The Effect of Bariatric Surgery on Microvascular Structure and Function, Peripheral Pressure Waveform and General Cardiovascular Risk: A Longitudinal Study
Abstract
:1. Introduction
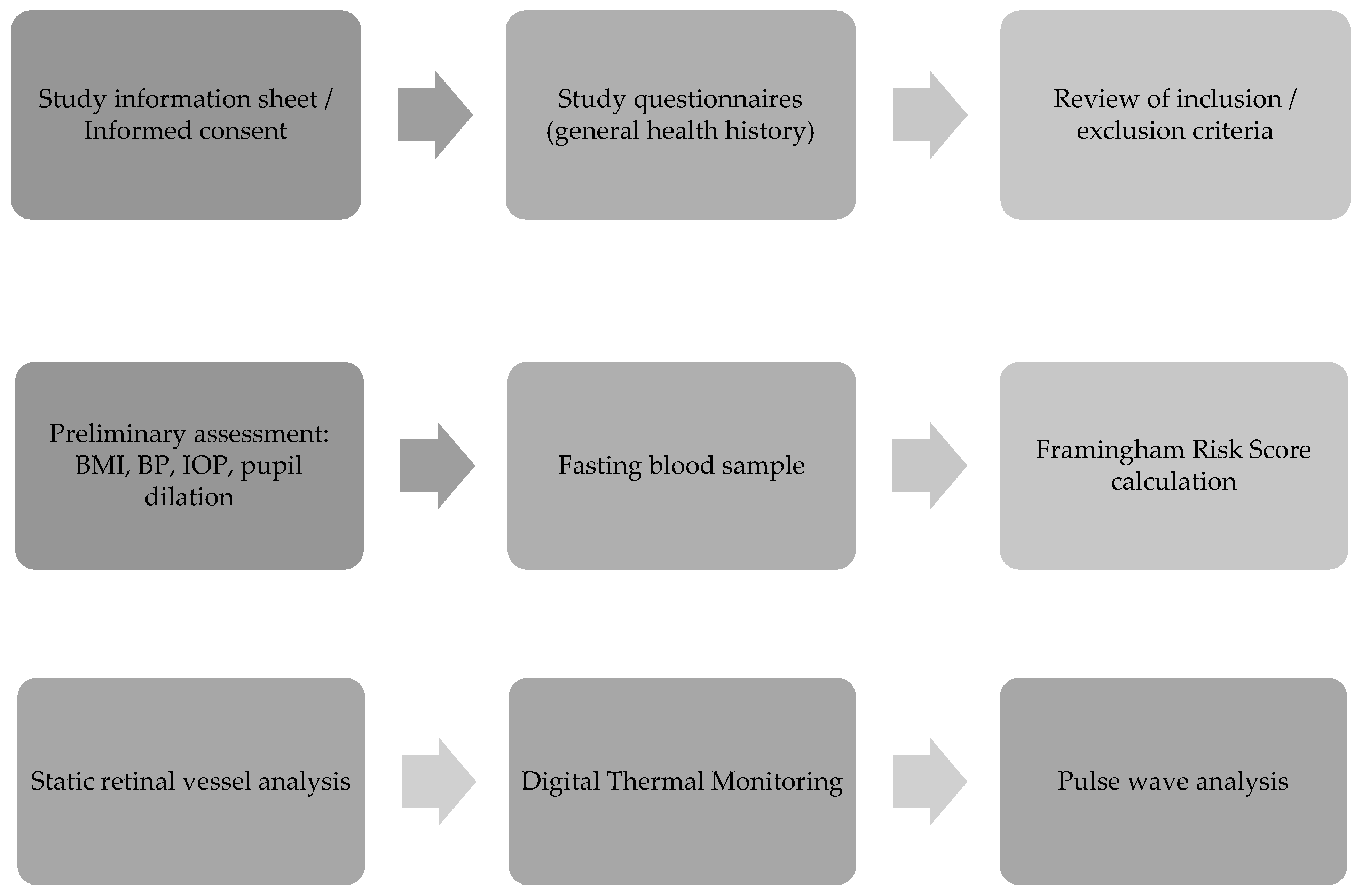
2. Materials and Methods
2.1. Patient Recruitment
2.2. Demographic and General Health History
2.3. Blood Analyses
2.4. Framingham Risk Score (FRS)
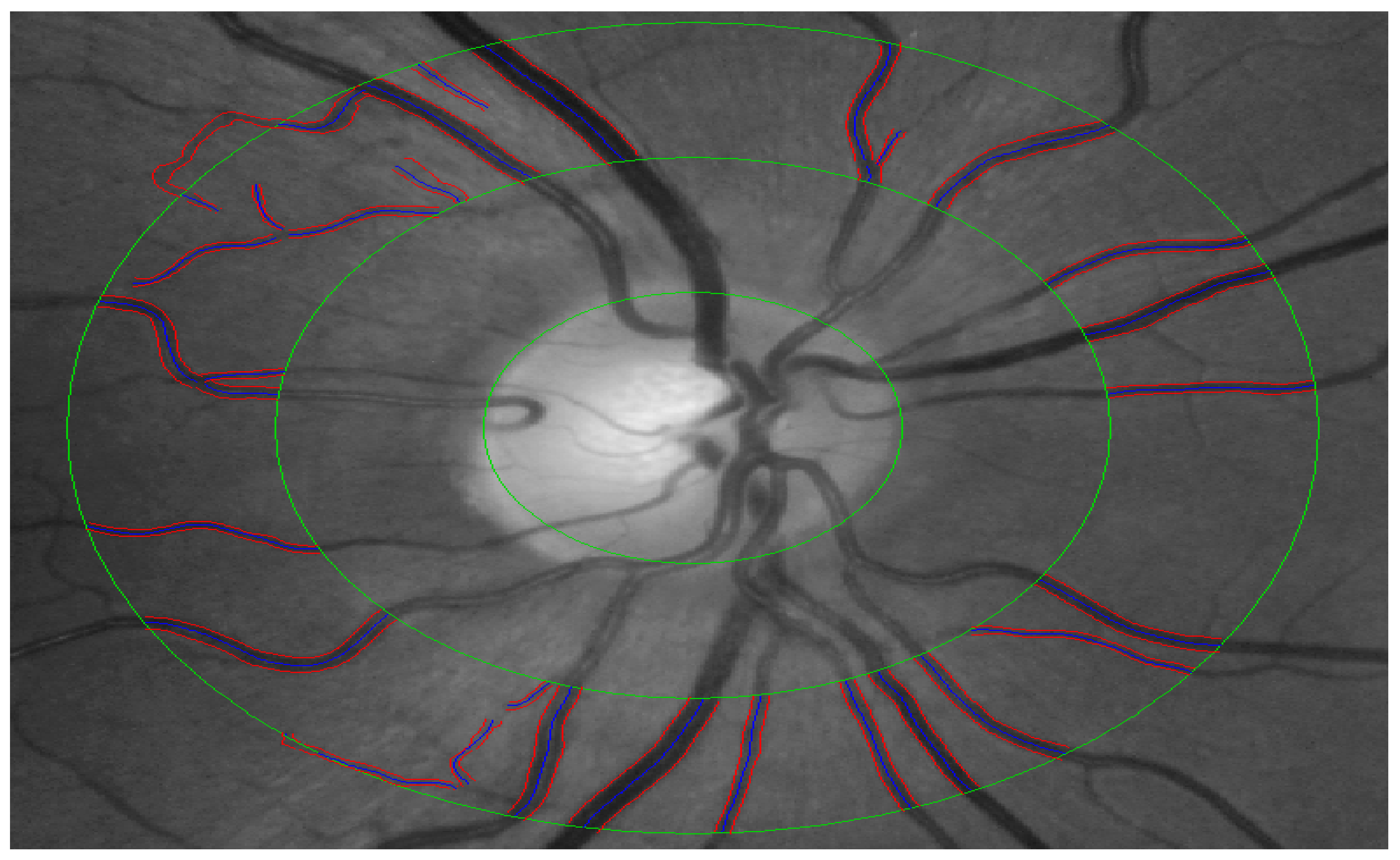
2.5. Quantification of Retinal Vessel Calibre (CRAE, CRVE and AVR)
2.6. Digital Thermal Monitoring (DTM)
2.7. Pulse Wave Analysis (PWA)
2.8. Statistical Analysis
2.9. Power Calculations
3. Results
3.1. Clinical Characteristics
3.2. Retinal Microcirculation Assessment
3.3. Assessments of Peripheral Vascular Function and Peripheral Pressure Waveforms
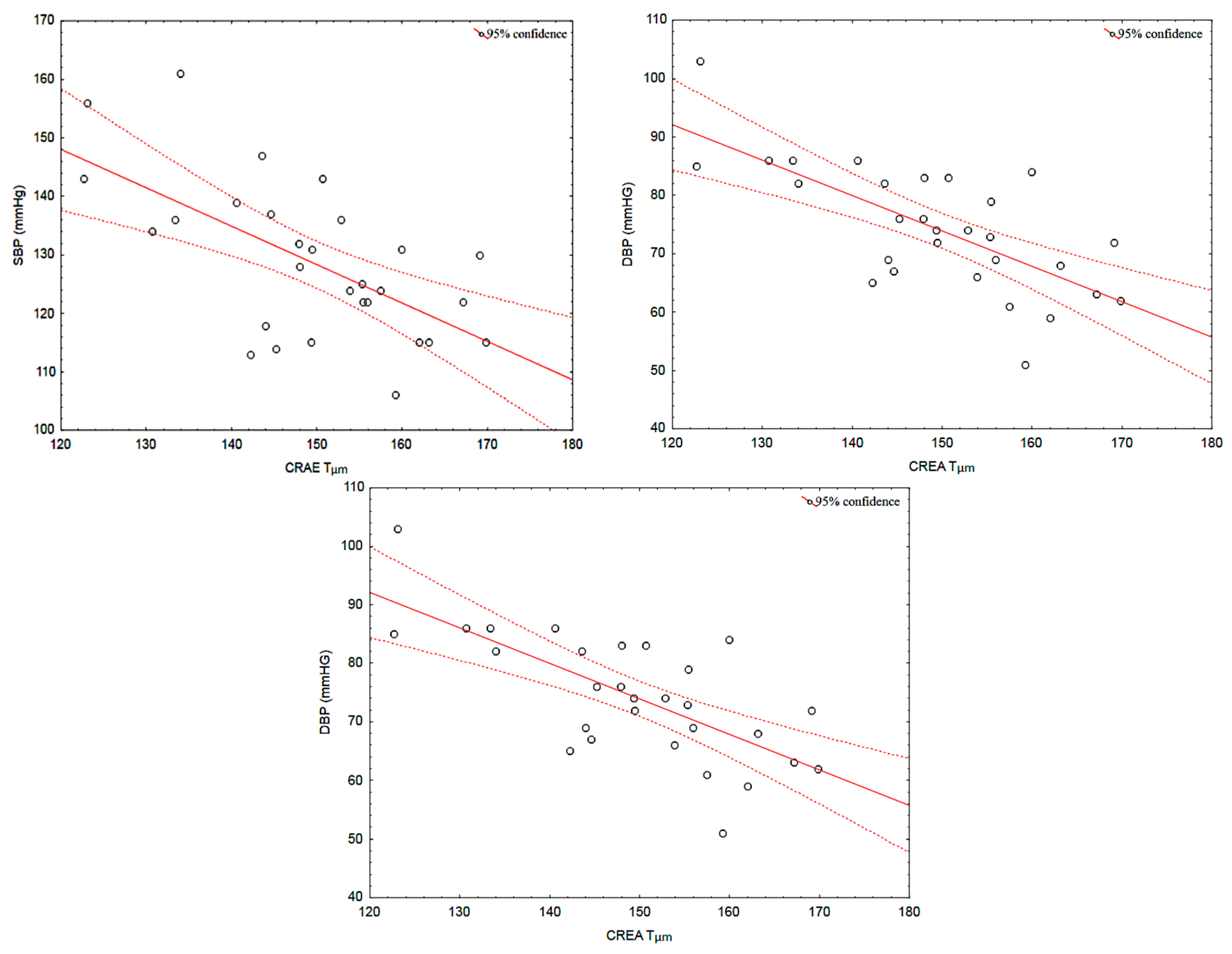
3.4. Correlations between Retinal Vessel Calibres and Systemic Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Clinical Implications of Obesity with Specific Focus on Cardiovascular Disease. Circulation 2004, 110, 2952–2967. [CrossRef]
- Shokr, H.; Dias, I.H.; Gherghel, D. Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants 2021, 10, 1756. [Google Scholar] [CrossRef]
- Karimzad, S.; Bilkhu, P.S.; Wolffsohn, J.S.; Bellary, S.; Shokr, H.; Singhal, R.; Gherghel, D. Impact of Bariatric Surgery-Induced Weight Loss on Anterior Eye Health in Patients with Obesity. Nutrients 2022, 14, 2462. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowska, S.; Shokr, H.; Benavente-Pérez, A.; Negi, A.; Bentham, P.; Gherghel, D. Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients. J. Clin. Med. 2022, 11, 6702. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Bellary, S.; Karimzad, S.; Gherghel, D. Overweight Status Is Associated with Extensive Signs of Microvascular Dysfunction and Cardiovascular Risk. Sci. Rep. 2016, 6, 32282. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H. Consensus Conference Panel Bariatric Surgery for Morbid Obesity: Health Implications for Patients, Health Professionals, and Third-Party Payers. J. Am. Coll. Surg. 2005, 200, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Consensus Development Conference Panel. Gastrointestinal Surgery for Severe Obesity. Consens. Statement 1991, 9, 1–20. [Google Scholar]
- Lammert, A.; Hasenberg, T.; Kräupner, C.; Schnülle, P.; Hammes, H.-P. Improved Arteriole-to-Venule Ratio of Retinal Vessels Resulting from Bariatric Surgery. Obesity 2012, 20, 2262–2267. [Google Scholar] [CrossRef]
- Bariatric Surgery: A Systematic Review and Meta-Analysis. JAMA 2004, 292, 1724–1737. [CrossRef] [PubMed]
- Pories, W.J.; Swanson, M.S.; MacDonald, K.G.; Long, S.B.; Morris, P.G.; Brown, B.M.; Barakat, H.A.; deRamon, R.A.; Israel, G.; Dolezal, J.M. Who Would Have Thought It? An Operation Proves to Be the Most Effective Therapy for Adult-Onset Diabetes Mellitus. Ann. Surg. 1995, 222, 339–352. [Google Scholar] [CrossRef]
- Sampalis, J.S.; Sampalis, F.; Christou, N. Impact of Bariatric Surgery on Cardiovascular and Musculoskeletal Morbidity. Surg. Obes. Relat. Dis. 2006, 2, 587–591. [Google Scholar] [CrossRef]
- Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [CrossRef] [PubMed]
- Bariatric Surgery: Remission of Inflammation, Cardiometabolic Benefits, and Common Adverse Effects. J. Endocr. Soc. 2020, 4, bvaa049. [CrossRef] [PubMed]
- Cardiovascular Effects of Bariatric Surgery. Nat. Rev. Cardiol. 2016, 13, 730–743. [CrossRef]
- Jamialahmadi, T.; Reiner, Ž.; Alidadi, M.; Kroh, M.; Simental-Mendia, L.E.; Pirro, M.; Sahebkar, A. Impact of Bariatric Surgery on Pulse Wave Velocity as a Measure of Arterial Stiffness: A Systematic Review and Meta-Analysis. Obes. Surg. 2021, 31, 4461–4469. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, T.; Alidadi, M.; Atkin, S.L.; Kroh, M.; Almahmeed, W.; Moallem, S.A.; Al-Rasadi, K.; Rodriguez, J.H.; Santos, R.D.; Ruscica, M.; et al. Effect of Bariatric Surgery on Flow-Mediated Vasodilation as a Measure of Endothelial Function: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4054. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Morris, R.W. Metabolic Syndrome vs. Framingham Risk Score for Prediction of Coronary Heart Disease, Stroke, and Type 2 Diabetes Mellitus. Arch. Intern. Med. 2005, 165, 2644–2650. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [CrossRef] [PubMed]
- SCORE2-OP working group and ESC Cardiovascular risk collaboration. SCORE2-OP Risk Prediction Algorithms: Estimating Incident Cardiovascular Event Risk in Older Persons in Four Geographical Risk Regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef] [PubMed]
- Drobnjak, D.; Munch, I.C.; Glümer, C.; Faerch, K.; Kessel, L.; Larsen, M.; Veiby, N.C.B.B. Retinal Vessel Diameters and Their Relationship with Cardiovascular Risk and All-Cause Mortality in the Inter99 Eye Study: A 15-Year Follow-Up. J. Ophthalmol. 2016, 2016, 6138659. [Google Scholar] [CrossRef]
- Gopinath, B.; Chiha, J.; Plant, A.J.H.; Thiagalingam, A.; Burlutsky, G.; Kovoor, P.; Liew, G.; Mitchell, P. Associations between Retinal Microvascular Structure and the Severity and Extent of Coronary Artery Disease. Atherosclerosis 2014, 236, 25–30. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Tielsch, J.M.; Klein, B.E.K.; Hubbard, L.D. Retinal Arteriolar Narrowing and Risk of Coronary Heart Disease in Men and WomenThe Atherosclerosis Risk in Communities Study. JAMA 2002, 287, 1153–1159. [Google Scholar] [CrossRef]
- Kawasaki, R.; Xie, J.; Cheung, N.; Lamoureux, E.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Sharrett, A.R.; Shea, S.; Wong, T.Y.; et al. Retinal Microvascular Signs and Risk of Stroke. Stroke 2012, 43, 3245–3251. [Google Scholar] [CrossRef]
- Witt, N.; Wong, T.Y.; Hughes, A.D.; Chaturvedi, N.; Klein, B.E.; Evans, R.; McNamara, M.; McG Thom, S.A.; Klein, R. Abnormalities of Retinal Microvascular Structure and Risk of Mortality from Ischemic Heart Disease and Stroke. Hypertension 2006, 47, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, A.; Soinio, M.; Cheung, C.Y.-L.; Hannukainen, J.C.; Karlsson, H.K.; Wong, T.Y.; Hughes, A.D.; Salminen, P.; Nuutila, P.; Vesti, E.; et al. Effects of Bariatric Surgery on Retinal Microvascular Architecture in Obese Patients. Int. J. Obes. 2019, 43, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Karki, S.; Dobyns, A.; Zizza, E.; Sroczynski, E.; Palmisano, J.N.; Mazzotta, C.; Hamburg, N.M.; Pernar, L.I.; Carmine, B.; et al. Association of Bariatric Surgery with Vascular Outcomes. JAMA Netw. Open 2021, 4, e2115267. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; Luca, M.D.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Eae, A.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Leenen, F.; Myers, M.G. Automated Office Blood Pressure Measurement in the Management of Hypertension–Fourth in Series. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/Automated-office-blood-pressure-measurement-in-the-management-of-hypertension (accessed on 31 October 2023).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Framingham Risk Score–An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/framingham-risk-score#:~:text=The%20FRS%20utilizes%20age%2C%20gender,cardiac%20event%20in%2010%20years (accessed on 12 October 2023).
- Hemann, B.A.; Bimson, W.F.; Taylor, A.J. The Framingham Risk Score: An Appraisal of Its Benefits and Limitations. Am. Heart Hosp. J. 2007, 5, 91–96. [Google Scholar] [CrossRef]
- Heitmar, R.; Kalitzeos, A.A.; Patel, S.R.; Prabhu-Das, D.; Cubbidge, R.P. Comparison of Subjective and Objective Methods to Determine the Retinal Arterio-Venous Ratio Using Fundus Photography. J. Optom. 2015, 8, 252–257. [Google Scholar] [CrossRef]
- Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal and Peripheral Vascular Function in Healthy Individuals with Low Cardiovascular Risk. Microvasc. Res. 2019, 126, 103908. [Google Scholar] [CrossRef]
- Pulse Wave Analysis. Br. J. Clin. Pharmacol. 2001, 51, 507–522. [CrossRef] [PubMed]
- Ikram, M.K.; Witteman, J.C.M.; Vingerling, J.R.; Breteler, M.M.B.; Hofman, A.; De Jong, P.T.V.M. Retinal Vessel Diameters and Risk of Hypertension: The Rotterdam Study. Hypertension 2006, 47, 189–194. [Google Scholar] [CrossRef]
- Ikram, M.K.; Ong, Y.T.; Cheung, C.Y.; Wong, T.Y. Retinal Vascular Caliber Measurements: Clinical Significance, Current Knowledge and Future Perspectives. Ophthalmologica 2013, 229, 3. [Google Scholar] [CrossRef] [PubMed]
- Daien, V.; Kawasaki, R.; Villain, M.; Ribstein, J.; Du Cailar, G.; Mimran, A.; Fesler, P. Retinal Vascular Caliber Is Associated with Renal Function in Apparently Healthy Subjects. Acta Ophthalmol. 2013, 91, e283–e288. [Google Scholar] [CrossRef] [PubMed]
- Assessments of Arterial Stiffness and Endothelial Function Using Pulse Wave Analysis. Int. J. Vasc. Med. 2013, 2012, 903107. [CrossRef]
- Use of Temperature Alterations to Characterize Vascular Reactivity. Clin. Physiol. Funct. Imaging 2010, 31, 66–72. [CrossRef] [PubMed]
- Knudtson, M.D.; Lee, K.E.; Hubbard, L.D.; Wong, T.Y.; Klein, R.; Klein, B.E.K. Revised Formulas for Summarizing Retinal Vessel Diameters. Curr. Eye Res. 2009, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Jacobi, D.; Pichelin, M.; Cariou, B.; Mirallié, E.; Blanchard, C. Improvement in Arterial Stiffness (pOpmètre®) after Bariatric Surgery. Results from a Prospective Study. Ann. Endocrinol. 2020, 81, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Relationships between Age, Blood Pressure, and Retinal Vessel Diameters in an Older Population. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2900–2904. [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Klein, B.E.K.; Hubbard, L.D.; Nieto, F.J. Retinal Arteriolar Diameter and Risk for Hypertension. Ann. Intern. Med. 2004, 140, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Rochtchina, E.; Liew, G.; Tan, A.G.; Wong, T.Y.; Leeder, S.R.; Smith, W.; Shankar, A.; Mitchell, P. The Long-Term Relation among Retinal Arteriolar Narrowing, Blood Pressure, and Incident Severe Hypertension. Am. J. Epidemiol. 2008, 168, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Shokr, H.; Gherghel, D. Heart Association Guidelines on the Cut-off Values for Early Hypertension: A Microvascular Perspective. Sci. Rep. 2021, 11, 3473. [Google Scholar] [CrossRef] [PubMed]
- Shokr, H.; Lush, V.; Dias, I.H.; Ekárt, A.; De Moraes, G.; Gherghel, D. The Use of Retinal Microvascular Function and Telomere Length in Age and Blood Pressure Prediction in Individuals with Low Cardiovascular Risk. Cells 2022, 11, 3037. [Google Scholar] [CrossRef]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Sharrett, A.R.; Klein, B.E.K.; Wang, J.J.; Chambless, L.E.; Wong, T.Y. Risk Prediction of Coronary Heart Disease Based on Retinal Vascular Caliber (from the Atherosclerosis Risk in Communities [ARIC] Study). Am. J. Cardiol. 2008, 102, 58–63. [Google Scholar] [CrossRef]
- Wang, J.J.; Liew, G.; Klein, R.; Rochtchina, E.; Knudtson, M.D.; Klein, B.E.K.; Wong, T.Y.; Burlutsky, G.; Mitchell, P. Retinal Vessel Diameter and Cardiovascular Mortality: Pooled Data Analysis from Two Older Populations. Eur. Heart J. 2007, 28, 1984–1992. [Google Scholar] [CrossRef]
- Tapp, R.J.; Ness, A.; Williams, C.; Howe, L.D.; Tilling, K.; Witt, N.; Chaturvedi, N.; McG Thom, S.A.; Hughes, A.D. Differential Effects of Adiposity and Childhood Growth Trajectories on Retinal Microvascular Architecture. Microcirculation 2013, 20, 609–616. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wang, J.J.; Islam, F.M.A.; Mitchell, P.; Tapp, R.J.; Zimmet, P.Z.; Simpson, R.; Shaw, J.; Wong, T.Y. Retinal Arteriolar Narrowing Predicts Incidence of Diabetes: The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes 2008, 57, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, H.; Nickel, T.; Drexel, V.; Hertel, G.; Emslander, I.; Sisic, Z.; Lorang, D.; Schuster, T.; Kotliar, K.E.; Pressler, A.; et al. Exercise-Induced Alterations of Retinal Vessel Diameters and Cardiovascular Risk Reduction in Obesity. Atherosclerosis 2011, 216, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, A.; Galceran, I.; Goday, A.; Vázquez, S.; Sans, L.; Riera, M.; Benaiges, D.; Pascual, J. Improvement of Arterial Stiffness One Month after Bariatric Surgery and Potential Mechanisms. J. Clin. Med. 2021, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Yang, C.; Tanaka, H.; Coresh, J.; Ndumele, C.E.; Matsushita, K. Increase in Arterial Stiffness Measures after Bariatric Surgery. Atherosclerosis 2021, 320, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Zeegers, M.P.; Mensink, R.P. Weight Loss Improves Fasting Flow-Mediated Vasodilation in Adults: A Meta-Analysis of Intervention Studies. Atherosclerosis 2015, 239, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Di Minno, M.N.D.; Guidone, C.; Cefalo, C.; Capaldo, B.; Riccardi, G.; Mingrone, G. Effects of Bariatric Surgery on Markers of Subclinical Atherosclerosis and Endothelial Function: A Meta-Analysis of Literature Studies. Int. J. Obes. 2016, 40, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bigornia, S.J.; Mott, M.M.; Hess, D.T.; Apovian, C.M.; McDonnell, M.E.; Duess, M.-A.; Kluge, M.A.; Fiscale, A.J.; Vita, J.A.; Gokce, N. Long-Term Successful Weight Loss Improves Vascular Endothelial Function in Severely Obese Individuals. Obesity 2010, 18, 754–759. [Google Scholar] [CrossRef]
- Gul, K.M.; Ahmadi, N.; Wang, Z.; Jamieson, C.; Nasir, K.; Metcalfe, R.; Hecht, H.S.; Hartley, C.J.; Naghavi, M. Digital Thermal Monitoring of Vascular Function: A Novel Tool to Improve Cardiovascular Risk Assessment. Vasc. Med. 2009, 14, 143–148. [Google Scholar] [CrossRef]
- Joannides, R.; Haefeli, W.E.; Linder, L.; Richard, V.; Bakkali, E.H.; Thuillez, C.; Lüscher, T.F. Nitric Oxide Is Responsible for Flow-Dependent Dilatation of Human Peripheral Conduit Arteries In Vivo. Circulation 1995, 91, 1314–1319. [Google Scholar] [CrossRef]
- Larijani, V.N.; Ahmadi, N.; Zeb, I.; Khan, F.; Flores, F.; Budoff, M. Beneficial Effects of Aged Garlic Extract and Coenzyme Q10 on Vascular Elasticity and Endothelial Function: The FAITH Randomized Clinical Trial. Nutrition 2013, 29, 71–75. [Google Scholar] [CrossRef]
- Chew, S.K.H.; Xie, J.; Wang, J.J. Retinal Arteriolar Diameter and the Prevalence and Incidence of Hypertension: A Systematic Review and Meta-Analysis of Their Association. Curr. Hypertens. Rep. 2012, 14, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, Inflammation and Innate Immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P. High-Density Lipoprotein and Cardiovascular Risk. Circulation 2004, 109, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Sato, H.; Hashimoto, K.; Osada, U.; Hariya, T.; Nakayama, H.; Asano, T.; Suzuki, N.; Okabe, T.; Yamazaki, M.; et al. Carotid Artery Intima-Media Thickness, HDL Cholesterol Levels, and Gender Associated with Poor Visual Acuity in Patients with Branch Retinal Artery Occlusion. PLoS ONE 2020, 15, e0240977. [Google Scholar] [CrossRef]
- Kim, J.; Lim, D.H.; Han, K.; Kang, S.W.; Ham, D.-I.; Kim, S.J.; Chung, T.-Y. Retinal Vein Occlusion Is Associated with Low Blood High-Density Lipoprotein Cholesterol: A Nationwide Cohort Study. Am. J. Ophthalmol. 2019, 205, 35–42. [Google Scholar] [CrossRef]
- Zheng, C.; Lin, Y.; Jiang, B.; Zhu, X.; Lin, Q.; Luo, W.; Tang, M.; Xie, L. Plasma Lipid Levels and Risk of Retinal Vascular Occlusion: A Genetic Study Using Mendelian Randomization. Front. Endocrinol. 2022, 13, 954453. [Google Scholar] [CrossRef]
- Yang, Y.; Han, K.; Park, S.H.; Kim, M.K.; Yoon, K.-H.; Lee, S.-H. High-Density Lipoprotein Cholesterol and the Risk of Myocardial Infarction, Stroke, and Cause-Specific Mortality: A Nationwide Cohort Study in Korea. J. Lipid. Atheroscler. 2021, 10, 74–87. [Google Scholar] [CrossRef]
- Habib, P.; Scrocco, J.D.; Terek, M.; Vanek, V.; Mikolich, J.R. Effects of Bariatric Surgery on Inflammatory, Functional and Structural Markers of Coronary Atherosclerosis. Am. J. Cardiol. 2009, 104, 1251–1255. [Google Scholar] [CrossRef]

| Variables | Baseline Mean (SD) | Follow Up Mean (SD) (12 Months after the RYGB Surgery) | p-Value |
|---|---|---|---|
| BMI (kg/m2) | 49.2 (7.69) | 38.38 (7.84) | <0.001 * |
| WC cm | 137.17 (20.42) | 112.04 (24.21) | <0.001 * |
| NC cm | 42.58 (4.74) | 38.03 (4.18) | <0.001 * |
| SBP (mmHg) | 144.24 (14.35) | 128.75 (13.23) | <0.001 * |
| DBP (mmHg) | 78.96 (11.35) | 73.34 (10.84) | 0.039 * |
| MAP (mmHg) | 100.72 (10.58) | 92.48 (10.94) | <0.001 * |
| HR (bpm) | 74.10 (13.45) | 69.17 (9.88) | 0.046 * |
| IOP (mmHg) | 15 (2.40) | 12.58 (1.95) | <0.001 * |
| OPP | 52.14 (8.01) | 49.06 (8.11) | 0.026 * |
| CHOL (mmol/L) | 4.90 (1.21) | 4.53 (0.97) | 0.003 * |
| HDL-C (mmol/L) | 1.24 (0.35) | 1.51 (0.40) | <0.001 * |
| LDL-C (mmol/L) | 2.97 (1.01) | 2.49 (0.81) | <0.001 * |
| TG (mmol/L) | 1.44 (0.72) | 1.13 (0.50) | 0.002 * |
| GLUC (mmol/L) | 5.75 (0.75) | 5.37 (0.42) | 0.004 * |
| FRS% | 12.00 (7.73) | 6.51 (5.17) | <0.001 * |
| Parameter | Baseline Mean (SD) | Follow Up Mean (SD) (12 Months after the RYGB Surgery) | p-Value |
|---|---|---|---|
| CRAE (μm) | 143.70 (13.97) | 149.31 (12.05) | 0.003 * |
| CRVE (μm) | 204.72 (23.26) | 213.24 (20.75) | 0.007 * |
| AVR | 0.67 (0.08) | 0.65 (0.08) | 0.068 |
| Parameter | Baseline Mean (SD) | Follow Up Mean (SD) (12 Months after the RYGB Surgery) | p-Value |
|---|---|---|---|
| aTR | 1.71 (1.02) | 2.39 (1.04) | 0.008 * |
| AUCtr | 264.51 (159.18) | 360.96 (181.40) | 0.025 * |
| Alx | 25.79 (8.85) | 20.10 (10.45) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimzad, S.; Shokr, H.; Bellary, S.; Singhal, R.; Gherghel, D. The Effect of Bariatric Surgery on Microvascular Structure and Function, Peripheral Pressure Waveform and General Cardiovascular Risk: A Longitudinal Study. J. Clin. Med. 2023, 12, 7379. https://doi.org/10.3390/jcm12237379
Karimzad S, Shokr H, Bellary S, Singhal R, Gherghel D. The Effect of Bariatric Surgery on Microvascular Structure and Function, Peripheral Pressure Waveform and General Cardiovascular Risk: A Longitudinal Study. Journal of Clinical Medicine. 2023; 12(23):7379. https://doi.org/10.3390/jcm12237379
Chicago/Turabian StyleKarimzad, Said, Hala Shokr, Srikanth Bellary, Rishi Singhal, and Doina Gherghel. 2023. "The Effect of Bariatric Surgery on Microvascular Structure and Function, Peripheral Pressure Waveform and General Cardiovascular Risk: A Longitudinal Study" Journal of Clinical Medicine 12, no. 23: 7379. https://doi.org/10.3390/jcm12237379
APA StyleKarimzad, S., Shokr, H., Bellary, S., Singhal, R., & Gherghel, D. (2023). The Effect of Bariatric Surgery on Microvascular Structure and Function, Peripheral Pressure Waveform and General Cardiovascular Risk: A Longitudinal Study. Journal of Clinical Medicine, 12(23), 7379. https://doi.org/10.3390/jcm12237379






