Asymmetric Properties of the Heart Rate Microstructure in Healthy Adults during 48 h ECG Recordings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. 48 h ECGs
2.3. Heart Rate Microstructure Measurement
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. The HR Microstructure during 48 h ECG Recordings
3.3. Sex Differences in the HR Microstructure during 48 h ECG Recordings
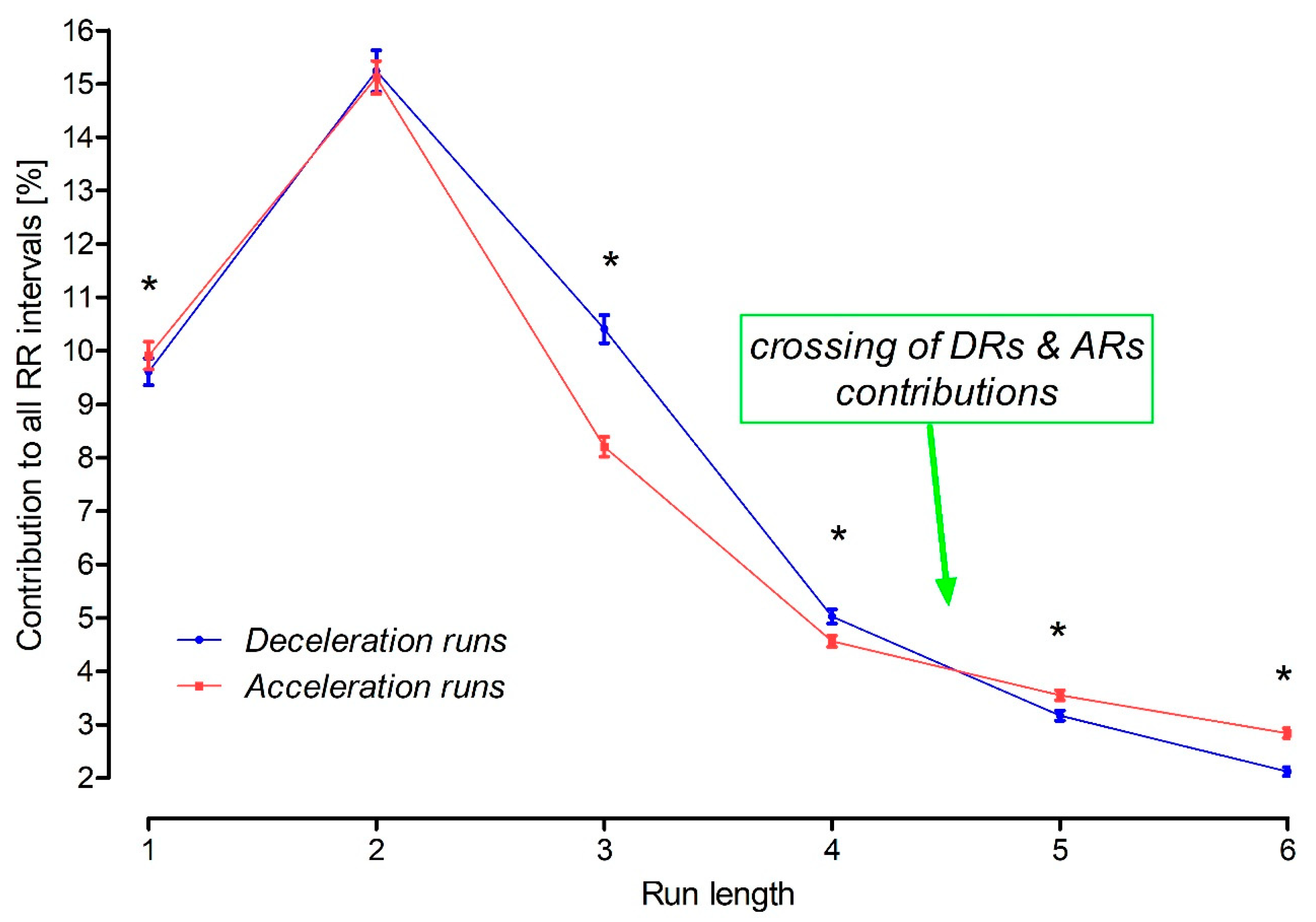
3.4. Crossing of the Acceleration and Deceleration Run Contributions
3.5. The Longest Runs
3.6. Neutral Runs
4. Discussion
4.1. HRA in Men and Women
4.2. The HR Microstructure in Healthy Individuals
4.3. Clinical Value of the HR Microstructure
4.4. Physiological Crossing of the Asymmetric Features of the HR Microstructure
4.5. Sampling Frequency
4.6. Study Limitations
4.7. Novelty, Potential Clinical Meanings and Conclusions
4.8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Opthof, T. The Normal Range and Determinants of the Intrinsic Heart Rate in Man. Cardiovasc. Res. 2000, 45, 177–184. [Google Scholar] [CrossRef]
- John, R.M.; Kumar, S. Sinus Node and Atrial Arrhythmias. Circulation 2016, 133, 1892–1900. [Google Scholar] [CrossRef]
- Boyett, M.R.; Yanni, J.; Tellez, J.; Bucchi, A.; Mesirca, P.; Cai, X.; Logantha, S.J.R.J.; Wilson, C.; Anderson, C.; Ariyaratnam, J.; et al. Regulation of Sinus Node Pacemaking and Atrioventricular Node Conduction by HCN Channels in Health and Disease. Prog. Biophys. Mol. Biol. 2021, 166, 61–85. [Google Scholar] [CrossRef]
- Moïse, N.S.; Flanders, W.H.; Pariaut, R. Beat-to-Beat Patterning of Sinus Rhythm Reveals Non-Linear Rhythm in the Dog Compared to the Human. Front. Physiol. 2019, 10, 1548. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; Cabrera, J.A.; Farré, J.; Climent, V.; Anderson, R.H.; Ho, S.Y. Sinus Node Revisited in the Era of Electroanatomical Mapping and Catheter Ablation. Heart Br. Card. Soc. 2005, 91, 189–194. [Google Scholar] [CrossRef]
- Boineau, J.P.; Canavan, T.E.; Schuessler, R.B.; Cain, M.E.; Corr, P.B.; Cox, J.L. Demonstration of a Widely Distributed Atrial Pacemaker Complex in the Human Heart. Circulation 1988, 77, 1221–1237. [Google Scholar] [CrossRef]
- Karemaker, J.M. Autonomic Integration: The Physiological Basis of Cardiovascular Variability. J. Physiol. 1999, 517 Pt 2, 316. [Google Scholar] [CrossRef]
- Crystal, G.J.; Salem, M.R. The Bainbridge and the “Reverse” Bainbridge Reflexes: History, Physiology, and Clinical Relevance. Anesth. Analg. 2012, 114, 520–532. [Google Scholar] [CrossRef]
- Eckberg, D.L. Sympathovagal Balance. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence Diagram of Physiological and Environmental Factors Affecting Heart Rate Variability: An Extended Literature Overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ. Arrhythm. Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef] [PubMed]
- Severi, S.; Cavalcanti, S.; Mancini, E.; Santoro, A. Effect of Electrolyte and pH Changes on the Sinus Node Pacemaking in Humans. J. Electrocardiol. 2002, 35, 115–124. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral Control of Sinoatrial Node Activity and Heart Rate: Insight From Experimental Models and Findings From Humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Harthoorn, L.F.; Dransfield, E. Periprandial Changes of the Sympathetic-Parasympathetic Balance Related to Perceived Satiety in Humans. Eur. J. Appl. Physiol. 2008, 102, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Harrison, N.A. Visceral Influences on Brain and Behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, W.; Wei, Z.; Zhang, H. Air Pollution and Cardiac Arrhythmias: From Epidemiological and Clinical Evidences to Cellular Electrophysiological Mechanisms. Front. Cardiovasc. Med. 2021, 8, 736151. [Google Scholar] [CrossRef]
- Guzik, P.; Piskorski, J.; Krauze, T.; Wykretowicz, A.; Wysocki, H. Heart Rate Asymmetry by Poincaré Plots of RR Intervals. Biomed. Tech. Eng. 2006, 51, 272–275. [Google Scholar] [CrossRef]
- Piskorski, J.; Guzik, P. Asymmetric Properties of Long-Term and Total Heart Rate Variability. Med. Biol. Eng. Comput. 2011, 49, 1289–1297. [Google Scholar] [CrossRef]
- Piskorski, J.; Guzik, P. Geometry of the Poincaré Plot of RR Intervals and Its Asymmetry in Healthy Adults. Physiol. Meas. 2007, 28, 287–300. [Google Scholar] [CrossRef]
- Piskorski, J.; Ellert, J.; Krauze, T.; Grabowski, W.; Wykretowicz, A.; Guzik, P. Testing Heart Rate Asymmetry in Long, Nonstationary 24 Hour RR-Interval Time Series. Physiol. Meas. 2019, 40, 105001. [Google Scholar] [CrossRef]
- Sibrecht, G.; Piskorski, J.; Krauze, T.; Guzik, P. Heart Rate Asymmetry, Its Compensation, and Heart Rate Variability in Healthy Adults during 48-h Holter ECG Recordings. J. Clin. Med. 2023, 12, 1219. [Google Scholar] [CrossRef]
- Piskorski, J.; Guzik, P. The Structure of Heart Rate Asymmetry: Deceleration and Acceleration Runs. Physiol. Meas. 2011, 32, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piskorski, J.; Barthel, P.; Bauer, A.; Müller, A.; Junk, N.; Ulm, K.; Malik, M.; Schmidt, G. Heart Rate Deceleration Runs for Postinfarction Risk Prediction. J. Electrocardiol. 2012, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Billois, R.; Porée, F.; Beuchée, A.; Carrault, G. Interest of RR Deceleration for Diagnosis of Late Onset Sepsis. In Proceedings of the 2012 Computing in Cardiology, Krakow, Poland, 9–12 September 2012; pp. 633–636. [Google Scholar]
- Guzik, P.; Piskorski, J.; Awan, K.; Krauze, T.; Fitzpatrick, M.; Baranchuk, A. Obstructive Sleep Apnea and Heart Rate Asymmetry Microstructure during Sleep. Clin. Auton. Res. 2013, 23, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, X.; Zhang, C.; Wang, G.; Fang, J.; Ma, J.; Zhang, J. Heart Rate Acceleration Runs and Deceleration Runs in Patients with Obstructive Sleep Apnea Syndrome. Sleep Breath. 2017, 21, 443–451. [Google Scholar] [CrossRef]
- Kong, Z.-B.; Wang, X.-D.; Shen, S.-R.; Liu, H.; Zhou, L.; Chen, B.; Chen, Z.; Yin, S.-J. Risk Prediction for Arrhythmias by Heart Rate Deceleration Runs in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 585–593. [Google Scholar] [CrossRef]
- Wang, X.-D.; Zhou, L.; Zhu, C.-Y.; Chen, B.; Chen, Z.; Wei, L. Autonomic Function as Indicated by Heart Rate Deceleration Capacity and Deceleration Runs in Type 2 Diabetes Patients with or without Essential Hypertension. Clin. Interv. Aging 2018, 13, 1169–1176. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piskorski, J. Asymmetric Properties of Heart Rate Microstructure. J. Med. Sci. 2020, 89, e436. [Google Scholar] [CrossRef]
- Guzik, P.; Więckowska, B. Data Distribution Analysis—A Preliminary Approach to Quantitative Data in Biomedical Research. J. Med. Sci. 2023, 92, e869. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex Differences in Healthy Human Heart Rate Variability: A Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Piskorski, J.; Guzik, P. Compensatory Properties of Heart Rate Asymmetry. J. Electrocardiol. 2012, 45, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Nieminen, T.; Piskorski, J.; Kaiser, W.; Viik, J.; Nikus, K.; Lehtinen, R.; Lehtimäki, T.; Kähönen, M. Increased Rate of Fast-Changing Microstructure of Heart Rate Asymmetry Predicts Mortality in Patients Undergoing a Clinically Indicated Exercise Test. Cardiol. J. 2011, 18, 593. [Google Scholar]
- Fisher, J.P.; Zera, T.; Paton, J.F.R. Chapter 10—Respiratory–Cardiovascular Interactions. In Handbook of Clinical Neurology; Chen, R., Guyenet, P.G., Eds.; Respiratory Neurobiology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 188, pp. 279–308. [Google Scholar]
- Ben-Tal, A.; Shamailov, S.S.; Paton, J.F.R. Evaluating the Physiological Significance of Respiratory Sinus Arrhythmia: Looking beyond Ventilation–Perfusion Efficiency. J. Physiol. 2012, 590, 1989–2008. [Google Scholar] [CrossRef]
- Hathorn, M.K. Respiratory Sinus Arrhythmia in New-Born Infants. J. Physiol. 1987, 385, 1–12. [Google Scholar] [CrossRef]
- Grossman, P.; Taylor, E.W. Toward Understanding Respiratory Sinus Arrhythmia: Relations to Cardiac Vagal Tone, Evolution and Biobehavioral Functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef]
- Barrow, A.; Pandit, J.J. Lung Ventilation and the Physiology of Breathing. Surg. Oxf. 2017, 35, 227–233. [Google Scholar] [CrossRef]
| Parameter (Median, IQR) | All Participants (n = 101) | Women (n = 54) | Men (n = 47) |
|---|---|---|---|
| Age [years] | 39 (28–44) | 40 (28–45) | 39 (28–43) |
| BMI [kg/m²] | 23.7 (21–25.6) | 22.5 (20.8–25) | 24.5 (22–26.8) |
| SBP [mmHg] | 118 (114–124) | 118 (114–124) | 117 (114–124) |
| DBP [mmHg] | 72 (67–79) | 75 (68–80) | 71 (66–78) |
| HR [beats/min] | 70 (65–75) | 70 (65–75) | 70 (63–75) |
| Length of Run | Acceleration Runs [%] | Deceleration Runs [%] | p-Value (Wilcoxon Test) | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| 1 | 9.80 | 7.87–11.41 | 9.22 | 7.99–10.88 | 0.046 |
| 2 | 14.70 | 12.52–17.20 | 14.63 | 12.59–18.16 | 0.750 |
| 3 | 8.17 | 6.80–9.67 | 10.48 | 8.64–12.06 | <0.001 |
| 4 | 4.42 | 3.76–5.32 | 4.97 | 4.06–5.78 | <0.001 |
| 5 | 3.52 | 2.92–4.12 | 3.12 | 2.52–3.94 | <0.001 |
| 6 | 2.72 | 2.24–3.41 | 2.14 | 1.52–2.67 | <0.001 |
| 7 | 2.14 | 1.62–2.66 | 1.24 | 0.85–1.60 | <0.001 |
| 8 | 1.54 | 1.05–1.89 | 0.64 | 0.45–0.90 | <0.001 |
| 9 | 0.98 | 1.67–1.32 | 0.31 | 0.21–0.50 | <0.001 |
| 10 | 0.60 | 0.40–0.93 | 0.16 | 0.10–0.26 | <0.001 |
| 11 | 0.42 | 0.27–0.60 | 0.08 | 0.05–0.14 | <0.001 |
| 12 | 0.25 | 0.17–0.42 | 0.04 | 0.02–0.08 | <0.001 |
| 13 | 0.20 | 0.11–0.28 | 0.03 | 0.01–0.05 | <0.001 |
| 14 | 0.14 | 0.07–0.22 | 0.01 | 0–0.03 | <0.001 |
| 15 | 0.10 | 0.05–0.15 | 0.01 | 0–0.02 | <0.001 |
| 16 | 0.06 | 0.04–0.10 | 0 | 0–0.01 | <0.001 |
| 17 | 0.05 | 0.2–0.08 | 0 | 0–0.01 | <0.001 |
| 18 | 0.03 | 0.01–0.07 | 0 | 0–0 | <0.001 |
| 19 | 0.03 | 0.01–0.05 | 0 | 0–0 | <0.001 |
| 20 | 0.02 | 0.01–0.04 | 0 | 0–0 | <0.001 |
| 21 | 0.01 | 0–0.03 | 0 | 0–0 | <0.001 |
| 22 | 0.01 | 0–0.02 | 0 | 0–0 | <0.001 |
| 23 | 0 | 0–0.01 | 0 | 0–0 | <0.001 |
| 24 | 0 | 0–0.01 | 0 | 0–0 | <0.001 |
| 25 | 0 | 0–0.01 | 0 | 0–0 | <0.001 |
| Run Length | Women ^ | Men ^ | * p-Value (M-W) | # p-Value (M-W) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceleration Runs * [%] | Deceleration Runs # [%] | ^ p-Value (Wilcoxon) Test) | Acceleration Runs * [%] | Deceleration Runs # [%] | ^ p-Value (Wilcoxon) | |||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||||
| 1 | 9.88 | 8.76–11.26 | 9.30 | 8.29–10.62 | 0.025 | 9.33 | 7.68–11.42 | 9.01 | 7.76–11.06 | 0.694 | 0.5040 | 0.8191 |
| 2 | 16.37 | 13.88–18.03 | 16.28 | 13.90–18.56 | 0.718 | 12.94 | 11.89–15.38 | 13.30 | 11.10–15.63 | 0.320 | 0.0001 | 0.1009 |
| 3 | 8.25 | 7.37–9.85 | 10.64 | 9.32–12.55 | <0.001 | 8.06 | 6.52–9.10 | 9.45 | 7.77–11.26 | <0.001 | 0.1978 | 0.4830 |
| 4 | 4.00 | 3.45–4.66 | 4.40 | 3.88–5.53 | <0.001 | 5.00 | 4.12–5.61 | 5.46 | 4.46–5.88 | <0.001 | 0.0004 | 0.6620 |
| 5 | 3.23 | 2.77–3.62 | 2.77 | 2.15–3.19 | <0.001 | 3.89 | 3.22–4.72 | 3.89 | 3.02–4.37 | <0.001 | 0.0001 | 0.0012 |
| 6 | 2.47 | 2.09–2.97 | 1.86 | 1.25–2.27 | <0.001 | 3.21 | 2.71–3.95 | 2.56 | 2.06–2.90 | <0.001 | 0.0001 | 0.0397 |
| 7 | 1.79 | 1.45–2.27 | 1.10 | 0.69–1.47 | <0.001 | 2.50 | 2.08–2.93 | 1.38 | 1.13–1.69 | <0.001 | 0.0001 | 0.0017 |
| 8 | 1.21 | 0.91–1.74 | 0.57 | 0.29–0.85 | <0.001 | 1.71 | 1.29–2.12 | 0.72 | 0.52–0.98 | <0.001 | 0.0016 | 0.0001 |
| 9 | 0.780 | 0.60–1.17 | 0.29 | 0.16–0.48 | <0.001 | 1.10 | 0.77–1.43 | 0.33 | 0.24–0.53 | <0.001 | 0.0063 | 0.0001 |
| 10 | 0.53 | 0.36–0.84 | 0.15 | 0.08–0.24 | <0.001 | 0.77 | 0.50–1.01 | 0.20 | 0.12–0.28 | <0.001 | 0.0119 | 0.0042 |
| 11 | 0.38 | 0.22–0.54 | 0.08 | 0.05–0.13 | <0.001 | 0.51 | 0.30–0.66 | 0.09 | 0.05–0.14 | <0.001 | 0.0189 | 0.0231 |
| 12 | 0.24 | 0.16–0.38 | 0.04 | 0.02–0.08 | <0.001 | 0.33 | 0.21–0.46 | 0.05 | 0.03–0.08 | <0.001 | 0.0989 | 0.0947 |
| 13 | 0.15 | 0.08–0.27 | 0.03 | 0.01–0.05 | <0.001 | 0.21 | 0.14–0.32 | 0.03 | 0.02–0.05 | <0.001 | 0.0492 | 0.2010 |
| 14 | 0.13 | 0.07–0.23 | 0.01 | 0–0.02 | <0.001 | 0.14 | 0.09–0.21 | 0.02 | 0.01–0.04 | <0.001 | 0.4253 | 0.5667 |
| 15 | 0.09 | 0.04–0.14 | 0.01 | 0–0.01 | <0.001 | 0.11 | 0.06–0.16 | 0.01 | 0–0.02 | <0.001 | 0.2973 | 0.3831 |
| 16 | 0.06 | 0.04–0.11 | 0 | 0–0.01 | <0.001 | 0.07 | 0.04–0.10 | 0 | 0–0.01 | <0.001 | 0.9999 | 0.3718 |
| 17 | 0.04 | 0.02–0.09 | 0 | 0–0.01 | <0.001 | 0.05 | 0.03–0.07 | 0 | 0–0.01 | <0.001 | 0.6768 | 0.0058 |
| 18 | 0.03 | 0.01–0.07 | 0 | 0–0 | <0.001 | 0.03 | 0.01–0.08 | 0 | 0–0 | <0.001 | 0.1865 | 0.0944 |
| 19 | 0.02 | 0.01–0.05 | 0 | 0–0 | <0.001 | 0.03 | 0.02–0.05 | 0 | 0–0 | <0.001 | 0.2222 | 0.9173 |
| 20 | 0.02 | 0.01–0.04 | 0 | 0–0 | <0.001 | 0.02 | 0–0.04 | 0 | 0–0 | <0.001 | 0.9593 | 0.2243 |
| 21 | 0.01 | 0–0.03 | 0 | 0–0 | <0.001 | 0.01 | 0–0.03 | 0 | 0–0 | <0.001 | 0.7515 | 0.9219 |
| 22 | 0.01 | 0–0.03 | 0 | 0–0 | <0.001 | 0 | 0–0.02 | 0 | 0–0 | <0.001 | 0.7396 | 0.8423 |
| 23 | 0 | 0–0.2 | 0 | 0–0 | <0.001 | 0.01 | 0–0.01 | 0 | 0–0 | <0.001 | 0.2039 | 0.6025 |
| 24 | 0 | 0–0.01 | 0 | 0–0 | <0.001 | 0 | 0–0.01 | 0 | 0–0 | <0.001 | 0.6253 | 0.5100 |
| 25 | 0 | 0–0.01 | 0 | 0–0 | <0.001 | 0 | 0–0.01 | 0 | 0–0 | <0.001 | 0.4414 | 0.4696 |
| Run Length | All Participants n = 101 [%] | Women * n = 54 [%] | Men * n = 47 [%] | * p-Value (M-W) | |||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| N1 | 0.50 | 0.39–0.69 | 0.57 | 0.43–0.73 | 0.48 | 0.38–0.67 | 0.08 |
| N2 | 0.01 | 0.01–0.02 | 0.01 | 0.01–0.02 | 0.01 | 0.01–0.02 | 0.08 |
| N3 | 0 | 0–0.001 | 0 | 0–0.001 | 0 | 0–0.001 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibrecht, G.; Piskorski, J.; Krauze, T.; Guzik, P. Asymmetric Properties of the Heart Rate Microstructure in Healthy Adults during 48 h ECG Recordings. J. Clin. Med. 2023, 12, 7472. https://doi.org/10.3390/jcm12237472
Sibrecht G, Piskorski J, Krauze T, Guzik P. Asymmetric Properties of the Heart Rate Microstructure in Healthy Adults during 48 h ECG Recordings. Journal of Clinical Medicine. 2023; 12(23):7472. https://doi.org/10.3390/jcm12237472
Chicago/Turabian StyleSibrecht, Greta, Jarosław Piskorski, Tomasz Krauze, and Przemysław Guzik. 2023. "Asymmetric Properties of the Heart Rate Microstructure in Healthy Adults during 48 h ECG Recordings" Journal of Clinical Medicine 12, no. 23: 7472. https://doi.org/10.3390/jcm12237472
APA StyleSibrecht, G., Piskorski, J., Krauze, T., & Guzik, P. (2023). Asymmetric Properties of the Heart Rate Microstructure in Healthy Adults during 48 h ECG Recordings. Journal of Clinical Medicine, 12(23), 7472. https://doi.org/10.3390/jcm12237472







