Post-Stroke Brain Health Monitoring and Optimization: A Narrative Review
Abstract
1. Introduction
2. Aims, Materials, Methods of Search Strategy, and Limitations
3. Defining and Framing Post-Stroke Brain Health
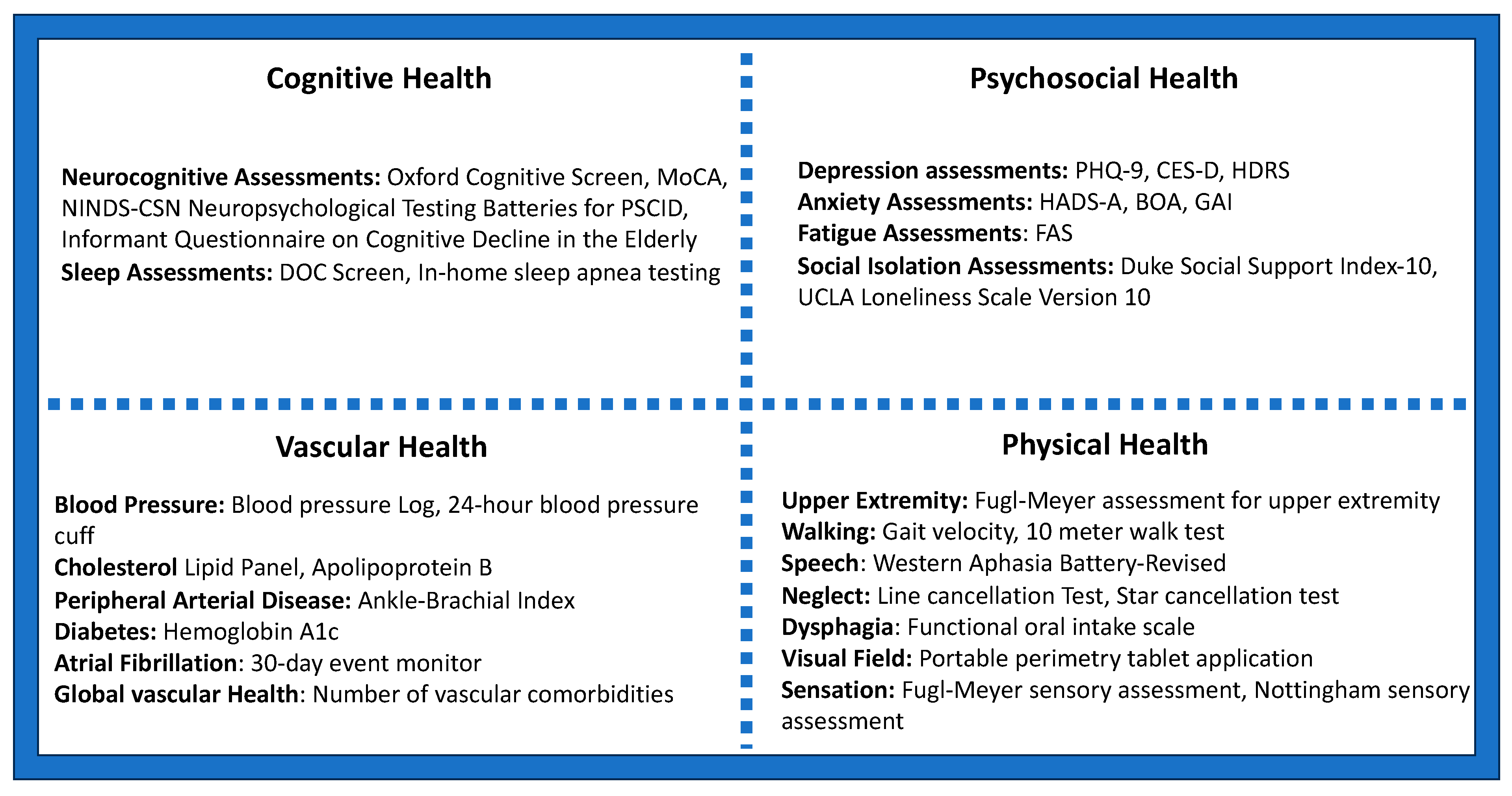
4. Monitoring Post-Stroke Brain Health and Neuroprognostication
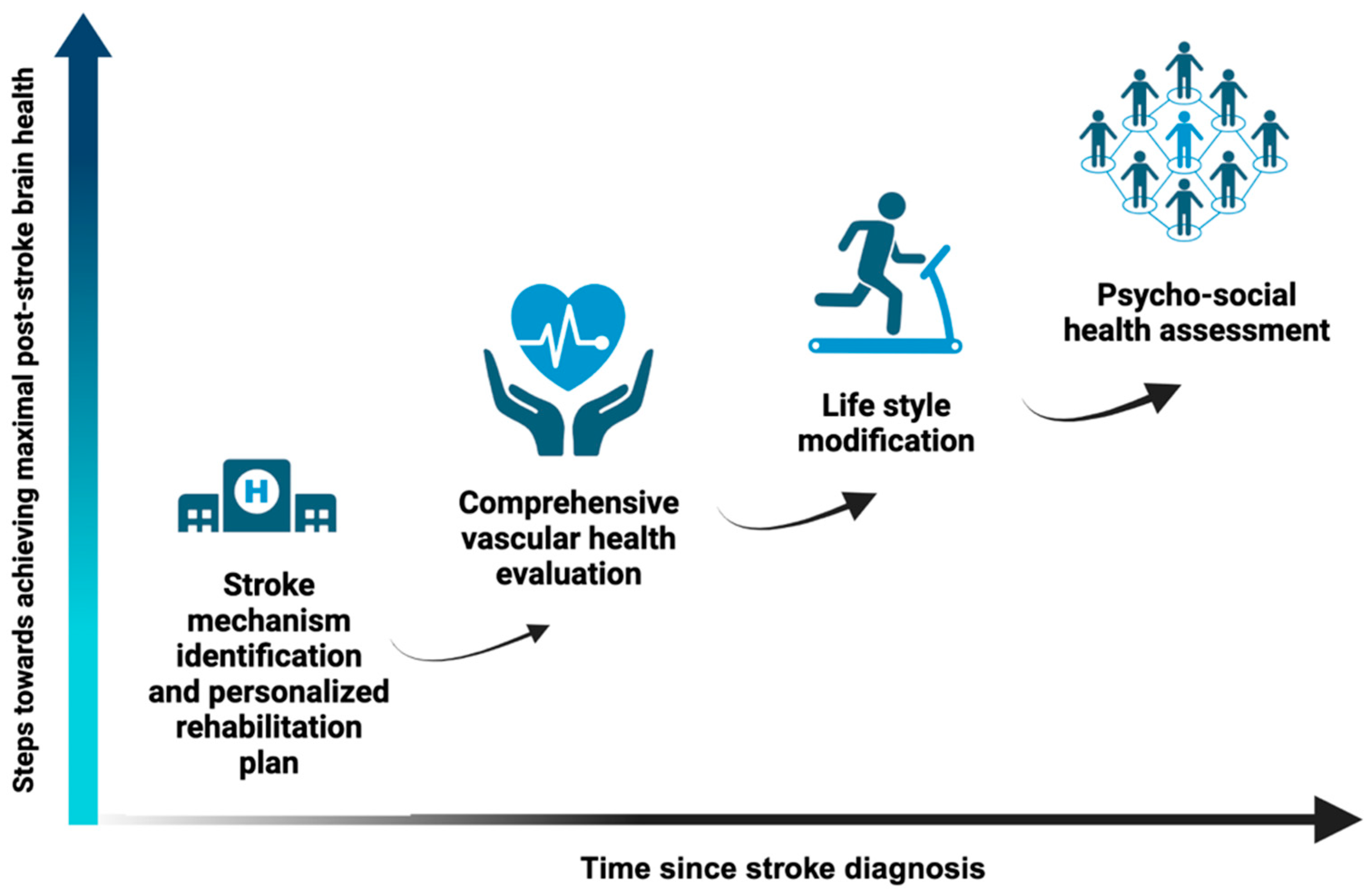
5. Approach to Optimizing Management of Post-Stroke Brain Health
5.1. Identification of Stroke Mechanism, Etiology-Specific Secondary Management, and Personalized Rehabilitation
5.2. Addressing Comprehensive Vascular Health
5.2.1. Blood Pressure
5.2.2. Diabetes and Glycemic Impairment
5.2.3. Hyperlipidemia
5.2.4. Heart Failure and Atrial Cardiopathy
5.2.5. Peripheral Arterial Disease and Carotid Disease
5.2.6. Atrial Fibrillation
5.2.7. Chronic Kidney Disease
| Target Area | Clinical Considerations | Level of Evidence | Areas for Future Research |
|---|---|---|---|
| Intracranial vascular integrity | Consider cilostazol and/or isosorbide mononitrate in lacunar stroke survivors to improve cognitive and functional outcomes [103]. | Phase II clinical trial | Develop additional medication regimens to maximize post-stroke neurovasculome function. |
| Global vascular health | Consider referring survivors of stroke for cardiac rehabilitation assessment and personalized interventions in addition to standard post-stroke rehabilitation to improve functional and motor recovery [44,45]. | Observational research | Develop pragmatic trials and real-world studies assessing multi-morbidity interventions. |
| Blood pressure | Aim for strict long-term outpatient blood pressure control in patients with stroke and hypertension, taking into account the degree of extra- and intracranial atherosclerosis [35,48,104]. | Secondary prevention guidelines, meta-analysis of clinical trials | Determine optimal long-term outpatient blood pressure goals with respect to post-stroke cognition and mood and if specific goals have differential effects based on various patient and stroke characteristics such as age, location of stroke, etiology of stroke, and comorbidities. |
| Aim for a long-term, outpatient blood pressure goal of <130/80 if no side effects or other contraindications such as frailty and fall risk [35,48,104]. | Determine the clinical significance of change in outpatient blood pressure following stroke as well as chronic outpatient blood pressure variability in post-stroke cognitive impairment, post-stroke physical recovery, and recurrent cardiovascular/cerebrovascular events. | ||
| Determine if certain blood pressure medication classes have neuroprotective effects independent of their effects on blood pressure reduction. | |||
| Diabetes | Consider an SGLT-2 inhibitor for those with heart failure or chronic kidney disease or a GLP-1 agonist for secondary stroke and cardiovascular prevention in stroke patients with diabetes in addition to metformin if no contraindications [35]. | Secondary prevention guidelines | Determine if there is a protective effect of GLP-1 agonists on post-stroke cognitive impairment. |
| Aim for a long-term HbA1C of ≤7% in most patients [35]. | Determine the optimal hemoglobin A1C target for prevention of post-stroke cognitive impairment. | ||
| Lipids | In patients with strokes caused by intracranial or extracranial atherosclerosis, or with a comorbid atherosclerotic condition such as coronary artery disease, more intensive LDL target of <40–55 and/or reduction in LDL by >50% can be considered for optimal secondary prevention [63,105]. | Secondary prevention guidelines, post hoc analysis of clinical trial | Determine how change in different hyperlipidemia markers like low-density lipoprotein, HDL/LDL ratio, and various apolipoproteins impacts post-stroke cognition and mood and if this impact varies by different stroke and patient characteristics. |
| Determine if certain medication classes such as statins or psck9-inhibtors have neuroprotective effects independent of their effects on hyperlipidemia biomarkers. | |||
| Heart Failure | Ensure guideline-directed medical therapy for heart failure is instituted, particularly that patients are on angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, or angiotensin receptor neprilysin inhibitor if no contraindication given possible cognitive protection [75,106]. | Observational research, heart failure guidelines | Determine if anticoagulation improves cognitive trajectories following strokes in patients with heart failure with varying ejection fractions without atrial fibrillation. |
| Consider early identification and management of comorbid atrial fibrillation in patients with heart failure. | |||
| Peripheral Arterial Disease and Carotid Disease | Consider screening for peripheral arterial disease with an ankle–brachial index in patients with functional limitations not explained by the stroke itself and/or in patients with significant intracranial or extracranial atherosclerosis [78,82]. | Observational research, post hoc analysis of clinical trial | Determine the utility of early stenting, bypass, angioplasty, aggressive lipid management, and various anti-thrombotic regimens in improving functional outcomes in patients with comorbid peripheral arterial disease and stroke. |
| If evidence of peripheral arterial disease, consider aggressive lipid management, anti-platelet management, and/or referral to peripheral vascular specialist. | Determine which, if any, patients with comorbid carotid disease and stroke would benefit cognitively from carotid revascularization. | ||
| Atrial Fibrillation | Early recognition of atrial fibrillation and timely initiation of anticoagulation in patients with stroke determined to be cardioembolic may lead to improved cognitive outcomes [88,89,93]. | Observational research | Determine what, if any, burden of atrial fibrillation (measured as time or percentage of time in atrial fibrillation) among patients with paroxysmal atrial fibrillation predisposes to cognitive decline following stroke. |
| Determine what, if any, treatment options for atrial fibrillation improve post-stroke cognitive trajectories including rate control, rhythm control, atrial appendage occlusion, and anticoagulation. | |||
| Determine the role of anticoagulation on cognitive outcomes in patients with embolic source of undetermined significance and/or atrial cardiopathy. | |||
| Chronic Kidney Disease | Aim for a blood pressure goal of <120/80 in patients with comorbid chronic kidney and stroke if there are no contraindications [97]. | Secondary prevention guidelines, expert consensus statement. | Determine if chronic kidney disease independently predisposes to atrial fibrillation and if prolonged monitoring for atrial fibrillation can impact post-stroke brain health following stroke. |
| Consider the addition of an SGLT-2 inhibitor in patients with diabetic CKD and comorbid stroke to prevent further progression of CKD [35,97]. | Determine how chronic uremic toxins and CKD treatments impact vascular function and intracranial hemodynamics as well as neuroinflammation, blood–brain barrier breakdown, and oxidative-stress-related neuronal damage. | ||
| Determine what, if any, treatment options following stroke can mitigate silent cerebral small vessel ischemia following stroke in patients with comorbid chronic kidney disease. |
5.3. Lifestyle Modification
5.3.1. Physical Activity
5.3.2. Diet and Nutrition
5.3.3. Smoking
5.3.4. Alcohol, Marijuana, and Substance Use Disorder
5.3.5. Sleep
| Target Area | Clinical Considerations | Level of Evidence | Areas for Future Research |
|---|---|---|---|
| Physical activity | Cardiopulmonary aerobic exercise can reduce multiple aspects of post-stroke disability and should be considered in all survivors of stroke [109]. | Cochrane review, expert recommendations, post hoc analysis of clinical trial | Determining type, timing, and dose of physical activity is most effective in mitigating post-stroke physical and cognitive disability and how this varies by specific stroke and patient characteristics. |
| Structured aerobic exercise should be conducted at least 3 days per week, lasting at least 20 min, and for at least 8 weeks in duration following stroke. However, there may be a dose–response effect; thus, any aerobic exercise is better than none, and more than the minimum recommendation may lead to additional benefits [110,142]. | |||
| Physical activity is one of the most important factors for secondary prevention in patients with stroke from intracranial atherosclerosis and likely also follows a dose-dependent relationship. Increased walking may be a sustainable recommendation to help with secondary prevention in the setting of intracranial atherosclerosis [108]. | |||
| Diet | Counsel on lowering daily sodium intake in survivors of stroke to a goal of <1500–2300 mg, particularly in patients with comorbid hypertension [53,114]. | Open-label cluster randomized clinical trial, guideline recommendations, observational research | Determine if certain diets can improve post-stroke cognitive trajectories and if this varies by stroke or patient characteristics. |
| Encourage survivors of stroke to minimize processed foods and consider following the MIND diet [115,116,118,119]. | |||
| Smoking cessation | Consider pharmacological management to assist with smoking cessation following stroke paired with behavioral interventions [35,121]. | Observational research, secondary prevention guidelines | Determine psychosocial, systemic, and other barriers to smoking cessation among survivors of stroke to more effectively intervene to improve smoking cessation rates following stroke. |
| Consider screening and treating comorbid mood disorders in survivors of stroke who smoke [125]. | |||
| Alcohol, Marijuana, and other substance use | In individuals meeting criteria for either alcohol use disorder or excessive alcohol use (8+ alcoholic drinks/week for women, 15+ alcoholic drinks/week for men, or engaging in binge drinking), we recommend referral to addiction services following stroke [130]. | Observational research | More research is needed to determine how light alcohol use impacts post-stroke functional and cognitive outcomes as well as recurrent stroke risk. |
| For individuals with any substance use disorder, we recommend referral to addiction services following stroke [132,142,143,144]. | More research is needed to determine how light THC and/or CBD use impacts post-stroke functional and cognitive outcomes. | ||
| Coordination of care with addiction support services with tailored medication and/or behavioral plan should be planned before patients with comorbid stroke and substance use disorder leave the inpatient setting, particularly those with strokes from drug-related endocarditis [142,145]. | More research is needed on how to improve post-stroke substance use disorder systems of care. | ||
| Sleep | Screen for and consider home sleep testing in patients with post-stroke cognitive decline or mood disorders; testing within 2 weeks following stroke may be the most beneficial time for testing and starting treatment, though more research is needed [134,139,140,141,143]. | Observational research, randomized clinical trial, meta-analysis of clinical trials, expert opinion | Determine whether treating sleep-disordered breathing reduces stroke recurrence in patients that wear CPAP or other mask-based interventions >4 h per night. |
| Consider screening for and testing for sleep apnea in patients with comorbid hypertension following stroke. | Determine real-world compliance rates with CPAP in patients with comorbid sleep apnea and history of stroke. | ||
| Determine the efficacy of newer non-CPAP treatments such as hypoglossal nerve stimulation therapy in improving post-stroke cognition, mood, and mitigating stroke recurrence. | |||
| Determine if certain patients with specific stroke or other characteristics are more likely to benefit from treatment of sleep-disordered breathing for mood, cognition, and functional recovery. |
5.4. Social and Psychological Factors
5.4.1. Depression
5.4.2. Other Post-Stroke Mood Sequelae
5.4.3. Social Interactions and Support
| Target Area | Clinical Considerations | Level of Evidence | Areas for Future Research |
|---|---|---|---|
| Depression | Consider whether pre-stroke depression is a modifiable factor affecting stroke survivors’ ability to meet the therapy requirements needed for inpatient rehabilitation. | Observational research, guideline recommendations, Cochrane review, expert scientific statement | Determine if and when the optimal time to screen for post-stroke depression is. |
| Screen for depression with the PHQ-9, CES-D, and HDRS in survivors of stroke if not meeting anticipated functional goals or experiencing cognitive decline [28,149]. | |||
| Recognize that aphasia can make diagnosing depression or another comorbid mood disorder difficult and may warrant discussing with caregivers and family. | Determine which patients, if any, may benefit from interventions aimed at preventing post-stroke depression. | ||
| Treat post-stroke depression with medication classes such as SSRIs, SNRIs, or Mirtazapine based off phenotype and comorbidities; consider referral for psychological therapy; and consider referral for neuromodulation treatments [146,149]. | Develop treatments tailored to different phenotypes and etiologies (i.e., from infarct eloquent mood networks vs. from post-stroke physical disability) | ||
| Other Post-Stroke Mood Sequelae | Consider screening for anxiety, fatigue, and other modifiable mood sequelae, particularly in those with unexpected cognitive/functional decline or plateau. | Phase II clinical trials, Cochrane review | Determine if and when the optimal time to screen for non-depression mood sequelae of stroke is. |
| Consider modafinil for the treatment of post-stroke fatigue if no underlying treatable condition found (i.e., hypothyroidism or sleep apnea) [160]. | |||
| Consider Dextromethorphan/Quinidine or SSRIs for the treatment of pseudobulbar affect [161,162]. | |||
| Social Isolation | Consider screening for social isolation, particularly in elderly stroke survivors or those with other mood disorders [146,175,176]. | Observational research, expert scientific statement, systematic review | Determine the role of peer-support groups in improving psychological well-being, functional status, and cognition following stroke. |
| Determine the efficacy of remote and telehealth interventions in mitigating social isolation following stroke. | |||
| Consider assessing caregiver well-being in caregivers of stroke survivors, particularly survivors of stroke with high levels of disability [171,177]. |
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Yahya, T.; Jilani, M.H.; Khan, S.U.; Mszar, R.; Hassan, S.Z.; Blaha, M.J.; Blankstein, R.; Virani, S.S.; Johansen, M.C.; Vahidy, F.; et al. Stroke in young adults: Current trends, opportunities for prevention and pathways forward. Am. J. Prev. Cardiol. 2020, 3, 100085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tong, X.; Schieb, L.; Vaughan, A.; Gillespie, C.; Wiltz, J.L.; King, S.C.; Odom, E.; Merritt, R.; Hong, Y.; et al. Vital Signs: Recent Trends in Stroke Death Rates—United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Andres, W.; Rothstein, A.; Elser, H.; Sloane, K.L.; Gottesman, R.F.; Kasner, S.E.; Schneider, A.L.C. Trends in the Prevalence of Stroke Among Community-Dwelling Individuals in the US, 1999–2018. JAMA Neurol. 2023, 80, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Doyle, S.; Rost, N.S.; Sorond, F.A.; Lakshminarayan, K.; Launer, L.J. The role of population-level preventive care for brain health in ageing. Lancet Healthy Longev. 2023, 4, E274–E283. [Google Scholar] [CrossRef]
- Singh, S.D.; Gutierrez-Martinez, L.; Newhouse, A.; Sonni, A.; Chemali, Z.; Rosand, J. Brain health begins with brain care. Lancet Neurol. 2022, 21, 961–962. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One brain, one life, one approach. Eur. J. Neurol. 2022, 29, 2559–2566. [Google Scholar] [CrossRef]
- Nakamura, A.; Sakai, S.; Taketomi, Y.; Tsuyama, J.; Miki, Y.; Hara, Y.; Arai, N.; Sugiura, Y.; Kawaji, H.; Murakami, M.; et al. PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke. Neuron 2023, 111, 2995–3010.e9. [Google Scholar] [CrossRef]
- Xing, C.; Hayakawa, K.; Lok, J.; Arai, K.; Lo, E.H. Injury and repair in the neurovascular unit. Neurol. Res. 2012, 34, 325–330. [Google Scholar] [CrossRef]
- Kline, D.K.; Lin, D.J.; Cloutier, A.; Sloane, K.; Parlman, K.; Ranford, J.; Picard-Fraser, M.; Fox, A.B.; Hochberg, L.R.; Kimberley, T.J. Arm Motor Recovery After Ischemic Stroke: A Focus on Clinically Distinct Trajectory Groups. J. Neurol. Phys. Ther. 2021, 45, 70–78. [Google Scholar] [CrossRef]
- Elgh, E.; Hu, X. Dynamic Trajectory of Long-Term Cognitive Improvement Up to 10 Years in Young Community-Dwelling Stroke Survivors: A Cohort Study. Front. Neurol. 2019, 10, 97. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs 2010, 11, 298–308. [Google Scholar] [PubMed]
- Ito, K.L.; Kim, B.; Liu, J.; Soekadar, S.R.; Winstein, C.; Yu, C.; Cramer, S.C.; Schweighofer, N.; Liew, S.L. Corticospinal Tract Lesion Load Originating from Both Ventral Premotor and Primary Motor Cortices Are Associated with Post-Stroke Motor Severity. Neurorehabil. Neural Repair 2022, 36, 179–182. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.D.; Le, V.; Der-Yeghiaian, L.; See, J.; Newton, J.M.; Ward, N.S.; Cramer, S.C. Anatomy of stroke injury predicts gains from therapy. Stroke 2011, 42, 421–426. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Foulon, C. The rise of a new associationist school for lesion-symptom mapping. Brain 2018, 141, 2–4. [Google Scholar] [CrossRef]
- van Assche, M.; Klug, J.; Dirren, E.; Richiardi, J.; Carrera, E. Preparing for a Second Attack: A Lesion Simulation Study on Network Resilience After Stroke. Stroke 2022, 53, 2038–2047. [Google Scholar] [CrossRef]
- Stefaniak, J.D.; Halai, A.D.; Lambon Ralph, M.A. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat. Rev. Neurol. 2020, 16, 43–55. [Google Scholar] [CrossRef]
- Rosenich, E.; Hordacre, B.; Paquet, C.; Koblar, S.A.; Hillier, S.L. Cognitive Reserve as an Emerging Concept in Stroke Recovery. Neurorehabil. Neural Repair. 2020, 34, 187–199. [Google Scholar] [CrossRef]
- Liew, S.L.; Schweighofer, N.; Cole, J.H.; Zavaliangos-Petropulu, A.; Lo, B.P.; Han, L.K.M.; Hahn, T.; Schmaal, L.; Donnelly, M.R.; Jeong, J.N.; et al. Association of Brain Age, Lesion Volume, and Functional Outcome in Patients with Stroke. Neurology 2023, 100, e2103–e2113. [Google Scholar] [CrossRef] [PubMed]
- Bretzner, M.; Bonkhoff, A.K.; Schirmer, M.D.; Hong, S.; Dalca, A.; Donahue, K.; Giese, A.K.; Etherton, M.R.; Rist, P.M.; Nardin, M.; et al. Radiomics-Derived Brain Age Predicts Functional Outcome After Acute Ischemic Stroke. Neurology 2023, 100, e822–e833. [Google Scholar] [CrossRef] [PubMed]
- Bowden, M.G.; Woodbury, M.L.; Duncan, P.W. Promoting neuroplasticity and recovery after stroke: Future directions for rehabilitation clinical trials. Curr. Opin. Neurol. 2013, 26, 37–42. [Google Scholar] [CrossRef]
- Brainin, M.; Tuomilehto, J.; Heiss, W.D.; Bornstein, N.M.; Bath, P.M.; Teuschl, Y.; Richard, E.; Guekht, A.; Quinn, T.; Post Stroke Cognition Study, G. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015, 22, 229-e16. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.G.; Heitsch, L.; Cole, J.W.; Lindgren, A.G.; de Havenon, A.; Dude, J.A.; Lohse, K.R.; Cramer, S.C.; Worrall, B.B.; Gpas Collaboration, P.C. Domain-Specific Outcomes for Stroke Clinical Trials: What the Modified Rankin Isn’t Ranking. Neurology 2021, 97, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Erler, K.S.; Wu, R.; DiCarlo, J.A.; Petrilli, M.F.; Gochyyev, P.; Hochberg, L.R.; Kautz, S.A.; Schwamm, L.H.; Cramer, S.C.; Finklestein, S.P.; et al. Association of Modified Rankin Scale with Recovery Phenotypes in Patients with Upper Extremity Weakness after Stroke. Neurology 2022, 98, e1877–e1885. [Google Scholar] [CrossRef]
- Cramer, S.C.; Lin, D.J.; Finklestein, S.P. Domain-Specific Outcome Measures in Clinical Trials of Therapies Promoting Stroke Recovery: A Suggested Blueprint. Stroke 2023, 54, e86–e90. [Google Scholar] [CrossRef]
- Lanctot, K.L.; Lindsay, M.P.; Smith, E.E.; Sahlas, D.J.; Foley, N.; Gubitz, G.; Austin, M.; Ball, K.; Bhogal, S.; Blake, T.; et al. Canadian Stroke Best Practice Recommendations: Mood, Cognition and Fatigue following Stroke, 6th edition update 2019. Int. J. Stroke 2020, 15, 668–688. [Google Scholar] [CrossRef]
- de Kort, F.A.S.; Coenen, M.; Weaver, N.A.; Kuijf, H.J.; Aben, H.P.; Bae, H.J.; Bordet, R.; Camma, G.; Chen, C.; Dewenter, A.; et al. White Matter Hyperintensity Volume and Poststroke Cognition: An Individual Patient Data Pooled Analysis of 9 Ischemic Stroke Cohort Studies. Stroke 2023, 54, 3021–3029. [Google Scholar] [CrossRef]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Jahangiri, F.R.; Liang, M.; Huckabey, M.; Baloney, N.; Sharifi, S. Carotid Endarterectomy Surgeries: A Multimodality Intraoperative Neurophysiological Monitoring Approach. Cureus 2022, 14, e26556. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Sagen, U.; Vik, T.G.; Moum, T.; Morland, T.; Finset, A.; Dammen, T. Screening for anxiety and depression after stroke: Comparison of the hospital anxiety and depression scale and the Montgomery and Asberg depression rating scale. J. Psychosom. Res. 2009, 67, 325–332. [Google Scholar] [CrossRef]
- Cramer, S.C.; Wolf, S.L.; Saver, J.L.; Johnston, K.C.; Mocco, J.; Lansberg, M.G.; Savitz, S.I.; Liebeskind, D.S.; Smith, W.; Wintermark, M.; et al. The Utility of Domain-Specific End Points in Acute Stroke Trials. Stroke 2021, 52, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Veazie, S.; Gilbert, J.; Winchell, K.; Paynter, R.; Guise, J.M. Addressing Social Isolation To Improve the Health of Older Adults: A Rapid Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2019. [Google Scholar]
- Leifheit, E.C.; Wang, Y.; Goldstein, L.B.; Lichtman, J.H. Trends in 1-Year Recurrent Ischemic Stroke in the US Medicare Fee-for-Service Population. Stroke 2022, 53, 3338–3347. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Rohweder, G.; Ellekjaer, H.; Salvesen, O.; Naalsund, E.; Indredavik, B. Functional outcome after common poststroke complications occurring in the first 90 days. Stroke 2015, 46, 65–70. [Google Scholar] [CrossRef]
- Saver, J.L. Cryptogenic Stroke. N. Engl. J. Med. 2016, 375, e26. [Google Scholar] [CrossRef]
- Elkind, M.S. Stroke Etiologic Classification-Moving from Prediction to Precision. JAMA Neurol. 2017, 74, 388–390. [Google Scholar] [CrossRef]
- Simpkins, A.N.; Janowski, M.; Oz, H.S.; Roberts, J.; Bix, G.; Dore, S.; Stowe, A.M. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 2020, 11, 615–627. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Grefkes, C. Precision medicine in stroke: Towards personalized outcome predictions using artificial intelligence. Brain 2022, 145, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.W.; Handlery, R.; Stewart, J.C.; Pearson, J.L.; Wilcox, S.; Fritz, S. Integrating Survivors of Stroke Into Exercise-Based Cardiac Rehabilitation Improves Endurance and Functional Strength. J. Am. Heart Assoc. 2021, 10, e017907. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, S.J.; Fleming, T.K.; Zinonos, S.; Cosgrove, N.M.; Cabrera, J.; Kostis, J.B.; Greiss, C.; Ray, A.R.; Eckert, A.; Scarpati, R.; et al. Stroke Recovery Program with Modified Cardiac Rehabilitation Improves Mortality, Functional & Cardiovascular Performance. J. Stroke Cerebrovasc. Dis. 2022, 31, 106322. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Toell, T.; Boehme, C.; Krebs, S.; Mayer, L.; Lang, C.; Seekircher, L.; Tschiderer, L.; Willeit, K.; Rumpold, G.; et al. STROKE-CARD care to prevent cardiovascular events and improve quality of life after acute ischaemic stroke or TIA: A randomised clinical trial. eClinicalMedicine 2020, 25, 100476. [Google Scholar] [CrossRef] [PubMed]
- Willmot, M.; Leonardi-Bee, J.; Bath, P.M. High blood pressure in acute stroke and subsequent outcome: A systematic review. Hypertension 2004, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Saver, J.L.; Ovbiagele, B.; Wu, Y.L.; Cheng, C.Y.; Lee, M. Association Between Magnitude of Differential Blood Pressure Reduction and Secondary Stroke Prevention: A Meta-analysis and Meta-Regression. JAMA Neurol. 2023, 80, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Tzourio, C.; Anderson, C.; Chapman, N.; Woodward, M.; Neal, B.; MacMahon, S.; Chalmers, J.; Group, P.C. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003, 163, 1069–1075. [Google Scholar] [CrossRef]
- Diener, H.C.; Sacco, R.L.; Yusuf, S.; Cotton, D.; Ounpuu, S.; Lawton, W.A.; Palesch, Y.; Martin, R.H.; Albers, G.W.; Bath, P.; et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: A double-blind, active and placebo-controlled study. Lancet Neurol. 2008, 7, 875–884. [Google Scholar] [CrossRef]
- Pearce, L.A.; McClure, L.A.; Anderson, D.C.; Jacova, C.; Sharma, M.; Hart, R.G.; Benavente, O.R.; Investigators, S.P.S. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: A secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014, 13, 1177–1185. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, D.; Shi, M.; Bu, X.; Xie, X.; Xu, T.; Han, Y.; Xu, T.; Geng, D.; Chen, J.; et al. Effect of immediate blood pressure reduction on post-stroke depression in ischemic stroke patients: A substudy of CATIS trial. J. Affect. Disord. 2022, 300, 195–202. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Lau, L.H.; Lew, J.; Borschmann, K.; Thijs, V.; Ekinci, E.I. Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J. Diabetes Investig. 2019, 10, 780–792. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Y.; Wang, C.; Jia, Q.; Zhang, N.; Zhao, X.; Liu, G.; Wang, Y.; Liu, L.; Wang, Y.; et al. Glycated hemoglobin independently predicts stroke recurrence within one year after acute first-ever non-cardioembolic strokes onset in A Chinese cohort study. PLoS ONE 2013, 8, e80690. [Google Scholar] [CrossRef]
- Lei, C.; Wu, B.; Liu, M.; Chen, Y. Association between hemoglobin A(1)C levels and clinical outcome in ischemic stroke patients with or without diabetes. J. Clin. Neurosci. 2015, 22, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.W.; Crawford, J.D.; Samaras, K.; Desmond, D.W.; Kohler, S.; Staals, J.; Verhey, F.R.J.; Bae, H.J.; Lee, K.J.; Kim, B.J.; et al. Association of Prediabetes and Type 2 Diabetes with Cognitive Function After Stroke: A STROKOG Collaboration Study. Stroke 2020, 51, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiong, Q.; Du, Y.; Huang, L.W.; Yu, M. Nonlinear relationship between glycated hemoglobin and cognitive impairment after acute mild ischemic stroke. BMC Neurol. 2023, 23, 116. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Rashidy-Pour, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. GLP-1 mimetics and cognition. Life Sci. 2021, 264, 118645. [Google Scholar] [CrossRef]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Z. Trends in lipid profile and lipid control among survivors of stroke or myocardial infarction among US adults, 2001–2018. Front. Endocrinol. 2023, 14, 1128878. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Park, J.G.; Ruzza, A.; Sever, P.S.; Keech, A.C.; Sabatine, M.S. Cardiovascular Benefit of Lowering Low-Density Lipoprotein Cholesterol Below 40 mg/dL. Circulation 2021, 144, 1732–1734. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Vitturi, B.K.; Gagliardi, R.J. Effects of statin therapy on outcomes of ischemic stroke: A real-world experience in Brazil. Arq. Neuropsiquiatr. 2020, 78, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Scutt, P.; Blackburn, D.J.; Ankolekar, S.; Krishnan, K.; Ballard, C.; Burns, A.; Mant, J.; Passmore, P.; Pocock, S.; et al. Intensive versus Guideline Blood Pressure and Lipid Lowering in Patients with Previous Stroke: Main Results from the Pilot ’Prevention of Decline in Cognition after Stroke Trial’ (PODCAST) Randomised Controlled Trial. PLoS ONE 2017, 12, e0164608. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, X.; Ma, W.; Yang, R.; Xu, F.; Han, D.; Huang, T.; Peng, M.; Xu, A.; Lyu, J. Associations of HDL-C/LDL-C with myocardial infarction, all-cause mortality, haemorrhagic stroke and ischaemic stroke: A longitudinal study based on 384 093 participants from the UK Biobank. Stroke Vasc. Neurol. 2023, 8, 119–126. [Google Scholar] [CrossRef]
- Doehner, W.; Ural, D.; Haeusler, K.G.; Celutkiene, J.; Bestetti, R.; Cavusoglu, Y.; Pena-Duque, M.A.; Glavas, D.; Iacoviello, M.; Laufs, U.; et al. Heart and brain interaction in patients with heart failure: Overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur. J. Heart Fail. 2018, 20, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Ozyuncu, N.; Gulec, S.; Kaya, C.T.; Goksuluk, H.; Tan, T.S.; Vurgun, V.K.; Us, E.; Erol, C. Relation of Acute Decompensated Heart Failure to Silent Cerebral Infarcts in Patients with Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2019, 123, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Kozdag, G.; Ciftci, E.; Ural, D.; Sahin, T.; Selekler, M.; Agacdiken, A.; Demirci, A.; Komsuoglu, S.; Komsuoglu, B. Silent cerebral infarction in chronic heart failure: Ischemic and nonischemic dilated cardiomyopathy. Vasc. Health Risk Manag. 2008, 4, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Parissis, J.; Frogoudaki, A.; Vrettou, A.R.; Ikonomidis, I.; Paraskevaidis, I.; Triantafyllou, N.; Kargiotis, O.; Voumvourakis, K.; Alexandrov, A.V.; et al. Heart failure and the risk of ischemic stroke recurrence: A systematic review and meta-analysis. J. Neurol. Sci. 2016, 362, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Witt, L.S.; Rotter, J.; Stearns, S.C.; Gottesman, R.F.; Kucharska-Newton, A.M.; Richey Sharrett, A.; Wruck, L.M.; Bressler, J.; Sueta, C.A.; Chang, P.P. Heart Failure and Cognitive Impairment in the Atherosclerosis Risk in Communities (ARIC) Study. J. Gen. Intern. Med. 2018, 33, 1721–1728. [Google Scholar] [CrossRef]
- Johansen, M.C.; Wang, W.; Zhang, M.; Knopman, D.S.; Ndumele, C.; Mosley, T.H.; Selvin, E.; Shah, A.M.; Solomon, S.D.; Gottesman, R.F.; et al. Risk of Dementia Associated with Atrial Cardiopathy: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e025646. [Google Scholar] [CrossRef]
- de Roos, A.; van der Grond, J.; Mitchell, G.; Westenberg, J. Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease? Circulation 2017, 135, 2178–2195. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; van Buchem, M.A.; Sigurdsson, S.; Zhang, Q.; Harris, T.B.; Gudnason, V.; Arai, A.E.; Launer, L.J. Cardiac hemodynamics are linked with structural and functional features of brain aging: The age, gene/environment susceptibility (AGES)-Reykjavik Study. J. Am. Heart Assoc. 2015, 4, e001294. [Google Scholar] [CrossRef]
- Zuccala, G.; Onder, G.; Marzetti, E.; Monaco, M.R.; Cesari, M.; Cocchi, A.; Carbonin, P.; Bernabei, R.; Group, G.S. Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur. Heart J. 2005, 26, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.A.; Shen, L.; Jhund, P.S.; Kristensen, S.L.; Kober, L.; Chen, F.; Gong, J.; Lefkowitz, M.P.; Rouleau, J.L.; Shi, V.C.; et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Gomes, A.C.; Rahimi, K. Management of blood pressure in heart failure. Heart 2019, 105, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Kolls, B.J.; Sapp, S.; Rockhold, F.W.; Jordan, J.D.; Dombrowski, K.E.; Fowkes, F.G.R.; Mahaffey, K.W.; Berger, J.S.; Katona, B.G.; Blomster, J.I.; et al. Stroke in Patients with Peripheral Artery Disease. Stroke 2019, 50, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, A.; Zhang, S.; Li, N.; Chen, S.; Zhang, Y.; Zhou, Y.; Wu, S.; Zhao, X. Asymptomatic polyvascular disease and the risks of cardiovascular events and all-cause death. Atherosclerosis 2017, 262, 1–7. [Google Scholar] [CrossRef]
- Shin, Y.Y.; Ha, S.H.; Woo, H.G.; Heo, S.H.; Chang, D.I.; Kim, B.J. Subclinical Peripheral Arterial Disease in Patients with Acute Ischemic Stroke: A Study with Ultrasonography. J. Stroke Cerebrovasc. Dis. 2019, 28, 104370. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Sang, Y.; Kalbaugh, C.; Loehr, L.R.; Hirsch, A.T.; Tanaka, H.; Heiss, G.; Windham, B.G.; Selvin, E.; et al. Ankle-brachial index and physical function in older individuals: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2017, 257, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Spiesser, J.; Hakimi, Z.; Bego, G.; Carita, P.; Gabriel, S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007, 68, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Singh, N.; Marko, M.; Hill, M.D.; Menon, B.K.; Demchuk, A.; Coutts, S.B.; Almekhlafi, M.A.; Ospel, J.M. Embolic Stroke of Undetermined Source and Symptomatic Nonstenotic Carotid Disease. Stroke 2020, 51, 1321–1325. [Google Scholar] [CrossRef]
- Naazie, I.N.; Cui, C.L.; Osaghae, I.; Murad, M.H.; Schermerhorn, M.; Malas, M.B. A Systematic Review and Meta-Analysis of Transcarotid Artery Revascularization with Dynamic Flow Reversal Versus Transfemoral Carotid Artery Stenting and Carotid Endarterectomy. Ann. Vasc. Surg. 2020, 69, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Talelli, P.; Ellul, J.; Terzis, G.; Lekka, N.P.; Gioldasis, G.; Chrysanthopoulou, A.; Papapetropoulos, T. Common carotid artery intima media thickness and post-stroke cognitive impairment. J. Neurol. Sci. 2004, 223, 129–134. [Google Scholar] [CrossRef]
- Marshall, R.S.; Lazar, R.M.; Liebeskind, D.S.; Connolly, E.S.; Howard, G.; Lal, B.K.; Huston, J., 3rd; Meschia, J.F.; Brott, T.G. Carotid revascularization and medical management for asymptomatic carotid stenosis—Hemodynamics (CREST-H): Study design and rationale. Int. J. Stroke 2018, 13, 985–991. [Google Scholar] [CrossRef]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; et al. Atrial Fibrillation and Dementia: A Report from the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409. [Google Scholar] [CrossRef]
- Krawczyk, M.; Fridman, S.; Cheng, Y.; Fang, J.; Saposnik, G.; Sposato, L.A. Atrial fibrillation diagnosed after stroke and dementia risk: Cohort study of first-ever ischaemic stroke patients aged 65 or older. Europace 2019, 21, 1793–1801. [Google Scholar] [CrossRef]
- Reiffel, J.A. Atrial fibrillation and stroke: Epidemiology. Am. J. Med. 2014, 127, e15–e16. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Zareifopoulos, N.; Theochari, C.A.; Arfaras-Melainis, A.; Papanastasiou, C.A.; Uppal, D.; Giannakoulas, G.; Kalogeropoulos, A.P.; Fontes, J.D.T. Association Between Atrial Fibrillation and Cognitive Impairment in Individuals with Prior Stroke: A Meta-Analysis and Meta-Regression Analysis. Stroke 2020, 51, 1662–1666. [Google Scholar] [CrossRef]
- Bernstein, R.A.; Kamel, H.; Granger, C.B.; Piccini, J.P.; Sethi, P.P.; Katz, J.M.; Vives, C.A.; Ziegler, P.D.; Franco, N.C.; Schwamm, L.H.; et al. Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients with Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA 2021, 325, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Rosenqvist, M. Less dementia with oral anticoagulation in atrial fibrillation. Eur. Heart J. 2018, 39, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.X.; Ang, E.; Lim, X.T.; Arain, S.J. Association of Risk of Dementia with Direct Oral Anticoagulants Versus Warfarin Use in Patients with Non-Valvular Atrial Fibrillation: A Systematic Review and Meta-analysis. J. Cardiovasc. Pharmacol. 2021, 77, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mohanty, P.; Trivedi, C.; Assadourian, J.; Mayedo, A.Q.; MacDonald, B.; Della Rocca, D.G.; Gianni, C.; Horton, R.; Al-Ahmad, A.; et al. Impact of Oral Anticoagulation Therapy Versus Left Atrial Appendage Occlusion on Cognitive Function and Quality of Life in Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e019664. [Google Scholar] [CrossRef] [PubMed]
- Bodagh, N.; Kotadia, I.; Gharaviri, A.; Zelaya, F.; Birns, J.; Bhalla, A.; Sommerville, P.; Niederer, S.; O’Neill, M.; Williams, S.E. The Impact of Atrial Fibrillation Treatment Strategies on Cognitive Function. J. Clin. Med. 2023, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Ademi, Z.; Doehner, W.; Lip, G.Y.H.; Mark, P.; Toyoda, K.; Wong, C.X.; Sarnak, M.; Cheung, M.; Herzog, C.A.; et al. Chronic Kidney Disease and Cerebrovascular Disease: Consensus and Guidance from a KDIGO Controversies Conference. Stroke 2021, 52, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Rothwell, P.M. Does Chronic Kidney Disease Predict Stroke Risk Independent of Blood Pressure?: A Systematic Review and Meta-Regression. Stroke 2019, 50, 3085–3092. [Google Scholar] [CrossRef]
- El Husseini, N.; Fonarow, G.C.; Smith, E.E.; Ju, C.; Schwamm, L.H.; Hernandez, A.F.; Schulte, P.J.; Xian, Y.; Goldstein, L.B. Renal Dysfunction Is Associated with Poststroke Discharge Disposition and In-Hospital Mortality: Findings from Get with The Guidelines-Stroke. Stroke 2017, 48, 327–334. [Google Scholar] [CrossRef]
- Harhay, M.N.; Xie, D.; Zhang, X.; Hsu, C.Y.; Vittinghoff, E.; Go, A.S.; Sozio, S.M.; Blumenthal, J.; Seliger, S.; Chen, J.; et al. Cognitive Impairment in Non-Dialysis-Dependent CKD and the Transition to Dialysis: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2018, 72, 499–508. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Wadley, V.; Yaffe, K.; McClure, L.A.; Howard, G.; Go, R.; Allman, R.M.; Warnock, D.G.; McClellan, W. Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. J. Kidney Dis. 2008, 52, 227–234. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirawa, N.; Yatsu, K.; Kobayashi, Y.; Yamamoto, Y.; Saka, S.; Andoh, D.; Toya, Y.; Yasuda, G.; Umemura, S. Relationship between silent brain infarction and chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Woodhouse, L.J.; Mhlanga, I.I.; Oatey, K.; Heye, A.K.; Bamford, J.; Cvoro, V.; Doubal, F.N.; England, T.; Hassan, A.; et al. Isosorbide Mononitrate and Cilostazol Treatment in Patients with Symptomatic Cerebral Small Vessel Disease: The Lacunar Intervention Trial-2 (LACI-2) Randomized Clinical Trial. JAMA Neurol. 2023, 80, 669–682. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: Comparisons, Reflections, and Recommendations. J. Am. Coll. Cardiol. 2022, 80, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Lavallee, P.C.; Kim, J.S.; Labreuche, J.; Charles, H.; Giroud, M.; Lee, B.C.; Mahagne, M.H.; Meseguer, E.; Nighoghossian, N.; et al. More Than 50 Percent Reduction in LDL Cholesterol in Patients with Target LDL <70 mg/dL After a Stroke. Stroke 2023, 54, 1993–2001. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Derdeyn, C.P.; Chimowitz, M.I.; Lynn, M.J.; Fiorella, D.; Turan, T.N.; Janis, L.S.; Montgomery, J.; Nizam, A.; Lane, B.F.; Lutsep, H.L.; et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): The final results of a randomised trial. Lancet 2014, 383, 333–341. [Google Scholar] [CrossRef]
- Turan, T.N.; Nizam, A.; Lynn, M.J.; Egan, B.M.; Le, N.A.; Lopes-Virella, M.F.; Hermayer, K.L.; Harrell, J.; Derdeyn, C.P.; Fiorella, D.; et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 2017, 88, 379–385. [Google Scholar] [CrossRef]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Johnson, L.; Kramer, S.; Carter, D.D.; Jarvis, H.; Brazzelli, M.; Mead, G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, 3, CD003316. [Google Scholar] [CrossRef] [PubMed]
- Buvarp, D.; Viktorisson, A.; Axelsson, F.; Lehto, E.; Lindgren, L.; Lundstrom, E.; Sunnerhagen, K.S. Physical Activity Trajectories and Functional Recovery After Acute Stroke Among Adults in Sweden. JAMA Netw. Open 2023, 6, e2310919. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Waiwood, A.M.; Cumming, T.B.; Marsland, A.L.; Bernhardt, J.; Erickson, K.I. Effects of Physical Activity on Poststroke Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Stroke 2017, 48, 3093–3100. [Google Scholar] [CrossRef]
- Eng, J.J.; Reime, B. Exercise for depressive symptoms in stroke patients: A systematic review and meta-analysis. Clin. Rehabil. 2014, 28, 731–739. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Falck, R.S.; Dao, E.; Best, J.R.; Davis, J.C.; Bennett, K.; Hall, P.A.; Hsiung, G.R.; Middleton, L.E.; Goldsmith, C.H.; et al. Effect of Exercise Training or Complex Mental and Social Activities on Cognitive Function in Adults with Chronic Stroke: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2236510. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Salari-Moghaddam, A.; Keshteli, A.H.; Mousavi, S.M.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J. Affect. Disord. 2019, 256, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. J. Prev. Alzheimers Dis. 2019, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cherian, L.; Agarwal, P.; Holland, T.; Schneider, J.; Aggarwal, N. Western diet associated with increased post-stroke depressive symptoms. J. Nutr. Sci. 2022, 11, e44. [Google Scholar] [CrossRef]
- Sato, M.; Ido, Y.; Yoshimura, Y.; Mutai, H. Relationship of Malnutrition During Hospitalization with Functional Recovery and Postdischarge Destination in Elderly Stroke Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1866–1872. [Google Scholar] [CrossRef]
- Parikh, N.S.; Zhang, C.; Salehi Omran, S.; Restifo, D.; Carpenter, M.J.; Schwamm, L.; Kamel, H. Smoking-Cessation Pharmacotherapy After Stroke and Transient Ischemic Attack: A Get with The Guidelines-Stroke Analysis. Stroke 2023, 54, e63–e65. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.S.; Parasram, M.; White, H.; Merkler, A.E.; Navi, B.B.; Kamel, H. Smoking Cessation in Stroke Survivors in the United States: A Nationwide Analysis. Stroke 2022, 53, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Cheon, D.Y.; Han, K.D.; Oh, M.S.; Yu, K.H.; Lee, B.C.; Kim, C.H.; Kim, Y.; Lee, S.H.; Kim, C.; Lim, J.S.; et al. Risk of dementia according to the smoking habit change after ischemic stroke: A nationwide population-based cohort study. Sci. Rep. 2022, 12, 22422. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, R.; Ago, T.; Kiyuna, F.; Sato, N.; Nakamura, K.; Kuroda, J.; Wakisaka, Y.; Kitazono, T.; Fukuoka Stroke Registry, I. Smoking Status and Functional Outcomes After Acute Ischemic Stroke. Stroke 2020, 51, 846–852. [Google Scholar] [CrossRef]
- Parikh, N.S.; Salehi Omran, S.; Kamel, H.; Elkind, M.S.V.; Willey, J. Symptoms of depression and active smoking among survivors of stroke and myocardial infarction: An NHANES analysis. Prev. Med. 2020, 137, 106131. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Lee, S.R.; Choi, E.K.; Park, S.H.; Lee, H.; Choi, J.; Han, M.; Ahn, H.J.; Kwon, S.; Lee, S.; et al. Cumulative Alcohol Consumption Burden and the Risk of Stroke in Young Adults: A Nationwide Population-Based Study. Neurology 2023, 100, e505–e515. [Google Scholar] [CrossRef]
- Ricci, C.; Wood, A.; Muller, D.; Gunter, M.J.; Agudo, A.; Boeing, H.; van der Schouw, Y.T.; Warnakula, S.; Saieva, C.; Spijkerman, A.; et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ 2018, 361, k934. [Google Scholar] [CrossRef]
- Rist, P.M.; Berger, K.; Buring, J.E.; Kase, C.S.; Gaziano, J.M.; Kurth, T. Alcohol consumption and functional outcome after stroke in men. Stroke 2010, 41, 141–146. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Jung, J.H.; Han, K.D.; Oh, S.; Lip, G.Y.H. Lower risk of stroke after alcohol abstinence in patients with incident atrial fibrillation: A nationwide population-based cohort study. Eur. Heart J. 2021, 42, 4759–4768. [Google Scholar] [CrossRef]
- Sacco, R.L.; Elkind, M.; Boden-Albala, B.; Lin, I.F.; Kargman, D.E.; Hauser, W.A.; Shea, S.; Paik, M.C. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA 1999, 281, 53–60. [Google Scholar] [CrossRef]
- Parikh, S.; George, P.; Wilson, K.; Rybin, D.; Hohler, A.D. Alcohol use and hospital readmissions following stroke: A safety net hospital experience. J. Neurol. Sci. 2018, 385, 83–86. [Google Scholar] [CrossRef]
- Marinelli, L.; Balestrino, M.; Mori, L.; Puce, L.; Rosa, G.M.; Giorello, L.; Curra, A.; Fattapposta, F.; Serrati, C.; Gandolfo, C.; et al. A randomised controlled cross-over double-blind pilot study protocol on THC:CBD oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open 2017, 7, e016843. [Google Scholar] [CrossRef] [PubMed]
- Sheikh Andalibi, M.S.; Rezaei Ardani, A.; Amiri, A.; Morovatdar, N.; Talebi, A.; Azarpazhooh, M.R.; Mokhber, N. The Association between Substance Use Disorders and Long-Term Outcome of Stroke: Results from a Population-Based Study of Stroke among 450,229 Urban Citizens. Neuroepidemiology 2021, 55, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Swartz, R.H.; Bayley, M.; Lanctot, K.L.; Murray, B.J.; Cayley, M.L.; Lien, K.; Sicard, M.N.; Thorpe, K.E.; Dowlatshahi, D.; Mandzia, J.L.; et al. Post-stroke depression, obstructive sleep apnea, and cognitive impairment: Rationale for, and barriers to, routine screening. Int. J. Stroke 2016, 11, 509–518. [Google Scholar] [CrossRef]
- Aaronson, J.A.; van Bennekom, C.A.; Hofman, W.F.; van Bezeij, T.; van den Aardweg, J.G.; Groet, E.; Kylstra, W.A.; Schmand, B. Obstructive Sleep Apnea is Related to Impaired Cognitive and Functional Status after Stroke. Sleep 2015, 38, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Gordon, C.; Wu, D.; Huang, H.C.; Yuliana, L.T.; Susatia, B.; Marta, O.F.D.; Chiu, H.Y. Dynamic Prevalence of Sleep Disorders Following Stroke or Transient Ischemic Attack: Systematic Review and Meta-Analysis. Stroke 2021, 52, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Khot, S.P.; Morgenstern, L.B. Sleep and Stroke. Stroke 2019, 50, 1612–1617. [Google Scholar] [CrossRef]
- Boulos, M.I.; Dharmakulaseelan, L.; Brown, D.L.; Swartz, R.H. Trials in Sleep Apnea and Stroke: Learning from the Past to Direct Future Approaches. Stroke 2021, 52, 366–372. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Huang, H.; Wu, H.; Huang, J.; Li, L.; Wang, L. Effect of CPAP on cognitive function in stroke patients with obstructive sleep apnoea: A meta-analysis of randomised controlled trials. BMJ Open 2023, 13, e060166. [Google Scholar] [CrossRef]
- Boulos, M.I.; Kamra, M.; Colelli, D.R.; Kirolos, N.; Gladstone, D.J.; Boyle, K.; Sundaram, A.; Hopyan, J.J.; Swartz, R.H.; Mamdani, M.; et al. SLEAP SMART (Sleep Apnea Screening Using Mobile Ambulatory Recorders After TIA/Stroke): A Randomized Controlled Trial. Stroke 2022, 53, 710–718. [Google Scholar] [CrossRef]
- Swartz, R.H.; Cayley, M.L.; Lanctot, K.L.; Murray, B.J.; Cohen, A.; Thorpe, K.E.; Sicard, M.N.; Lien, K.; Sahlas, D.J.; Herrmann, N. The "DOC" screen: Feasible and valid screening for depression, Obstructive Sleep Apnea (OSA) and cognitive impairment in stroke prevention clinics. PLoS ONE 2017, 12, e0174451. [Google Scholar] [CrossRef]
- MacKay-Lyons, M.; Billinger, S.A.; Eng, J.J.; Dromerick, A.; Giacomantonio, N.; Hafer-Macko, C.; Macko, R.; Nguyen, E.; Prior, P.; Suskin, N.; et al. Aerobic Exercise Recommendations to Optimize Best Practices in Care After Stroke: AEROBICS 2019 Update. Phys. Ther. 2020, 100, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.I. The case for improving the detection and treatment of obstructive sleep apnea following stroke. CMAJ 2023, 195, E374–E375. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Levin, R.L.; Heller, W. The cognitive, emotional, and social sequelae of stroke: Psychological and ethical concerns in post-stroke adaptation. Top. Stroke Rehabil. 2006, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Townend, B.S.; Whyte, S.; Desborough, T.; Crimmins, D.; Markus, R.; Levi, C.; Sturm, J.W. Longitudinal prevalence and determinants of early mood disorder post-stroke. J. Clin. Neurosci. 2007, 14, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S.; et al. Poststroke Depression: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Lillia, N.; Lax, A.; Crocamo, C.; Mantero, V.; Carra, G.; Agostoni, E.; Clerici, M. Depression after stroke and risk of mortality: A systematic review and meta-analysis. Stroke Res. Treat. 2013, 2013, 862978. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Lee, S.Y.; Song, M.K.; et al. Post-Stroke Depression and Cognitive Aging: A Multicenter, Prospective Cohort Study. J. Pers. Med. 2022, 12, 389. [Google Scholar] [CrossRef]
- Allida, S.; Cox, K.L.; Hsieh, C.F.; Lang, H.; House, A.; Hackett, M.L. Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke. Cochrane Database Syst. Rev. 2020, 1, CD003437. [Google Scholar] [CrossRef]
- Almeida, O.P.; Hankey, G.J.; Ford, A.; Etherton-Beer, C.; Flicker, L.; Hackett, M.; Assessment of Fluoxetine in Stroke Recovery Trial, C. Depression Outcomes Among Patients Treated with Fluoxetine for Stroke Recovery: The AFFINITY Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1072–1079. [Google Scholar] [CrossRef]
- Collaboration, E.T. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020, 19, 661–669. [Google Scholar] [CrossRef]
- Stulberg, E.L.; Dong, L.; Zheutlin, A.R.; Kim, S.; Claflin, E.S.; Skolarus, L.E.; Morgenstern, L.B.; Lisabeth, L.D. Associations of Self-Reported History of Depression and Antidepressant Use Before Stroke Onset with Poststroke Post-Acute Rehabilitation Care-An Exploratory Study: The BASIC (Brain Attack Surveillance in Corpus Christi) Project. J. Am. Heart Assoc. 2019, 8, e013382. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, B.K.; Mitre, L.P.; Kim, A.I.H.; Gagliardi, R.J. Prevalence and Predictors of Fatigue and Neuropsychiatric Symptoms in Patients with Minor Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105964. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.L.; Becker, K.J.; Kim, J.S.; Choi-Kwon, S.; Saban, K.L.; McNair, N.; Mead, G.E.; American Heart Association Council on Cardiovascular and Stroke Nursing and Stroke Council. Poststroke Fatigue: Emerging Evidence and Approaches to Management: A Scientific Statement for Healthcare Professionals from the American Heart Association. Stroke 2017, 48, e159–e170. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, S.; Zhang, Z.; Wang, C.; Wang, A.; Zhu, W. Related risk factors associated with post-stroke fatigue: A systematic review and meta-analysis. Neurol. Sci. 2021, 42, 1463–1471. [Google Scholar] [CrossRef]
- Bivard, A.; Lillicrap, T.; Krishnamurthy, V.; Holliday, E.; Attia, J.; Pagram, H.; Nilsson, M.; Parsons, M.; Levi, C.R. MIDAS (Modafinil in Debilitating Fatigue After Stroke): A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Stroke 2017, 48, 1293–1298. [Google Scholar] [CrossRef]
- Tay, J.; Morris, R.G.; Markus, H.S. Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment. Int. J. Stroke 2021, 16, 510–518. [Google Scholar] [CrossRef]
- Mikami, K.; Jorge, R.E.; Moser, D.J.; Arndt, S.; Jang, M.; Solodkin, A.; Small, S.L.; Fonzetti, P.; Hegel, M.T.; Robinson, R.G. Prevention of poststroke apathy using escitalopram or problem-solving therapy. Am. J. Geriatr. Psychiatry 2013, 21, 855–862. [Google Scholar] [CrossRef]
- Lapchak, P.A. Neuronal Dysregulation in Stroke-Associated Pseudobulbar Affect (PBA): Diagnostic Scales and Current Treatment Options. J. Neurol. Neurophysiol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Allida, S.; House, A.; Hackett, M.L. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database Syst. Rev. 2022, 11, CD003690. [Google Scholar] [CrossRef]
- Hammond, F.M.; Alexander, D.N.; Cutler, A.J.; D’Amico, S.; Doody, R.S.; Sauve, W.; Zorowitz, R.D.; Davis, C.S.; Shin, P.; Ledon, F.; et al. PRISM II: An open-label study to assess effectiveness of dextromethorphan/quinidine for pseudobulbar affect in patients with dementia, stroke or traumatic brain injury. BMC Neurol. 2016, 16, 89. [Google Scholar] [CrossRef]
- Sloane, K.L.; Kasner, S.E.; Favilla, C.G.; Rothstein, A.; Witsch, J.; Hamilton, R.H.; Schneider, A.L.C. Always Look on the Bright Side: Associations of Optimism with Functional Outcomes After Stroke. J. Am. Heart Assoc. 2023, 12, e027959. [Google Scholar] [CrossRef]
- Venna, V.R.; McCullough, L.D. Role of social factors on cell death, cerebral plasticity and recovery after stroke. Metab. Brain Dis. 2015, 30, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Glymour, M.M.; Weuve, J.; Fay, M.E.; Glass, T.; Berkman, L.F. Social ties and cognitive recovery after stroke: Does social integration promote cognitive resilience? Neuroepidemiology 2008, 31, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Cene, C.W.; Beckie, T.M.; Sims, M.; Suglia, S.F.; Aggarwal, B.; Moise, N.; Jimenez, M.C.; Gaye, B.; McCullough, L.D.; the American Heart Association Social Determinants of Health Committee of the Council on Epidemiology and Prevention and Council on Quality of Care and Outcomes Research; et al. Effects of Objective and Perceived Social Isolation on Cardiovascular and Brain Health: A Scientific Statement from the American Heart Association. J. Am. Heart Assoc. 2022, 11, e026493. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Laumeier, I.; Ihl, T.; Steinicke, M.; Ferse, C.; Endres, M.; Grau, A.; Hastrup, S.; Poppert, H.; Palm, F.; et al. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): An open-label, randomised controlled trial. Lancet Neurol. 2020, 19, 49–60. [Google Scholar] [CrossRef]
- Hughes, R.; Fleming, P.; Henshall, L. Peer support groups after acquired brain injury: A systematic review. Brain Inj. 2020, 34, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, S.; Wilson, W.; Gowan, N.; Ferreira, L.; Phadke, C.; Udler, E.; Bontempo, T. Experiences of Occupational Performance in Survivors of Stroke Attending Peer Support Groups. Can. J. Occup. Ther. 2020, 87, 173–181. [Google Scholar] [CrossRef]
- Gorenko, J.A.; Moran, C.; Flynn, M.; Dobson, K.; Konnert, C. Social Isolation and Psychological Distress Among Older Adults Related to COVID-19: A Narrative Review of Remotely-Delivered Interventions and Recommendations. J. Appl. Gerontol. 2021, 40, 3–13. [Google Scholar] [CrossRef]
- Rigby, H.; Gubitz, G.; Phillips, S. A systematic review of caregiver burden following stroke. Int. J. Stroke 2009, 4, 285–292. [Google Scholar] [CrossRef]
- Em, S.; Bozkurt, M.; Caglayan, M.; Ceylan Cevik, F.; Kaya, C.; Oktayoglu, P.; Nas, K. Psychological health of caregivers and association with functional status of stroke patients. Top. Stroke Rehabil. 2017, 24, 323–329. [Google Scholar] [CrossRef]
- Tooth, L.; McKenna, K.; Barnett, A.; Prescott, C.; Murphy, S. Caregiver burden, time spent caring and health status in the first 12 months following stroke. Brain Inj. 2005, 19, 963–974. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.E.; Chang, A.M. Predictors of quality of life following stroke. Disabil. Rehabil. 2002, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, H.J.; Gao, Y.; Zhou, J.; Zhou, M.; Wan, L.; Xiong, F.; Zhao, J.; He, Q.Q.; Wang, Y. Combined association of multiple chronic diseases and social isolation with the functional disability after stroke in elderly patients: A multicenter cross-sectional study in China. BMC Geriatr. 2021, 21, 495. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, E.T.; de Witte, L.P.; Schure, L.M.; Sanderman, R.; Meyboom-de Jong, B. Risk factors for burn-out in caregivers of stroke patients, and possibilities for intervention. Clin. Rehabil. 2001, 15, 669–677. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.; Guo, L.; Wang, J. A remote quantitative Fugl-Meyer assessment framework for stroke patients based on wearable sensor networks. Comput. Methods Programs Biomed. 2016, 128, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Wyke, S.; Brewster, S.; Sattar, N.; Gill, J.M.; Alexander, G.; Rafferty, D.; McFadyen, A.K.; Ramsay, A.; Dybus, A. Increasing physical activity in stroke survivors using STARFISH, an interactive mobile phone application: A pilot study. Top. Stroke Rehabil. 2016, 23, 170–177. [Google Scholar] [CrossRef]
- Grau-Pellicer, M.; Lalanza, J.F.; Jovell-Fernandez, E.; Capdevila, L. Impact of mHealth technology on adherence to healthy PA after stroke: A randomized study. Top. Stroke Rehabil. 2020, 27, 354–368. [Google Scholar] [CrossRef]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Cramer, S.C.; Dodakian, L.; Le, V.; McKenzie, A.; See, J.; Augsburger, R.; Zhou, R.J.; Raefsky, S.M.; Nguyen, T.; Vanderschelden, B.; et al. A Feasibility Study of Expanded Home-Based Telerehabilitation After Stroke. Front. Neurol. 2020, 11, 611453. [Google Scholar] [CrossRef]
- Garcia, A.; Mayans, B.; Margeli, C.; Pamplona, A.; Molas, C.; Monras, J.; Alpiste, F.; Torner, J.; Serrancoli, G. A feasibility study to assess the effectiveness of Muvity: A telerehabilitation system for chronic post-stroke subjects. J. Stroke Cerebrovasc. Dis. 2022, 31, 106791. [Google Scholar] [CrossRef]
- Gerber, S.M.; Schutz, N.; Uslu, A.S.; Schmidt, N.; Rothlisberger, C.; Wyss, P.; Perny, S.; Wyss, C.; Koenig-Bruhin, M.; Urwyler, P.; et al. Therapist-Guided Tablet-Based Telerehabilitation for Patients with Aphasia: Proof-of-Concept and Usability Study. JMIR Rehabil. Assist. Technol. 2019, 6, e13163. [Google Scholar] [CrossRef]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef]
- Burridge, J.H.; Lee, A.C.W.; Turk, R.; Stokes, M.; Whitall, J.; Vaidyanathan, R.; Clatworthy, P.; Hughes, A.M.; Meagher, C.; Franco, E.; et al. Telehealth, Wearable Sensors, and the Internet: Will They Improve Stroke Outcomes Through Increased Intensity of Therapy, Motivation, and Adherence to Rehabilitation Programs? J. Neurol. Phys. Ther. 2017, 41 (Suppl. S3), S32–S38. [Google Scholar] [CrossRef]
- Dawson, J.; Liu, C.Y.; Francisco, G.E.; Cramer, S.C.; Wolf, S.L.; Dixit, A.; Alexander, J.; Ali, R.; Brown, B.L.; Feng, W.; et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. Lancet 2021, 397, 1545–1553. [Google Scholar] [CrossRef]
- Dimyan, M.A.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil. Neural Repair 2010, 24, 125–135. [Google Scholar] [CrossRef]
- Smith, M.C.; Stinear, C.M. Transcranial magnetic stimulation (TMS) in stroke: Ready for clinical practice? J. Clin. Neurosci. 2016, 31, 10–14. [Google Scholar] [CrossRef]
- Breining, B.L.; Sebastian, R. Neuromodulation in post-stroke aphasia treatment. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 44–56. [Google Scholar] [CrossRef]
- Shen, X.; Liu, M.; Cheng, Y.; Jia, C.; Pan, X.; Gou, Q.; Liu, X.; Cao, H.; Zhang, L. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: A systematic review and meta-analysis of randomized controlled clinical trials. J. Affect. Disord. 2017, 211, 65–74. [Google Scholar] [CrossRef]
- Valiengo, L.C.; Goulart, A.C.; de Oliveira, J.F.; Bensenor, I.M.; Lotufo, P.A.; Brunoni, A.R. Transcranial direct current stimulation for the treatment of post-stroke depression: Results from a randomised, sham-controlled, double-blinded trial. J. Neurol. Neurosurg. Psychiatry 2017, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, L.; Braschi, C.; Spolidoro, M.; Begenisic, T.; Sale, A.; Maffei, L. Nurturing brain plasticity: Impact of environmental enrichment. Cell Death Differ. 2010, 17, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Li, M.Z.; Yang, L.; Feng, X.F.; Lei, J.F.; Zhang, N.; Zhao, Y.Y.; Zhao, H. The three-phase enriched environment paradigm promotes neurovascular restorative and prevents learning impairment after ischemic stroke in rats. Neurobiol. Dis. 2020, 146, 105091. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Dong, L.L.; Zhang, Y.J.; Zhao, X.M.; He, H.Y. Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy. Neural Regen. Res. 2021, 16, 813–819. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, M.; Yang, S.; Chen, X.; Wu, J.; Wen, M.; Yan, K.; Bi, X. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int. J. Neurosci. 2021, 131, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Chernenko, G.; Corbett, D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 2004, 24, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.; Ada, L.; Middleton, S.; Pollack, M.; Nilsson, M.; Churilov, L.; Blennerhassett, J.; Faux, S.; New, P.; McCluskey, A.; et al. Altering the rehabilitation environment to improve stroke survivor activity: A Phase II trial. Int. J. Stroke 2022, 17, 299–307. [Google Scholar] [CrossRef]
- Matz, K.; Teuschl, Y.; Firlinger, B.; Dachenhausen, A.; Keindl, M.; Seyfang, L.; Tuomilehto, J.; Brainin, M.; Group, A.S. Multidomain Lifestyle Interventions for the Prevention of Cognitive Decline After Ischemic Stroke: Randomized Trial. Stroke 2015, 46, 2874–2880. [Google Scholar] [CrossRef]
- Yaghi, S.; Siegler, J.E.; Nguyen, T.N. Pitfalls of Randomized Controlled Trials in Stroke: How Can We Do Better? Stroke Vasc. Interv. Neurol. 2023, 3, e000807. [Google Scholar] [CrossRef]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; van Veluw, S.J.; et al. The Neurovasculome: Key Roles in Brain Health and Cognitive Impairment: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e251–e271. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Wadley, V.G.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Howard, G.; Howard, V.J.; Cushman, M.; Judd, S.E.; et al. Risk Factors for Poststroke Cognitive Decline: The REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke 2018, 49, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, Y.; Guo, L.; Li, S. Association of Income with Post-Stroke Cognition and the Underlying Neuroanatomical Mechanism. Brain Sci. 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Stulberg, E.L.; Twardzik, E.; Kim, S.; Hsu, C.W.; Xu, Y.; Clarke, P.; Morgenstern, L.B.; Lisabeth, L.D. Association of Neighborhood Socioeconomic Status with Outcomes in Patients Surviving Stroke. Neurology 2021, 96, e2599–e2610. [Google Scholar] [CrossRef]
- Skolarus, L.E.; Burke, J.F. Towards an Understanding of Racial Differences in Post-stroke Disability. Curr. Epidemiol. Rep. 2015, 2, 191–196. [Google Scholar] [CrossRef]
- Khan, S.U.; Yedlapati, S.H.; Khan, M.Z.; Virani, S.S.; Blaha, M.J.; Sharma, G.; Jordan, J.E.; Kash, B.A.; Vahidy, F.S.; Arshad, A.; et al. Clinical and Economic Profile of Homeless Young Adults with Stroke in the United States, 2002–2017. Curr. Probl. Cardiol. 2023, 48, 101190. [Google Scholar] [CrossRef]
- Towfighi, A.; Boden-Albala, B.; Cruz-Flores, S.; El Husseini, N.; Odonkor, C.A.; Ovbiagele, B.; Sacco, R.L.; Skolarus, L.E.; Thrift, A.G.; American Heart Association Stroke, C.; et al. Strategies to Reduce Racial and Ethnic Inequities in Stroke Preparedness, Care, Recovery, and Risk Factor Control: A Scientific Statement from the American Heart Association. Stroke 2023, 54, e371–e388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stulberg, E.L.; Sachdev, P.S.; Murray, A.M.; Cramer, S.C.; Sorond, F.A.; Lakshminarayan, K.; Sabayan, B. Post-Stroke Brain Health Monitoring and Optimization: A Narrative Review. J. Clin. Med. 2023, 12, 7413. https://doi.org/10.3390/jcm12237413
Stulberg EL, Sachdev PS, Murray AM, Cramer SC, Sorond FA, Lakshminarayan K, Sabayan B. Post-Stroke Brain Health Monitoring and Optimization: A Narrative Review. Journal of Clinical Medicine. 2023; 12(23):7413. https://doi.org/10.3390/jcm12237413
Chicago/Turabian StyleStulberg, Eric L., Perminder S. Sachdev, Anne M. Murray, Steven C. Cramer, Farzaneh A. Sorond, Kamakshi Lakshminarayan, and Behnam Sabayan. 2023. "Post-Stroke Brain Health Monitoring and Optimization: A Narrative Review" Journal of Clinical Medicine 12, no. 23: 7413. https://doi.org/10.3390/jcm12237413
APA StyleStulberg, E. L., Sachdev, P. S., Murray, A. M., Cramer, S. C., Sorond, F. A., Lakshminarayan, K., & Sabayan, B. (2023). Post-Stroke Brain Health Monitoring and Optimization: A Narrative Review. Journal of Clinical Medicine, 12(23), 7413. https://doi.org/10.3390/jcm12237413





