Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position
Abstract
:1. Introduction
2. Materials and Methods
3. Results
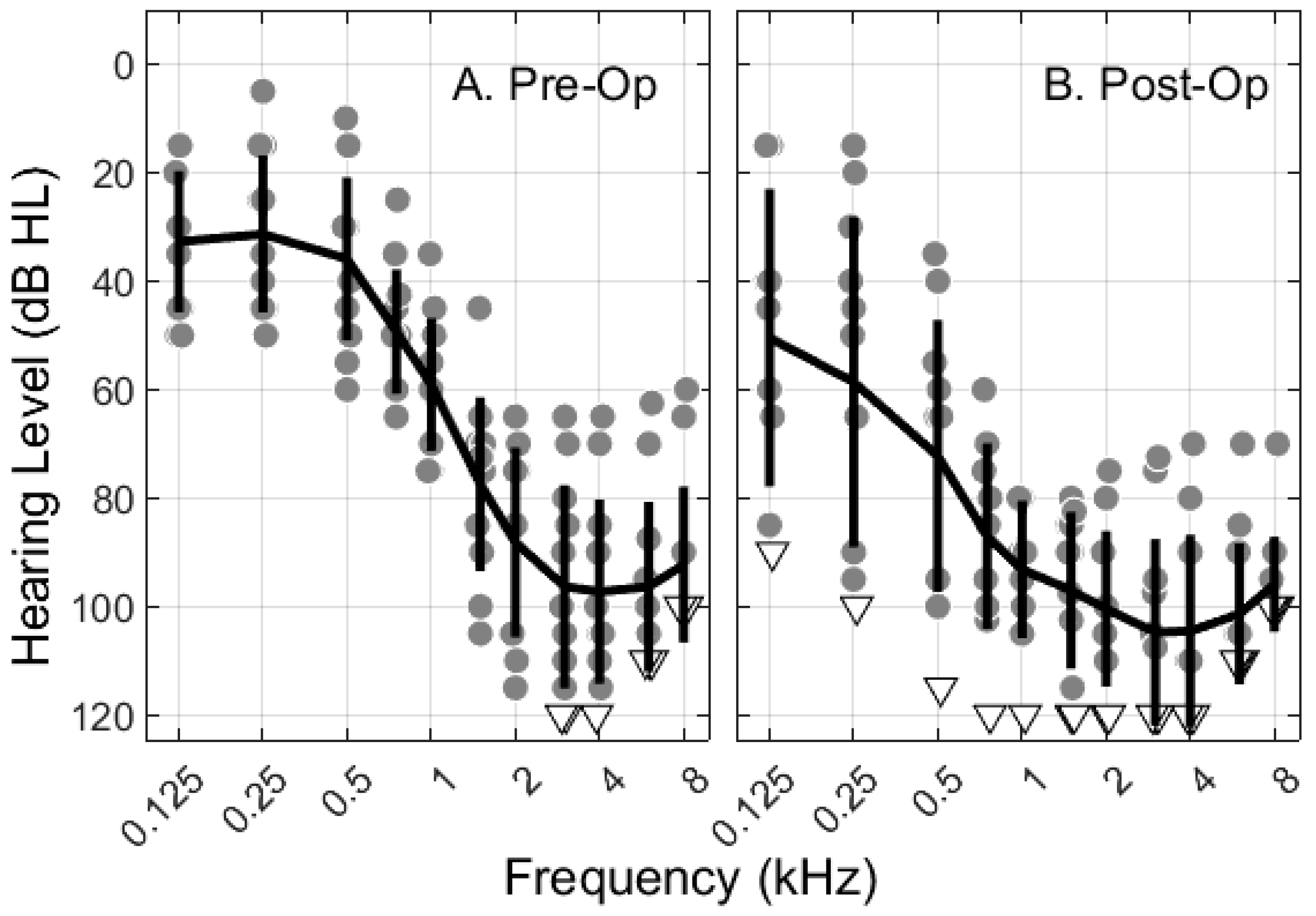
3.1. Participant Information
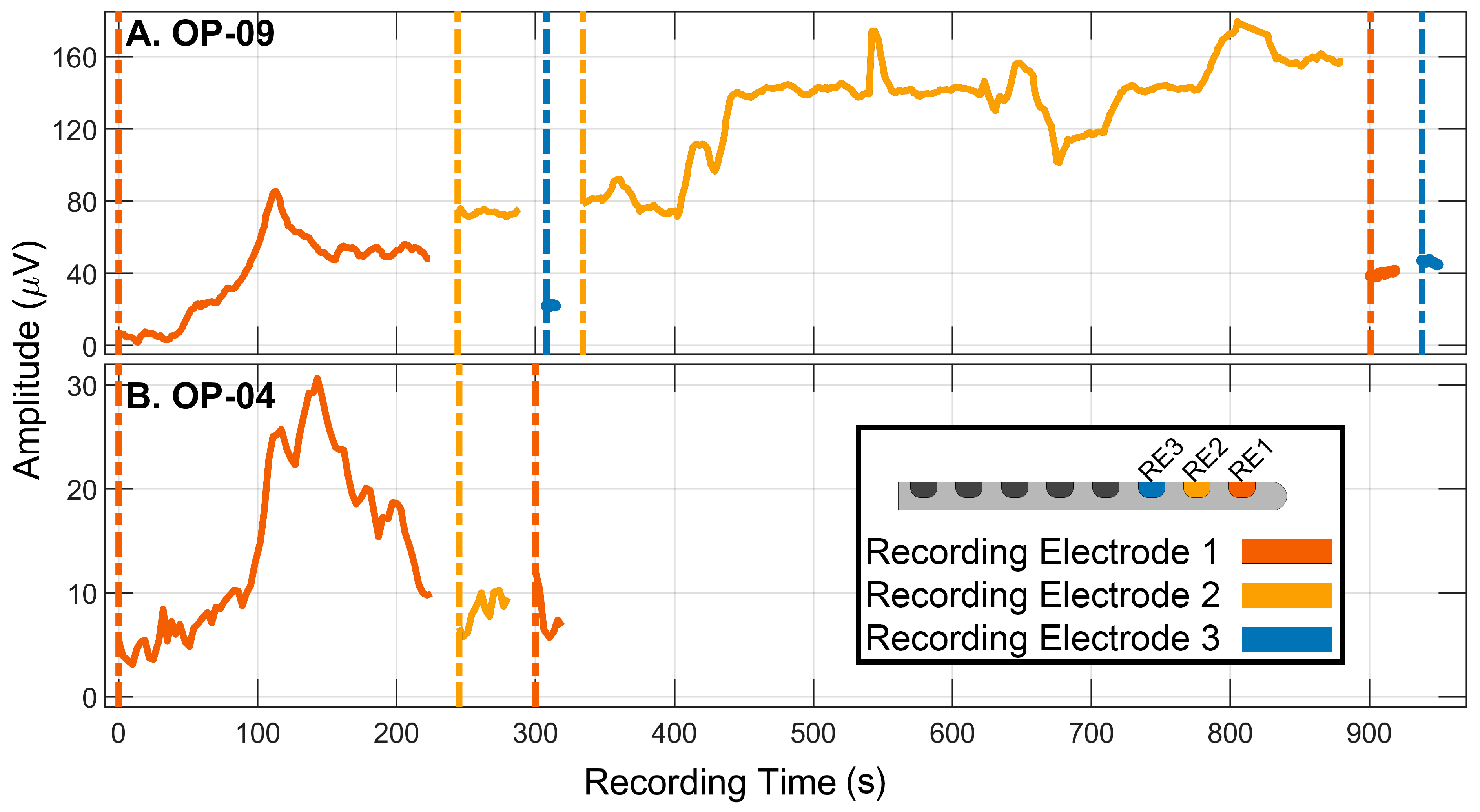
3.2. Monitoring Strategy: Adjusting the Recording Electrode
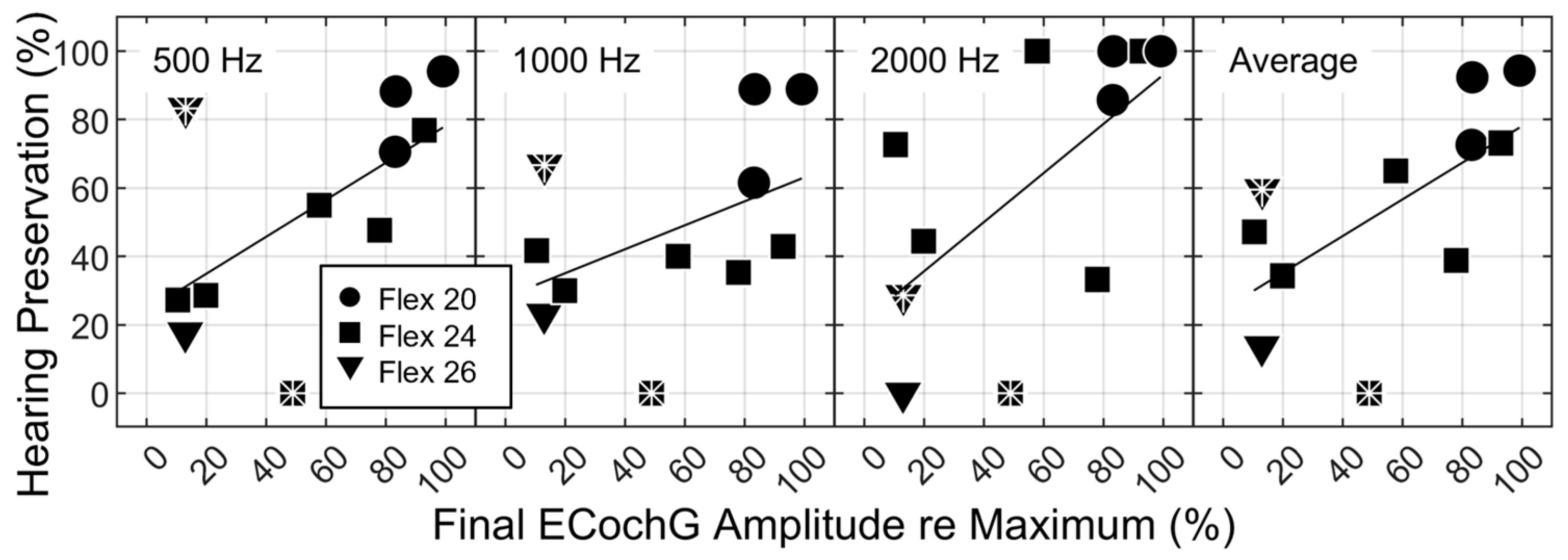
3.3. ECochG Amplitude Decrements as a Predictor of Hearing Preservation
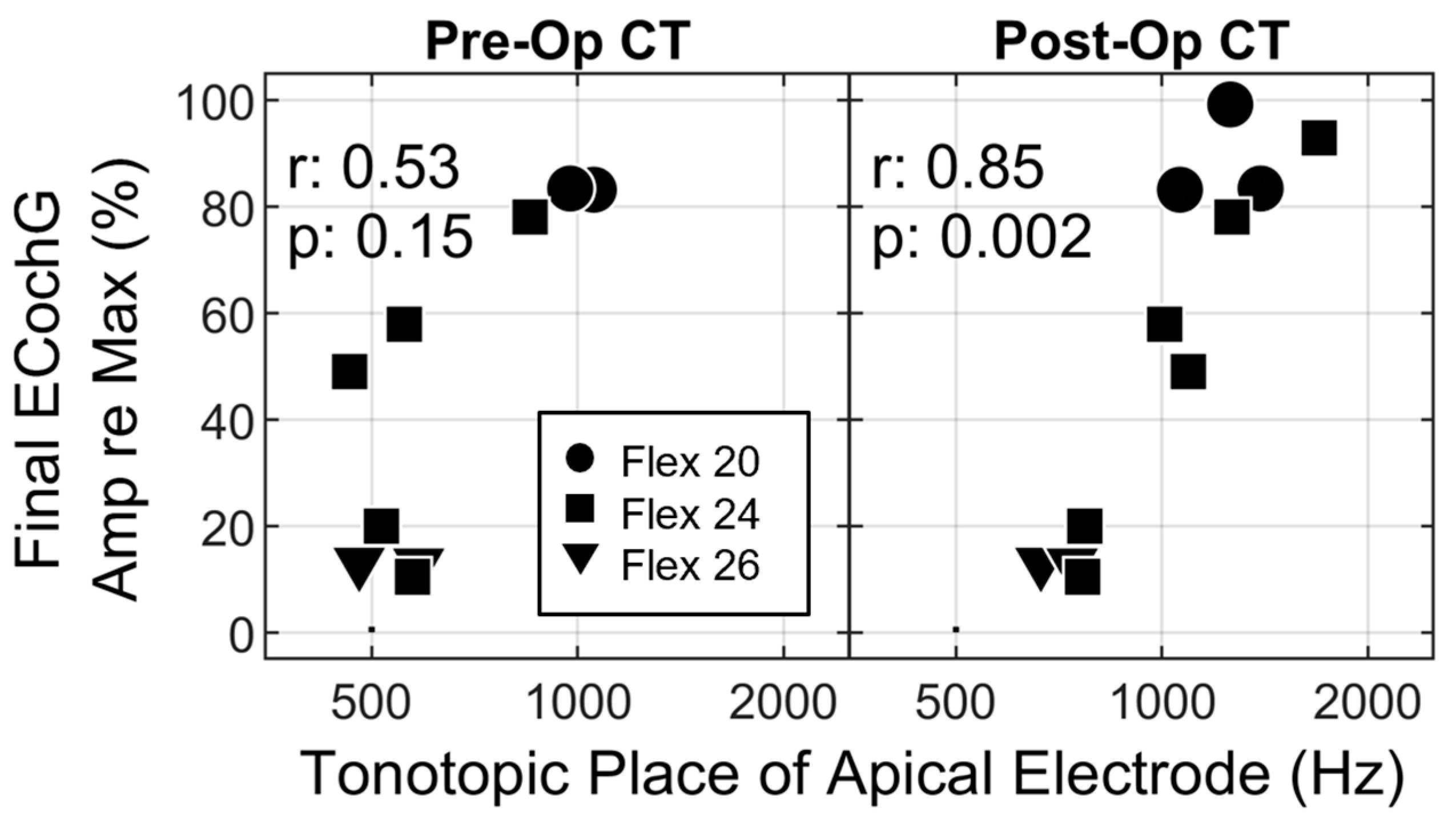
3.4. The Impact of Recording Site on ECochG Amplitude
4. Discussion
4.1. Real-Time Recording Electrode Adjustments
4.2. Basalward Spread of the Traveling Wave at High Stimulus Levels
4.3. Intracochlear Location of the Recording Electrode
4.4. Partial Insertions
4.5. Real-Time Feedback
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, M.L.; Driscoll, C.L.W.; Gifford, R.H.; Service, G.J.; Tombers, N.M.; Hughes-Borst, B.J.; Neff, B.A.; Beatty, C.W. Implications of minimizing trauma during conventional cochlear implantation. Otol. Neurotol. 2011, 32, 962–968. [Google Scholar] [CrossRef]
- Finley, C.C.; Skinner, M.W.; Holden, T.A.; Holden, L.K.; Whiting, B.R.; Chole, R.A.; Neely, G.J.; Hullar, T.E. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol. Neurotol. 2008, 29, 920–928. [Google Scholar] [CrossRef]
- Skinner, M.W.; Holden, T.A.; Whiting, B.R.; Voie, A.H.; Brunsden, B.; Neely, J.G.; Saxon, E.A.; Hullar, T.E.; Finley, C.C. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann. Otol. Rhinol. Laryngol. 2007, 116, 2–24. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Roland, P.S.; Gstöttner, W.; Adunka, O. Method for hearing preservation in cochlear implant surgery. Oper. Tech. Otolaryngol. 2005, 16, 93–100. [Google Scholar] [CrossRef]
- Dhanasingh, A. The rationale for FLEX (cochlear implant) electrode with varying array lengths. World J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 45–53. [Google Scholar] [CrossRef]
- Labadie, R.F.; Noble, J.H. Preliminary results with image-guided cochlear implant insertion techniques. Otol. Neurotol. 2018, 39, 922–928. [Google Scholar] [CrossRef]
- Lenarz, T.; Timm, M.E.; Salcher, R.; Büchner, A. Individual hearing preservation cochlear implantation using the concept of partial insertion. Otol. Neurotol. 2019, 40, E326–E335. [Google Scholar] [CrossRef]
- De Seta, D.; Daoudi, H.; Torres, R.; Ferrary, E.; Sterkers, O.; Nguyen, Y. Robotics, automation, active electrode arrays, and new devices for cochlear implantation: A contemporary review. Hear. Res. 2022, 414, 108425. [Google Scholar] [CrossRef]
- Trecca, E.M.C.; Riggs, W.J.; Mattingly, J.K.; Hiss, M.M.; Cassano, M.; Adunka, O.F. Electrocochleography and cochlear implantation: A systematic review. Otol. Neurotol. 2020, 41, 864–878. [Google Scholar] [CrossRef]
- Dallos, P. The Auditory Periphery; Biophysics and Physiology; Academic Press, Inc.: New York, NY, USA, 1973. [Google Scholar]
- Forgues, M.; Koehn, H.A.; Dunnon, A.K.; Pulver, S.H.; Buchman, C.A.; Adunka, O.F.; Fitzpatrick, D.C. Distinguishing hair cell from neural potentials recorded at the round window. J. Neurophysiol. 2014, 111, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.L.; Schreiner, C.E. The auditory neurophonic: Basic properties. Hear. Res. 1984, 15, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Calloway, N.H.; Fitzpatrick, D.C.; Campbell, A.P.; Iseli, C.; Pulver, S.; Buchman, C.A.; Adunka, O.F. Intracochlear electrocochleography during cochlear implantation. Otol. Neurotol. 2014, 35, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Kaicer, A.; Briggs, R.; O’leary, S. Cochlear Response Telemetry: Intracochlear Electrocochleography via Cochlear Implant Neural Response Telemetry Pilot Study Results. Otol. Neurotol. 2015, 36, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Kaicer, A.; Sly, D.; Iseli, C.; Wei, B.; Briggs, R.; O’Leary, S. Intraoperative real-time cochlear response telemetry predicts hearing preservation in cochlear implantation. Otol. Neurotol. 2016, 37, 332–338. [Google Scholar] [CrossRef]
- Adunka, O.F.; Mlot, S.; Suberman, T.A.; Campbell, A.P.; Surowitz, J.; Buchman, C.A.; Fitzpatrick, D.C. Intracochlear recordings of electrophysiological parameters indicating cochlear damage. Otol. Neurotol. 2010, 31, 1233–1241. [Google Scholar] [CrossRef]
- Mandalà, M.; Colletti, L.; Tonoli, G.; Colletti, V. Electrocochleography during cochlear implantation for hearing preservation. Otolaryngol. Head Neck Surg. 2012, 146, 774–781. [Google Scholar] [CrossRef]
- Campbell, A.P.; Suberman, T.A.; Buchman, C.A.; Fitzpatrick, D.C.; Adunka, O.F. Correlation of early auditory potentials and intracochlear electrode insertion properties: An animal model featuring near real-time monitoring. Otol. Neurotol. 2010, 31, 1391–1398. [Google Scholar] [CrossRef]
- Choudhury, B.; Adunka, O.F.; DeMason, C.E.; Ahmad, F.I.; Buchman, C.A.; Fitzpatrick, D.C. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol. Neurotol. 2011, 32, 1370–1378. [Google Scholar] [CrossRef]
- Jensen, M.J.; Isaac, H.; Hernandez, H.; Oleson, J.; Dunn, C.; Gantz, B.J.; Hansen, M.R. Timing of acoustic hearing changes after cochlear implantation. Laryngoscope 2022, 132, 2036–2043. [Google Scholar] [CrossRef]
- O’Leary, S.; Briggs, R.; Gerard, J.-M.; Iseli, C.; Wei, B.P.C.; Tari, S.; Rousset, A.; Bester, C. Intraoperative observational real-time electrocochleography as a predictor of hearing loss after cochlear implantation: 3 and 12 month outcomes. Otol. Neurotol. 2020, 41, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Mylanus, E.; Venail, F.; Lenarz, T.; Birman, C.; Di Lella, F.; Roland, J.T.; Gantz, B.; Beynon, A.; Sicard, M.; et al. Monitoring cochlear health with intracochlear electrocochleography during cochlear implantation: Findings from an international clinical investigation. Ear Hear. 2023, 44, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Saoji, A.A.; Graham, M.K.; Adkins, W.J.; Koka, K.; Carlson, M.L.; Neff, B.A.; Driscoll, C.L.W.; Fitzpatrick, D.C. Multi-frequency electrocochleography and electrode scan to identify electrode insertion trauma during cochlear implantation. Brain Sci. 2023, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Shew, M.A.; Lefler, S.M.; Kallogjeri, D.; Wick, C.C.; Holden, T.A.; Durakovic, N.; Ortmann, A.J.; Herzog, J.A.; Buchman, C.A. Is characteristic frequency limiting real-time electrocochleography during cochlear implantation? Front. Neurosci. 2022, 16, 915302. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Gifford, R.H.; Noble, J.H.; Bennett, M.L.; Rivas, A.; Wanna, G.B.; Haynes, D.S.; Labadie, R.F. Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front. Neurosci. 2017, 11, 291. [Google Scholar] [CrossRef]
- Lenarz, T.; Buechner, A.; Gantz, B.; Hansen, M.; Tejani, V.; Labadie, R.F.; O’Connell, B.P.; Buchman, C.A.; Valenzuela, C.V.; Adunka, O.; et al. Relationship Between Intraoperative Electrocochleography and Hearing Preservation. Otol. Neurotol. 2022, 43, e72–e78. [Google Scholar] [CrossRef]
- Riggs, W.J.; Dwyer, R.T.; Holder, J.T.; Mattingly, J.K.; Ortmann, A.; Noble, J.H.; Dawant, B.M.; Valenzuela, C.V.; O’Connell, B.P.; Harris, M.S.; et al. Intracochlear electrocochleography: Influence of scalar position of the cochlear implant electrode on postinsertion results. Otol. Neurotol. 2019, 40, E503–E510. [Google Scholar] [CrossRef]
- Dalbert, A.; Pfiffner, F.; Röösli, C.; Thoele, K.; Sim, J.H.; Gerig, R.; Huber, A.M. Extra-and intracochlear electrocochleography in cochlear implant recipients. Audiol. Neurotol. 2015, 20, 339–348. [Google Scholar] [CrossRef]
- Giardina, C.K.; Brown, K.D.; Adunka, O.F.; Buchman, C.A.; Hutson, K.A.; Pillsbury, H.C.; Fitzpatrick, D.C. Intracochlear electrocochleography: Response patterns during cochlear implantation and hearing preservation. Ear Hear. 2019, 40, 833–848. [Google Scholar] [CrossRef]
- Harris, M.S.; Riggs, W.J.; Koka, K.; Litvak, L.M.; Malhotra, P.; Moberly, A.C.; O’Connell, B.P.; Holder, J.; Di Lella, F.A.; Boccio, C.M.; et al. Real-time intracochlear electrocochleography obtained directly through a cochlear implant. Otol. Neurotol. 2017, 38, E107–E113. [Google Scholar] [CrossRef]
- Lorens, A.; Walkowiak, A.; Polak, M.; Kowalczuk, A.; Furmanek, M.; Skarzynski, H.; Obrycka, A. Cochlear microphonics in hearing preservation cochlear implantees. J. Int. Adv. Otol. 2019, 15, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.M.; Sears, R.E.; Tonndorf, J. Some properties of longitudinal shear waves: A study by computer simulation. J. Acoust. Soc. Am. 1968, 43, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; Naik, K.; Dallos, P. Using the cochlear microphonic as a tool to evaluate cochlear function in mouse models of hearing. J. Assoc. Res. Otolaryngol. 2011, 12, 113–125. [Google Scholar] [CrossRef]
- Weder, S.; Bester, C.; Collins, A.; Shaul, C.; Briggs, R.J.; O’Leary, S. Toward a better understanding of electrocochleography: Analysis of real-time recordings. Ear Hear. 2020, 41, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Riggs, W.J.; Dwyer, R.; Holder, J.T.; Noble, J.H.; Dawant, B.M.; Ortmann, A.; Valenzuela, C.V.; Mattingly, J.K.; Harris, M.M.; et al. Intra-cochlear electrocochleography during cochlear implant electrode insertion is predictive of final scalar location. Otol. Neurotol. 2018, 39, E654–E659. [Google Scholar] [CrossRef] [PubMed]
- Weder, S.; Bester, C.; Collins, A.; Shaul, C.; Briggs, R.J.; O’Leary, S. Real time monitoring during cochlear implantation: Increasing the accuracy of predicting residual hearing outcomes. Otol. Neurotol. 2021, 42, e1030–e1036. [Google Scholar] [CrossRef]
- Saoji, A.A.; Patel, N.S.; Carlson, M.L.; Neff, B.A.; Koka, K.; Tarigoppula, V.S.A.; Driscoll, C.L.W. Multi-frequency electrocochleography measurements can be used to monitor and optimize electrode placement during cochlear implant surgery. Otol. Neurotol. 2019, 40, 1287–1291. [Google Scholar] [CrossRef]
- Skarżyński, P.H.; Lorens, A.; Walkowiak, A.; Polak, M.; Skarżyński, H. Multi-frequency intraoperative monitoring of hearing preservation during cochlear implantation. Life 2022, 12, 636. [Google Scholar] [CrossRef]
- Banakis Hartl, R.M.; Kaufmann, C.; Hansen, M.R.; Tollin, D.J. Intracochlear Pressure Transients during Cochlear Implant Electrode Insertion: Effect of Micro-mechanical Control on Limiting Pressure Trauma. Otol. Neurotol. 2019, 40, 736–741. [Google Scholar] [CrossRef]
- Claussen, A.D.; Shibata, S.B.; Kaufmann, C.R.; Henslee, A.; Hansen, M.R. Comparative Analysis of Robotics-Assisted and Manual Insertions of Cochlear Implant Electrode Arrays. Otol. Neurotol. 2022, 43, 1155–1161. [Google Scholar] [CrossRef]
- Gantz, J.A.; Gantz, B.J.; Kaufmann, C.R.; Henslee, A.M.; Dunn, C.C.; Hua, X.; Hansen, M.R. A Steadier Hand: The First Human Clinical Trial of a Single-Use Robotic-Assisted Surgical Device for Cochlear Implant Electrode Array Insertion. Otol. Neurotol. 2023, 44, 34–39. [Google Scholar] [CrossRef]
- Kaufmann, C.R.; Henslee, A.M.; Claussen, A.; Hansen, M.R. Evaluation of insertion forces and cochlea trauma following robotics-assisted cochlear implant electrode array insertion. Otol. Neurotol. 2020, 41, 631–638. [Google Scholar] [CrossRef]
- Rajan, G.P.; Kontorinis, G.; Kuthubutheen, J. The effects of insertion speed on inner ear function during cochlear implantation: A comparison study. Audiol. Neurotol. 2012, 18, 17–22. [Google Scholar] [CrossRef]
- Skarzynski, H.; van de Heyning, P.; Agrawal, S.; Arauz, S.L.; Atlas, M.; Baumgartner, W.; Caversaccio, M.; De Bodt, M.; Gavilan, J.; Godey, B.; et al. Towards a consensus on a hearing preservation classification system. Acta Oto-Laryngol. 2013, 133, 3–13. [Google Scholar] [CrossRef]
- Skarzynski, P.H.; Lorens, A.; Gos, E.; Kolodziejak, A.; Obrycka, A.; Skarżyńska, M.B.; Czajka, N.; Porowski, M.; Skarzynski, H. Outcomes of cochlear implantation using FLEX26 electrode: Audiological results and quality of life after 12 months. Audiol. Neurotol. 2023, 1–8. [Google Scholar] [CrossRef]
- Russell, I.J.; Nilsen, K.E. The location of the cochlear amplifier: Spatial representation of a single tone on the guinea pig basilar membrane. Proc. Natl. Acad. Sci. USA 1997, 94, 2660–2664. [Google Scholar] [CrossRef]
- Honrubia, V.; Ward, P.H. Longitudinal distribution of the cochlear microphonics inside the cochlear duct (guinea pig). J. Acoust. Soc. Am. 1968, 44, 951–958. [Google Scholar] [CrossRef]
- Campbell, L.; Bester, C.; Iseli, C.; Sly, D.; Dragovic, A.; Gummer, A.W.; O’Leary, S. Electrophysiological evidence of the basilar-membrane travelling wave and frequency place coding of sound in cochlear implant recipients. Audiol. Neurotol. 2017, 22, 180–189. [Google Scholar] [CrossRef]
- Walia, A.; Ortmann, A.J.; Lefler, S.; Holden, T.A.; Puram, S.V.; Herzog, J.A.; Buchman, C.A. Place coding in the human cochlea. medRxiv 2023. [Google Scholar] [CrossRef]
- Roland, J.T.; Gantz, B.J.; Waltzman, S.B.; Parkinson, A.J. Long-term outcomes of cochlear implantation in patients with high-frequency hearing loss. Laryngoscope 2018, 128, 1939–1945. [Google Scholar] [CrossRef]
- Gantz, B.J.; Dunn, C.C.; Oleson, J.; Hansen, M.R. Acoustic plus electric speech processing: Long-term results. Laryngoscope 2018, 128, 473–481. [Google Scholar] [CrossRef]
- Gantz, B.J.; Dunn, C.; Oleson, J.; Hansen, M.; Parkinson, A.; Turner, C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope 2016, 126, 962–973. [Google Scholar] [CrossRef]
- Kobler, J.-P.; Dhanasingh, A.; Kiran, R.; Jolly, C.; Ortmaier, T. Cochlear dummy electrodes for insertion training and research purposes: Fabrication, mechanical characterization, and experimental validation. BioMed. Res. Int. 2015, 2015, 574209. [Google Scholar] [CrossRef]
- Harris, M.S.; Koka, K.; Riggs, W.J.; Saleh, S.; Holder, J.T.; Dwyer, R.T.; Prentiss, S.; Lefler, S.; Kozlowski, K.; Hiss, M.M.; et al. Can electrocochleography help preserve hearing after cochlear implantation with full electrode insertion? Otol. Neurotol. 2022, 43, 789–796. [Google Scholar] [CrossRef]
- Bester, C.; Collins, A.; Razmovski, T.; Weder, S.; Briggs, R.J.; Wei, B.; Zakaria, A.F.; Gerard, J.-M.; Mitchell-Innes, A.; Tykocinski, M.; et al. Electrocochleography triggered intervention successfully preserves residual hearing during implantation: Results of a randomised clinical trial. Hear. Res. 2022, 426, 108353. [Google Scholar] [CrossRef]
- Henslee, A.M.; Kaufmann, C.R.; Andrick, M.D.; Reineke, P.T.; Tejani, V.D.; Hansen, M.R. Development and Characterization of an Electrocochleography-Guided Robotics-Assisted Cochlear Implant Array Insertion System. Otolaryngol. Head Neck Surg. 2022, 167, 334–340. [Google Scholar] [CrossRef]
| ID | Age (Years) | Ear | Etiological Considerations | Onset | Flex Array | e1 Tonotopic Frequency (Hz) | e1 Insertion Angle (Degrees) Post-Op | Extracochlear Electrodes | RE | Final ECochG Amplitude (% max) | 500-Hz HP (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Op | Post-Op | RE1 | RE* | ||||||||||
| 09 † | 79 | L | Noise | Adult | 24 | 463.9 | 1093.4 | 331.9 | 10 ▪,11,12 | 1, 2 *, 3 | 22 | 86 | 0 |
| 04 † | 35 | L | Idiopathic | Teenager | 26 | 479.4 | 665.3 | 422.1 | 12 | 1, 2 * | 13 | 23 | 83 |
| 16 | 86 | R | Idiopathic | Adult | 24 | 516.6 | 772.2 | 394.5 | 12 ▪ | 1, 2, 3 * | 20 | 98 | 29 |
| 03 | 41 | R | Idiopathic | Early Adult | 24 | 557.8 | 1010 | 347.3 | 11,12 | 1,2 *, 3 | 58 | 36 | 55 |
| 10 | 72 | L | Noise | Adult | 24 | 574 | 765.4 | 393.9 | 11 ▪,12 | 1, 2 *, 3 | 10 | 76 | 27 |
| 08 | 72 | L | Idiopathic | Adult | 26 | 585.7 | 739.1 | 403 | None | 1, 2, 3 * | 13 | 75 | 18 |
| 07 | 78 | R | Noise, Family History | Adult | 24 | 852.8 | 1264.4 | 311.8 | 12 | 1 | 78 | 48 | |
| 02 | 71 | L | Suspected Genetic | Childhood | 20 | 975.2 | 1393.6 | 296.5 | None | 1 | 83 | 88 | |
| 01 | 48 | L | Idiopathic | Teenager | 20 | 1055.8 | 1062.3 | 383.3 | None | 1 | 83 | 71 | |
| 17 | 74 | L | Noise | Adult | 20 | poor scan | 1258.3 | 321.1 | None | 1 | 99 | 94 | |
| 15 | 52 | L | Vestibular schwannoma | Adult | 24 | no scan | 1699.4 | 267.6 | 10, 11, 12 | 1 | 93 | 77 | |
| ECochG Recording Site | Hearing Preservation Frequency | Intercept (p-Value) | Slope (p-Value) | r2 |
|---|---|---|---|---|
| RE1 | 500 Hz | 24.13 (0.15) | 0.54 (0.05) | 0.36 |
| RE* | 500 Hz | 66.36 (0.08) | −0.17 (0.70) | 0.02 |
| RE1 | Average of 500, 1000, 2000 Hz | 24.50 (0.12) | 0.54 (0.04) | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheperle, R.; Etler, C.; Oleson, J.; Dunn, C.; Kashani, R.; Claussen, A.; Gantz, B.J.; Hansen, M.R. Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position. J. Clin. Med. 2023, 12, 7409. https://doi.org/10.3390/jcm12237409
Scheperle R, Etler C, Oleson J, Dunn C, Kashani R, Claussen A, Gantz BJ, Hansen MR. Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position. Journal of Clinical Medicine. 2023; 12(23):7409. https://doi.org/10.3390/jcm12237409
Chicago/Turabian StyleScheperle, Rachel, Christine Etler, Jacob Oleson, Camille Dunn, Rustin Kashani, Alexander Claussen, Bruce J. Gantz, and Marlan R. Hansen. 2023. "Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position" Journal of Clinical Medicine 12, no. 23: 7409. https://doi.org/10.3390/jcm12237409
APA StyleScheperle, R., Etler, C., Oleson, J., Dunn, C., Kashani, R., Claussen, A., Gantz, B. J., & Hansen, M. R. (2023). Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position. Journal of Clinical Medicine, 12(23), 7409. https://doi.org/10.3390/jcm12237409








