrome—A Multicenter Observational Study. Front. Med. 2020, 7, 599533. [Google Scholar] [CrossRef] [PubMed]
- Al-Yousif, N.; Komanduri, S.; Qurashi, H.; Korzhuk, A.; Lawal, H.O.; Abourizk, N.; Schaefer, C.; Mitchell, K.J.; Dietz, C.M.; Hughes, E.K.; et al. Radiographic Assessment of Lung Edema (RALE) Scores are Highly Reproducible and Prognostic of Clinical Outcomes for Inpatients with COVID-19. BMJ Open 2022. [Google Scholar] [CrossRef]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef]
- Mushtaq, J.; Pennella, R.; Lavalle, S.; Colarieti, A.; Steidler, S.; Martinenghi, C.M.A.; Palumbo, D.; Esposito, A.; Rovere-Querini, P.; Tresoldi, M.; et al. Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in COVID-19 patients: Analysis of 697 Italian patients. Eur. Radiol. 2021, 31, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Sensusiati, A.; Amin, M.; Nasronudin, N.; Rosyid, A.; Ramadhan, N.; Lathifah, R.; Puspitasari, E.; Wahyuningtyas, R.; Soebakti, E. Age, neutrophil lymphocyte ratio, and radiographic assessment of the quantity of lung edema (RALE) score to predict in-hospital mortality in COVID-19 patients: A retrospective study [version 2; peer review: 2 approved]. F1000Research 2021, 9, 1286. [Google Scholar] [CrossRef]
- Kerpel, A.; Apter, S.; Nissan, N.; Houri-Levi, E.; Klug, M.; Amit, S.; Konen, E.; Marom, E.M. Diagnostic and Prognostic Value of Chest Radiographs for COVID-19 at Presentation. West J. Emerg. Med. 2020, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Pham, T.; Laffey, J.G. Missed or delayed diagnosis of ARDS: A common and serious problem. Intensive Care Med. 2020, 46, 1180–1183. [Google Scholar] [CrossRef]
- Goddard, S.L.; Rubenfeld, G.D.; Manoharan, V.; Dev, S.P.; Laffey, J.; Bellani, G.; Pham, T.; Fan, E. The Randomized Educational Acute Respiratory Distress Syndrome Diagnosis Study: A Trial to Improve the Radiographic Diagnosis of Acute Respiratory Distress Syndrome. Crit. Care Med. 2018, 46, 743–748. [Google Scholar] [CrossRef]
- Hagens, L.A.; Heijnen, N.F.L.; Smit, M.R.; Verschueren, A.R.M.; Nijsen, T.M.E.; Geven, I.; Schultz, M.J.; Bergmans, D.C.J.J.; Schnabel, R.M.; Bos, L.D.J. Diagnosis of acute respiratory distress syndrome (DARTS) by bedside exhaled breath octane measurements in invasively ventilated patients: Protocol of a multicentre observational cohort study. Ann. Transl. Med. 2021, 9, 1262. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. Jama 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Hagens, L.A.; Van der Ven, F.; Heijnen, N.F.L.; Smit, M.R.; Gietema, H.A.; Gerretsen, S.C.; Schultz, M.J.; Bergmans, D.; Schnabel, R.M.; Bos, L.D.J. Improvement of an interobserver agreement of ARDS diagnosis by adding additional imaging and a confidence scale. Front. Med. 2022, 9, 950827. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Atmowihardjo, L.N.; Heijnen, N.F.L.; Smit, M.R.; Hagens, L.A.; Filippini, D.F.L.; Zimatore, C.; Schultz, M.J.; Schnabel, R.M.; Bergmans, D.; Aman, J.; et al. Biomarkers of alveolar epithelial injury and endothelial dysfunction are associated with scores of pulmonary edema in invasively ventilated patients. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L38–L47. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Algera, A.G.; Serpa Neto, A.; Ahsan, A.; Beane, A.; Chittawatanarat, K.; Faiz, A.; Haniffa, R.; Hashemian, S.M.; Hashmi, M.; et al. Epidemiological Characteristics, Ventilator Management, and Clinical Outcome in Patients Receiving Invasive Ventilation in Intensive Care Units from 10 Asian Middle-Income Countries (PRoVENT-iMiC): An International, Multicenter, Prospective Study. Am. J. Trop. Med. Hyg. 2021, 104, 1022–1033. [Google Scholar] [CrossRef]
- Graat, M.E.; Hendrikse, K.A.; Spronk, P.E.; Korevaar, J.C.; Stoker, J.; Schultz, M.J. Chest radiography practice in critically ill patients: A postal survey in the Netherlands. BMC Med. Imaging 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Vercesi, V.; Costantino, F.; Chandrapatham, K.; Pelosi, P. Lung imaging: How to get better look inside the lung. Ann. Transl. Med. 2017, 5, 294. [Google Scholar] [CrossRef]
- Heldeweg, M.L.A.; Smit, M.R.; Kramer-Elliott, S.R.; Haaksma, M.E.; Smit, J.M.; Hagens, L.A.; Heijnen, N.F.L.; Jonkman, A.H.; Paulus, F.; Schultz, M.J.; et al. Lung Ultrasound Signs to Diagnose and Discriminate Interstitial Syndromes in ICU Patients: A Diagnostic Accuracy Study in Two Cohorts. Crit. Care Med. 2022, 50, 1607–1617. [Google Scholar] [CrossRef]
- Chiumello, D.; Froio, S.; Bouhemad, B.; Camporota, L.; Coppola, S. Clinical review: Lung imaging in acute respiratory distress syndrome patients--an update. Crit. Care 2013, 17, 243. [Google Scholar] [CrossRef]
- Beckmann, U.; Gillies, D.M.; Berenholtz, S.M.; Wu, A.W.; Pronovost, P. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med. 2004, 30, 1579–1585. [Google Scholar] [CrossRef]
- Moloney, F.; Fama, D.; Twomey, M.; O’Leary, R.; Houlihane, C.; Murphy, K.P.; O’Neill, S.B.; O’Connor, O.J.; Breen, D.; Maher, M.M. Cumulative radiation exposure from diagnostic imaging in intensive care unit patients. World J. Radiol. 2016, 8, 419–427. [Google Scholar] [CrossRef]
- Villar, J.; Pérez-Méndez, L.; Blanco, J.; Añón, J.M.; Blanch, L.; Belda, J.; Santos-Bouza, A.; Fernández, R.L.; Kacmarek, R.M.; Spanish Initiative for Epidemiology, S.; et al. A universal definition of ARDS: The PaO2/FiO2 ratio under a standard ventilatory setting—A prospective, multicenter validation study. Intensive Care Med. 2013, 39, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Sjoding, M.W.; Hofer, T.P.; Co, I.; Courey, A.; Cooke, C.R.; Iwashyna, T.J. Interobserver Reliability of the Berlin ARDS Definition and Strategies to Improve the Reliability of ARDS Diagnosis. Chest 2018, 153, 361–367. [Google Scholar] [CrossRef] [PubMed]

| All n = 422 | Survived at 30 Days n = 268 | Deceased at 30 Days n = 153 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years (SD)) | 62 (14) | 60 (14) | 66 (12) | <0.001 |
| Male (%) | 290 (68.7) | 189 (70.5) | 100 (65.4) | 0.323 |
| BMI (kg m−2) | 26.1 (23.4, 29.9) | 26.1 (23.6, 29.4) | 26.3 (22.9, 30.2) | 0.811 |
| Admission characteristics | ||||
| ICU stay inclusion (days) | 1 (0, 1.75) | 1 (0, 2) | 1 (0, 1) | 0.298 |
| Admission type (%) | 0.007 | |||
| - Medical | 307 (72.7) | 195 (72.8) | 2.5) | |
| - Emergency surgical | 60 (14.2) | 30 (11.2) | 30 (19.6) | |
| - Planned surgical | 55 (13.0) | 43 (16.0) | 12 (7.8) | |
| COVID-19 (%) | 50 (11.8) | 33 (12.3) | 17 (11.1) | 0.834 |
| Respiratory | ||||
| Maximum airway pressure (cmH2O) | 21 (16, 26) | 21 (16, 26) | 21 (17, 27) | 0.655 |
| Driving Pressure (cmH2O) | 14 (9, 18) | 14 (9, 18) | 14 (10, 18) | 0.651 |
| PEEP (cmH2O) | 8 (5, 10) | 8 (5, 10) | 8 (5, 10) | 0.625 |
| Severity | ||||
| ARDS category (%) | 0.997 | |||
| - No ARDS | 265 (62.8) | 169 (63.1) | 96 (62.7) | |
| - Mild ARDS | 23 (5.5) | 15 (5.6) | 8 (5.2) | |
| - Moderate ARDS | 83 (19.7) | 52 (19.4) | 30 (19.6) | |
| - Severe ARDS | 51 (12.1) | 32 (11.9) | 19 (12.4) | |
| APACHE II score | 20 (15, 25) | 20 (15, 24) | 23 (18, 26) | <0.001 |
| SOFA score | 9 (7, 11) | 9 (7, 11) | 10 (7, 11) | 0.043 |
| PaO2/FiO2 (mmHg) | 179 (113, 270) | 188 (119, 287) | 154 (109, 244) | 0.040 |
| Lactate (mmol/L) | 1.6 (1.2, 2.5) | 1.60 (1.1, 2.2) | 1.90 (1.3, 3.3) | <0.001 |
| Outcomes | ||||
| ICU length of stay (days) | 7 (3, 13) | 7 (3, 13) | 6 (3, 12.25) | 0.237 |
| ICU mortality (%) | 132 (32.5) | 5 (1.9) | 127 (84.7) | <0.001 |
| All Patients n = 421 | No ARDS n = 265 | Non-COVID-ARDS n = 108 | COVID-ARDS n = 48 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (CI) | p-Value | OR (CI) | p-Value | OR (CI) | p-Value | OR (CI) | p-Value | |
| Univariable analyses | ||||||||
| Baseline RALE | 1.01 (0.98–1.03) | 0.66 | 0.99 (0.96–1.03) | 0.77 | 1.04 (1–1.1) | 0.07 | 0.97 (0.91–1.03) | 0.37 |
| Multivariable analyses (adjusted for confounders: age, gender APACHE II) | ||||||||
| Baseline RALE | 1 (0.98–1.02) | 0.92 | 0.99 (0.95–1.02) | 0.47 | 1.05 (1–1.1) | 0.07 | 0.99 (0.91–1.07) | 0.80 |
| All Patients n = 84 | No ARDS n = 48 | ARDS n = 36 | ||||
|---|---|---|---|---|---|---|
| OR (CI) | p-Value | OR (CI) | p-Value | OR (CI) | p-Value | |
| Univariable analyses | ||||||
| ∆RALE * | 1 (0.93–1.07) | 0.98 | 0.97 (0.88–1.07) | 0.55 | 1.02 (0.92–1.14) | 0.64 |
| ∆RALE > 0 | 1.26 (0.44–3.54) | 0.64 | 0.64 (0.16–2.42) | 0.56 | 3.51 (0.5–25.74) | 0.18 |
| Multivariable analyses (corrected for confounders) | ||||||
| ∆RALE * | 1.02 (0.94–1.1) | 0.64 | 0.97 (0.87–1.08) | 0.60 | 1.21 (1.02–1.51) | 0.04 |
| ∆RALE > 0 | 1.32 (0.49–3.56) | 0.58 | 0.61 (0.17–2.09) | 0.44 | 17.38 (1.72–42.39) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, D.F.L.; Hagens, L.A.; Heijnen, N.F.L.; Zimatore, C.; Atmowihardjo, L.N.; Schnabel, R.M.; Schultz, M.J.; Bergmans, D.C.J.J.; Bos, L.D.J.; Smit, M.R. Prognostic Value of the Radiographic Assessment of Lung Edema Score in Mechanically Ventilated ICU Patients. J. Clin. Med. 2023, 12, 1252. https://doi.org/10.3390/jcm12041252
Filippini DFL, Hagens LA, Heijnen NFL, Zimatore C, Atmowihardjo LN, Schnabel RM, Schultz MJ, Bergmans DCJJ, Bos LDJ, Smit MR. Prognostic Value of the Radiographic Assessment of Lung Edema Score in Mechanically Ventilated ICU Patients. Journal of Clinical Medicine. 2023; 12(4):1252. https://doi.org/10.3390/jcm12041252
Chicago/Turabian StyleFilippini, Daan F. L., Laura A. Hagens, Nanon F. L. Heijnen, Claudio Zimatore, Leila N. Atmowihardjo, Ronny M. Schnabel, Marcus J. Schultz, Dennis C. J. J. Bergmans, Lieuwe D. J. Bos, and Marry R. Smit. 2023. "Prognostic Value of the Radiographic Assessment of Lung Edema Score in Mechanically Ventilated ICU Patients" Journal of Clinical Medicine 12, no. 4: 1252. https://doi.org/10.3390/jcm12041252
APA StyleFilippini, D. F. L., Hagens, L. A., Heijnen, N. F. L., Zimatore, C., Atmowihardjo, L. N., Schnabel, R. M., Schultz, M. J., Bergmans, D. C. J. J., Bos, L. D. J., & Smit, M. R. (2023). Prognostic Value of the Radiographic Assessment of Lung Edema Score in Mechanically Ventilated ICU Patients. Journal of Clinical Medicine, 12(4), 1252. https://doi.org/10.3390/jcm12041252







