Splanchnic Vein Thrombosis in Inflammatory Bowel Disease: An Observational Study from the ENEIDA Registry and Systematic Review
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
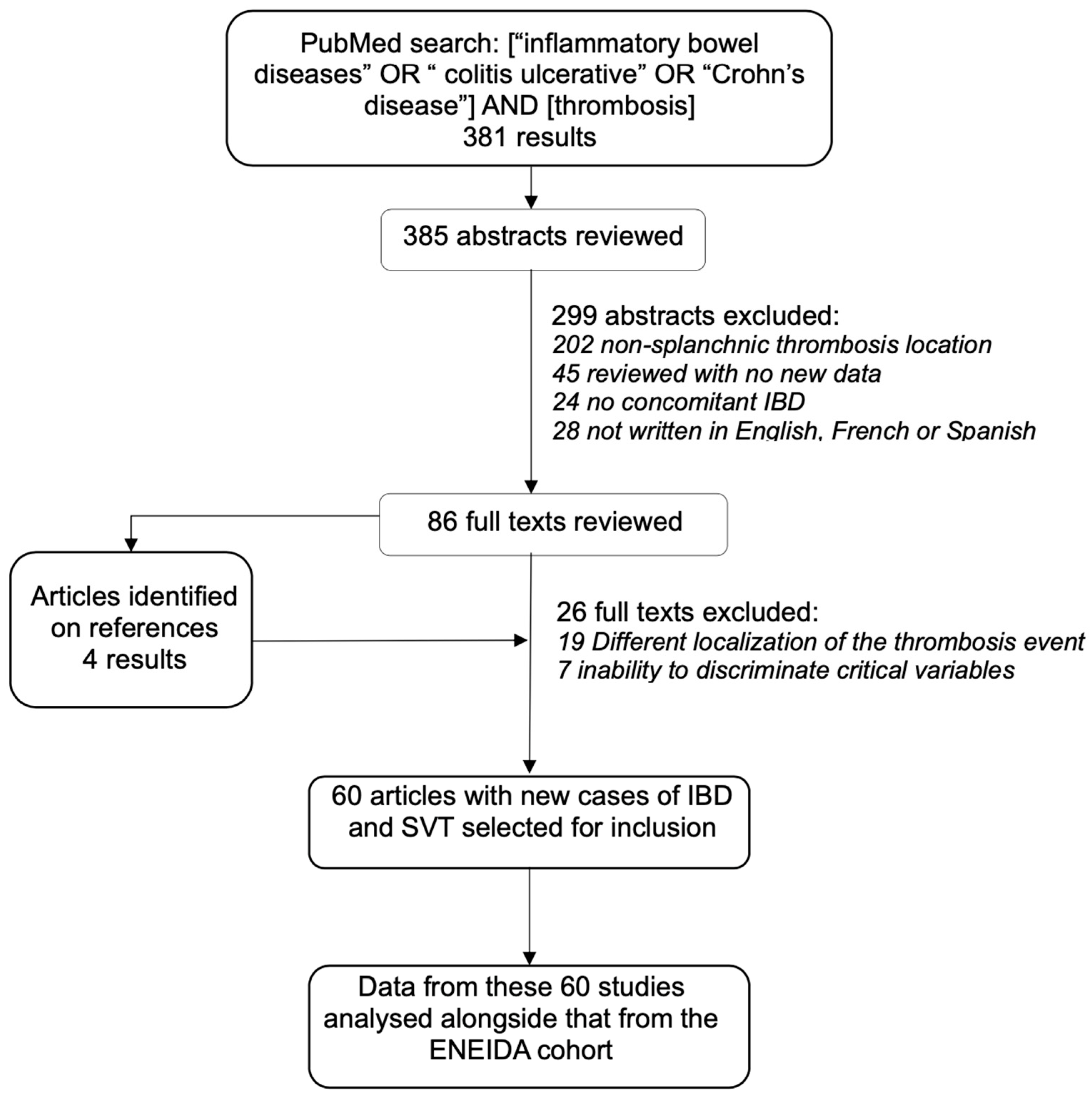
2.4. Systematic Review
2.5. Data Collection and Analysis
3. Results
3.1. ENEIDA Cohort
3.2. Systematic Review
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| IBD | Inflammatory bowel disease |
| LMWH | Low-molecular-weight heparin |
| MRI | Magnetic resonance imaging |
| SVT | Splanchnic venous thrombosis |
Appendix A. Complete Affiliations of the ENEIDA-GETECCU Investigators
References
- Intagliata, N.M.; Caldwell, S.H.; Tripodi, A. Diagnosis, development and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology 2019, 156, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Condat, B.; Valla, D. Nonmalignant portal vein thrombosis in adults. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Grainge, M.J.; West, J.; Card, T.R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Violi, N.; Schoepfer, A.M.; Fournier, N.; Guiu, B.; Bize, P.; Denys, A. Swiss Inflammatory Bowel Disease Cohort Study Group. Prevalence and clinical Importance of mesenteric venous thrombosis in the Swiss Inflammatory Bowel Disease Cohort. AJR Am. J. Roentgenol. 2014, 203, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Talbot, R.W.; Heppell, J.; Dozois, R.R.; Beart, R.W. Vascular complications of inflammatory bowel disease. Mayo Clin. Proc. 1986, 61, 140–145. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Sam, J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 2008, 103, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Papa, A. Review article: Inherited thrombophilia in inflammatory bowel disease. Am. J. Gastroenterol. 2003, 98, 1247–1251. [Google Scholar] [CrossRef]
- Murthy, S.K.; Nguyen, G.C. Venous thromboembolism in inflammatory bowel disease: An epidemiological review. Am. J. Gastroenterol. 2011, 106, 713–718. [Google Scholar] [CrossRef]
- Papay, P.; Miehsler, W.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; Fuchssteiner, H.; et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 723–729. [Google Scholar] [CrossRef]
- Menchén, L.; Marín-Jiménez, I.; Arias-Salgado, E.G.; Fontela, T.; Hernández-Sampelayo, P.; Rodríguez, M.C.; Butta, N.V. Matrix metalloproteinase 9 is involved in Crohn’s disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut 2009, 58, 920–928. [Google Scholar] [CrossRef]
- Landman, C.; Nahon, S.; Cosnes, J.; Bouhnik, Y.; Brixi-Benmansour, H.; Bouguen, G.; Colombel, J.F.; Savoye, G.; Coffin, B.; Abitbol, V.; et al. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Remzi, F.H.; Fazio, V.W.; Oncel, M.; Fazio, V.W.; Oncel, M.; Baker, M.E.; Church, J.M.; Ooi, B.S.; Connor, J.T.; Preen, M.; et al. Portal vein thrombi after restorative proctocolectomy. Surgery 2002, 132, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Edelman, R.R.; Chopra, S. Portal vein thrombosis: A review. Am. J. Med. 1992, 92, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, O.A.; Spinelli, K.S.; Abu-Hajir, M.; Attila, T.; Franco, J.; Otterson, M.F.; Telford, G.L.; Binion, D.G. Mesenteric Venous Thrombosis in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2005, 39, 27–31. [Google Scholar] [PubMed]
- Bruining, D.H.; Siddiki, H.A.; Fletcher, J.G.; Tremaine, W.J.; Sandborn, W.J.; Loftus, E.V., Jr. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm. Bowel Dis. 2008, 14, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fujiya, M.; Nomura, Y.; Inaba, Y.; Sugiyama, Y.; Iwama, T.; Ijiri, M.; Takahashi, K.; Tanaka, K.; Sakatani, A.; et al. The incidence and risk factors of venous thromboembolism in Japanese inpatients with inflammatory bowel disease: A retrospective cohort study. Intest. Res. 2018, 16, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-De Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, M.; Dick, A.D.; et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Zabana, Y.; Panés, J.; Nos, P.; Gomollón, F.; Esteve, M.; García-Sánchez, V.; Gisbert, J.P.; Barreiro-de Acosta, M.; Domènech, E. The ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU: Design, monitoring and functions. Gastroenterol. Hepatol. 2020, 43, 551–558. [Google Scholar] [CrossRef]
- Naymagon, L.; Tremblay, D.; Zubizarreta, N.; Moshier, E.; Naymagon, S.; Mascarenhas, J.; Schiano, T. The Natural History, Treatments, and Outcomes of Portal Vein Thrombosis in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 215–223. [Google Scholar] [CrossRef]
- Arora, Z.; Wu, X.; Navaneethan, U.; Shen, B. Non-surgical porto-mesenteric vein thrombosis is associated with worse long-term outcomes in inflammatory bowel diseases. Gastroenterol. Rep. 2015, 4, 210–215. [Google Scholar] [CrossRef]
- Fichera, A.; Cicchiello, L.A.; Mendelson, D.S.; Greenstein, A.J.; Heimann, T.M. Superior mesenteric vein thrombosis after colectomy for inflammatory bowel disease: A not uncommon cause of postoperative acute abdominal pain. Dis. Colon. Rectum 2003, 46, 643–648. [Google Scholar] [CrossRef]
- Leung, G.G.; Sivasankaran, M.V.; Choi, J.J.; Divino, C.M. Risk factors of portal vein thrombosis in Crohn’s disease patients. J. Gastrointest. Surg. 2012, 16, 1199–1203. [Google Scholar] [CrossRef]
- Antiel, R.M.; Hashim, Y.; Moir, C.R.; Rodriguez, V.; Elraiyah, T.; Zarroug, A.E. Intra-abdominal venous thrombosis after colectomy in pediatric patients with chronic ulcerative colitis: Incidence, treatment, and outcomes. J. Pediatr. Surg. 2014, 49, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Kayal, M.; Radcliffe, M.; Plietz, M.; Rosman, A.; Greenstein, A.; Khaitov, S.; Sylla, P.; Dubinsky, M.C. Portomesenteric venous thrombosis in patients undergoing surgery for medically refractory ulcerative colitis. Inflamm. Bowel Dis. 2020, 26, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Capron, J.P.; Remond, A.; Lebrec, D.; Delamarre, J.; Dupas, J.L.; Lorriaux, A. Gastrointestinal bleeding due to chronic portal vein thrombosis in ulcerative colitis. Dig. Dis. Sci. 1979, 24, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Reh, T.E.; Srivisal, S.; Schmidt, E.H. III Portal venous thrombosis in ulcerative colitis: CT diagnosis with angiographic correlation. J. Comput. Assist. Tomogr. 1980, 4, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Chesner, I.M.; Muller, S.; Newman, J. Ulcerative colitis complicated by Budd-Chiari syndrome. Gut 1986, 27, 1096–1100. [Google Scholar] [CrossRef]
- Brinson, R.R.; Curtis, W.D.; Schuman, B.M.; Mills, L.R. Recovery from hepatic vein thrombosis (Budd-Chiari syndrome) complicating ulcerative colitis. Dig. Dis. Sci. 1988, 33, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Brinberg, D.E.; Stefansson, T.B.; Greicius, F.A.; Kahlam, S.S.; Molin, C. Portal vein thrombosis in Crohn’s disease. Gastrointest. Radiol. 1991, 16, 245–247. [Google Scholar] [CrossRef]
- Crowe, A.; Taffinder, N.; Layer, G.T.; Irvine, A.; Nicholls, R.J. Portal vein thrombosis in a complicated case of Crohn’s disease. Postgrad. Med. J. 1992, 68, 291–293. [Google Scholar] [CrossRef]
- Mathieu, E.; Fain, O.; Trinchet, J.C.; Aurousseau, M.H.; Stérin, D.; Thomas, M. La thrombose porte: Une complication exceptionnelle de la maladie de Crohn. Rev. Med. Interne 1994, 15, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.Y.; Johnson, J.L.; Liacouras, C.A. Portal-mesenteric pylephlebitis with hepatic abscesses in a patient with Crohn’s disease treated successfully with anticoagulation and antibiotics. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 474–478. [Google Scholar] [CrossRef]
- Martin, E.C.; Laffey, K.J.; Bixon, R. Recanalization of the superior mesenteric vein for massive bleeding in Crohn disease. J. Vasc. Interv. Radiol. 1995, 6, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Ihara, T.; Sasaki, M.; Inoue, H.; Fujiyama, Y.; Bamba, T. Effectiveness of combined anticoagulant therapy for extending portal vein thrombosis in Crohn’s disease. Report. of a case. Dis. Colon. Rectum 1996, 39, 823–825. [Google Scholar] [CrossRef]
- Kraut, J.; Berman, J.H.; Gunasekaran, T.S.; Allen, R.; McFadden, J.; Messersmith, R.; Pelletiere, E. Hepatic vein thrombosis (Budd-Chiari syndrome) in an adolescent with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 417–420. [Google Scholar] [CrossRef]
- Yada, S.; Hizawa, K.; Aoyagi, K.; Hashizume, M.; Matsumoto, T.; Koga, H.; Fujishima, M. Portal hypertensive gastropathy due to chronic portal vein occlusion in Crohn’s disease. Am. J. Gastroenterol. 1998, 93, 1376–1377. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, M.H.; Moritz, M.J.; Ferber, A.; Fry, R.D. Budd-chiari syndrome complicating restorative proctocolectomy for ulcerative colitis: Report of a case. Dis. Colon. Rectum 1999, 42, 1220–1224. [Google Scholar] [CrossRef]
- Baddley, J.W.; Singh, D.; Correa, P.; Persich, N.J. Crohn’s disease presenting as septic thrombophlebitis of the portal vein (pylephlebitis): Case report and review of the literature. Am. J. Gastroenterol. 1999, 94, 847–849. [Google Scholar] [CrossRef]
- Farkas, L.M.; Nelson, R.L.; Abcarian, H. A case of portal venous system thrombosis in ulcerative colitis. J. Am. Coll. Surg. 2000, 190, 94. [Google Scholar] [CrossRef]
- Hagimoto, T.; Seo, M.; Okada, M.; Shirotani, T.; Tanaka, K.; Tomita, A.; Oda, T.; Iida, T. Portal vein thrombosis successfully treated with a colectomy in active ulcerative colitis: Report of a case. Dis. Colon. Rectum 2001, 44, 587–590. [Google Scholar] [CrossRef]
- Lambley, J.; Ho, Y.-H. Mesenteric vein thrombosis after proctocolectomy for inflammatory bowel disease. Dis. Colon. Rectum 2003, 46, 1715. [Google Scholar] [CrossRef]
- Decaens, T.; Maitre, S.; Marfaing, A.; Naveau, S.; Chaput, J.-C.; Mathurin, P. Inflammatory bowel disease and latent thrombocythemia: A novel cause of hepatic vein thrombosis? Gastroenterol. Clin. Biol. 2004, 28, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Larghi, A.; Faddoul, S.G.; Stein, J.A.; Worman, H.J. Portal vein thrombosis associated with Fusobacterium nucleatum septicemia in a patient with ulcerative colitis. J. Clin. Gastroenterol. 2004, 38, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Fior, F.; Halmos, O.; Veraldi, G.F.; Rossaro, L.; Ruzzenente, A.; Cordiano, C. Transhepatic fibrinolysis of mesenteric and portal vein thrombosis in a patient with ulcerative colitis: A case report. World J. Gastroenterol. 2005, 11, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.S.; Gasparaitis, A.; Hurst, R.; Hanauer, S.B.; Rubin, D.T. Superior mesenteric vein thrombosis after colectomy in a patient with Crohn’s disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Sanz-García, C.; Catalina-Rodríguez, M.V.; Nogales-Rincón, O.; Matilla-Peña, A.; Núñez-Martínez, O.; Clemente-Ricote, G. Massive abdominal vein thrombosis with acute liver failure and toxic megacolon as onset of ulcerative colitis. Gastroenterol. Hepatol. 2005, 28, 551–554. [Google Scholar] [CrossRef]
- Rahhal, R.M.; Pashankar, D.S.; Bishop, W.P. Ulcerative colitis complicated by ischemic colitis and Budd Chiari syndrome. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 94–97. [Google Scholar] [CrossRef]
- Shaked, G.; Czeiger, D.; Rozental, A. Acute portal vein occlusion in a patient with Crohn’s disease. J. Gastroenterol. Hepatol. 2005, 20, 1472–1473. [Google Scholar] [CrossRef]
- Ng, S.S.-M. Portal venous gas and thrombosis in a Chinese patient with fulminant Crohn’s colitis: A case report with literature review. World J. Gastroenterol. 2006, 12, 5582. [Google Scholar] [CrossRef]
- Aguas, M.; Bastida, G.; Nos, P.; Beltrán, B.; Grueso, J.L.; Grueso, J. Septic thrombophlebitis of the superior mesenteric vein and multiple liver abscesses in a patient with Crohn’s disease at onset. BMC Gastroenterol. 2007, 7, 22. [Google Scholar] [CrossRef]
- Palkovits, J.; Häfner, M.; Rand, T.; Vogelsang, H.; Kutilek, M.; Gangl, A.; Novacek, G. Portal vein thrombosis in ulcerative colitis complicated by bleeding from gastric varices. Inflamm. Bowel Dis. 2007, 13, 365–366. [Google Scholar] [CrossRef]
- Racine, A.; Nahon, S.; Jouannaud, V.; Caugant, H.; Lesgourgues, B. Portal vein thrombosis in a patient with quiescent Crohn’s disease associated with hyperhomocysteinemia and antiphospholipid antibody syndrome 1-year after an ileocecal resection. Am. J. Gastroenterol. 2008, 103, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Latzman, G.S.; Kornbluth, A.; Murphy, S.J.; Legnani, P.; George, J.; Guller, J.; Lewin, S.; Carroccio, A.; Harris, M.T. Use of an intravascular thrombectomy device to treat life-threatening venous thrombosis in a patient with Crohn’s disease and G20210A prothrombin gene mutation. Inflamm. Bowel Dis. 2007, 13, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Ryzko, J.; Janczyk, W.; Dzik, E.; Iwanczak, B.; Krzesiek, E. Hepatic vein thrombosis as a complication of ulcerative colitis in a 12-year-old patient. Dig. Dis. Sci. 2007, 52, 1293–1298. [Google Scholar] [CrossRef]
- Di Fabio, F.; Obrand, D.; Satin, R.; Gordon, P.H. Successful treatment of extensive splanchnic arterial and portal vein thrombosis associated with ulcerative colitis. Colorectal Dis. 2009, 11, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, T.; Mpoumponaris, A.; Giouleme, O.; Hatzidakis, A.; Patsiaoura, K.; Zezos, P.; Vakalopoulou, S.; Kargiotis, K.; Gkisakis, D.; Katsinelos, P.; et al. Late onset ulcerative colitis complicating a patient with Budd-Chiari syndrome: A case report and review of the literature. Eur. J. Gastroenterol. Hepatol. 2009, 21, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Valdés Mas, M.; Martínez Pascual, C.; Egea Valenzuela, J.; Martínez Bonil, M.C.; Vargas Acosta, A.M.; Ortiz Sánchez, M.L.; Miras López, M.; Carballo Alvarez, F. Bilateral pulmonary thromboembolism and Budd-Chiari syndrome in a patient with Crohn’s disease on oral contraceptives. Rev. Esp. Enferm. 2009, 101, 645–652. [Google Scholar] [CrossRef]
- Georgescu, E.; Dumitrescu, D.; Ionescu, R. Portal cavernoma in a patient with Crohn’s disease associated with factor V Leiden mutation and antiphospholipid syndrome. J. Gastrointestin Liver Dis. 2010, 19, 449–452. [Google Scholar]
- Ibele, A.R.; Kennedy, G.D.; Lund, D.P.; Nichol, P.F. Portal vein thrombus after pediatric proctocolectomy with ileoanal anastomosis. J. Pediatr. Surg. 2010, 45, 1026–1029. [Google Scholar] [CrossRef]
- Lefèvre, A.; Soyer, P.; Vahedi, K.; Guerrache, Y.; Bellucci, S.; Gault, V.; Boudiaf, M. Multiple intra-abdominal venous thrombosis in ulcerative colitis: Role of MDCT for detection. Clin. Imaging 2011, 35, 68–72. [Google Scholar] [CrossRef]
- Hagel, S.; Bruns, T.; Stallmach, A.; Schmidt, C. A confocal view of the intestinal microcirculation in a patient with Crohn disease and portal vein thrombosis. Endoscopy 2011, 43 (Suppl. S2), E126–E127. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Amitai, M.M.; Lubetsky, A.; Eliakim, R.; Chowers, Y.; Ben-Horin, S. Clinical and radiographic presentation of superior mesenteric vein thrombosis in Crohn’s disease: A single center experience. J. Crohns Colitis 2012, 6, 543–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maconi, G.; Bolzacchini, E.; Dell’Era, A.; Russo, U.; Ardizzone, S.; De Franchis, R. Portal vein thrombosis in inflammatory bowel diseases: A single-centre case series. J. Crohns Colitis 2012, 6, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Jaqua, N.T.; Stratton, A.; Yaccobe, L.; Tahir, U.; Kenny, P.; Kerns, T. A review of the literature on three extraintestinal complications of ulcerative colitis: An ulcerative colitis flare complicated by Budd-Chiari syndrome, cerebral venous thrombosis and idiopathic thrombocytopenia. Acta Gastroenterol. Belg. 2013, 76, 311–316. [Google Scholar] [PubMed]
- Maggi, U.; Rossi, G.; Avesani, E.C.; Artoni, A.; Caprioli, F.; Napolitano, L.; Martinelli, I. Thrombotic storm in a teenager with previously undiagnosed ulcerative colitis. Pediatrics 2013, 131, e1288–e1291. [Google Scholar] [CrossRef] [PubMed]
- Mogrovejo, E.E.; Manickam, P.; Cappell, M.S. Portal vein thrombosis after colectomy for ulcerative colitis: Two case reports and proposed novel pathophysiology for this association. J. Crohns Colitis 2013, 7, 933–934. [Google Scholar] [CrossRef]
- Yılmaz, B.; Köklü, S.; Bayraktar, Y. Ulcerative colitis presenting with Budd-Chiari syndrome. J. Crohns Colitis 2013, 7, e74–e75. [Google Scholar] [CrossRef] [PubMed]
- Mian, H.S.; Lawlor, R. Venous thrombosis in inflammatory bowel disease. CMAJ 2015, 187, 55. [Google Scholar] [CrossRef][Green Version]
- Ma, A.S.C.; Ewing, I.; Murray, C.D.; Hamilton, M.I. Hepatic portal venous gas and portal venous thrombosis following colonoscopy in a patient with terminal ileal Crohn’s disease. BMJ Case Rep. 2015, 2015, bcr2014206854. [Google Scholar] [CrossRef]
- Simoes, C.C.; Ghouri, Y.A.; Merwat, S.N.; Stevenson, H.L. Budd-Chiari syndrome: A rare and life-threatening complication of Crohn’s disease. BMJ Case Rep. 2018, 2018, bcr2017222946. [Google Scholar] [CrossRef]
- Bohra, G.K.; Chhabra, V.; Midha, N.; Sureka, B. Budd-Chiari syndrome in a patient with ulcerative colitis. BMJ Case Rep. 2018, 2018, bcr2017222300. [Google Scholar] [CrossRef]
- Ito, S.; Higashiyama, M.; Horiuchi, K.; Mizoguchi, A.; Soga, S.; Tanemoto, R.; Nishii, S.; Terada, H.; Wada, A.; Sugihara, N.; et al. Atypical clinical presentation of Crohn’s disease with superior mesenteric vein obstruction and protein-losing enteropathy. Intern. Med. 2019, 58, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Hedrick, T. Postoperative Evaluation and Management of Portomesenteric Venous Thrombosis in Patients With IBD. Dis. Colon. Rectum 2022, 65, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.S.; Fryer, J.; Danese, S.; Vanagunas, A.; Polensky, S.; Buchman, A.L. Mesenteric vascular thromboembolism in inflammatory bowel Disease: A single center experience. J. Gastrointest. Surg. 2011, 15, 97–100. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Bernstein, C.N.; Bitton, A.; Chan, A.K.; Griffiths, A.M.; Leontiadis, G.I.; Geerts, W.; Bressler, B.; Butzner, J.D.; Carrier, M.; et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014, 146, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e419S–e496S. [Google Scholar] [CrossRef]
- Ageno, W.; Beyer-Westendorf, J.; Garcia, D.A.; Lazo-Langner, A.; McBane, R.D.; Paciaroni, M. Guidance for the management of venous thrombosis in unusual sites. J. Thromb. Thrombolysis 2016, 41, 129–143. [Google Scholar] [CrossRef]
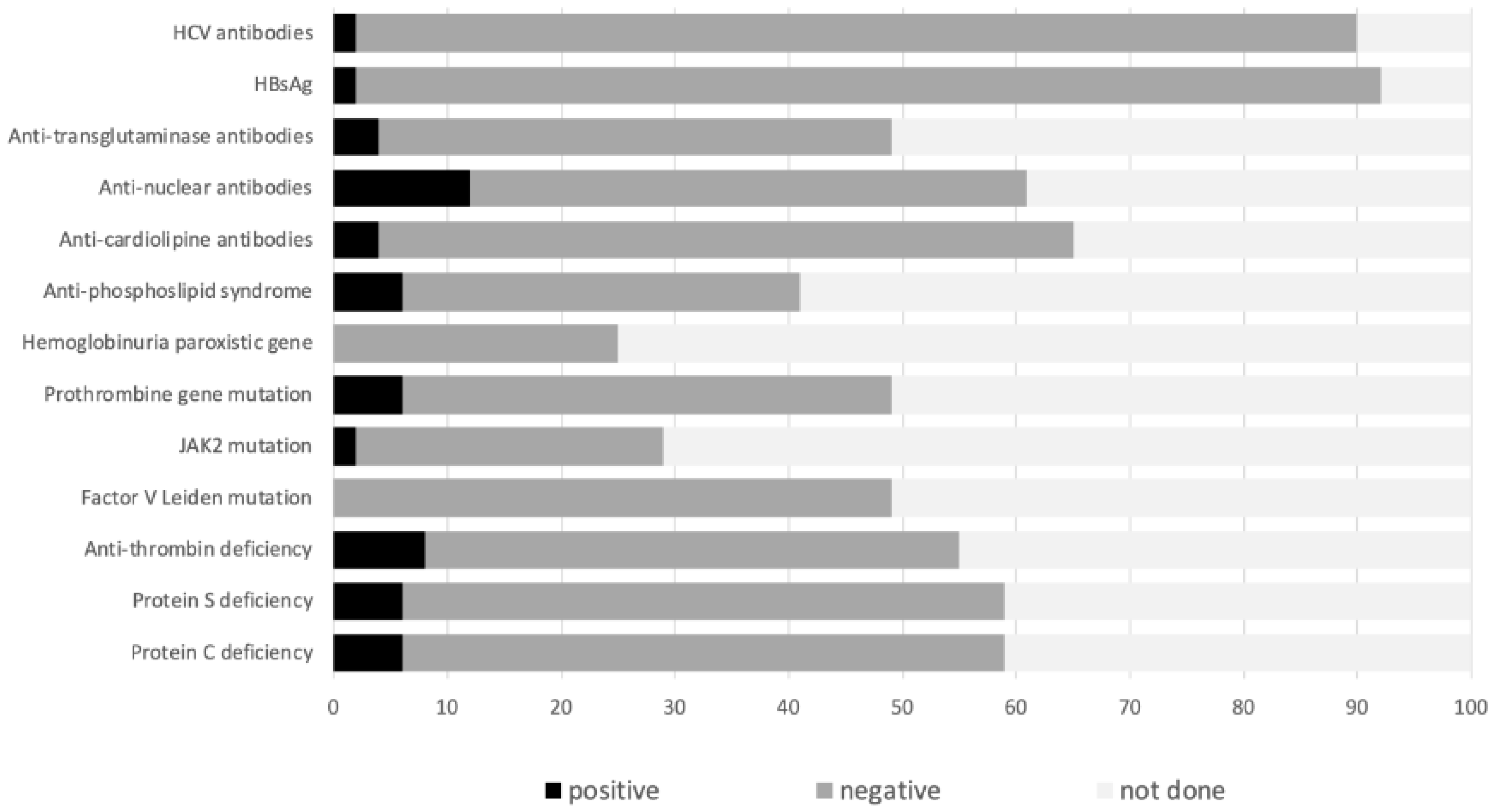
| ENEIDA Cohort (n = 49) | Systematic Review (n = 318) (available cases) | |
|---|---|---|
| Age (years) * | 42 (34–49) | 34 (25–44) (78) |
| Male gender | 34 (69) | 163 (51) (318) |
| Ethnicity Caucasian/Black | 48 (98)/1 (2) | 14 (74)/0 (0) (19) |
| Active alcoholism | 4 (8) | None available |
| Active smoking | 10 (20) | 56 (30) (189) |
| Crohn’s disease/Ulcerative colitis | 35/14 (71/29) | 164/153 (52/48) (317) |
| Ulcerative colitis extent proctitis/left-sided/extensive | 1/1/12 (7/7/86) | 0/12/69 (0/14/85) (81) |
| Crohn’s disease location Ileal/colic/ileocolic/upper gastrointestinal | 12/3/17/4 (33/8/47/8) | 13/24/11 (27/50/23) (48) |
| Crohn’s disease behaviour inflammatory/structuring/penetrating | 13/8/13 (37/23/37) | None available |
| Perianal disease | 10 (22) | 11 (18) (63) |
| Extraintestinal manifestations | 20 (41) | 3 (13) (24) |
| ENEIDA Cohort (n = 49) | Systematic Review (n = 318) (available cases) | |
|---|---|---|
| Active IBD | 25 (68) | 98 (57) (173) |
| Partial Mayo score (ulcerative colitis) | 2 (0–5) | NA |
| Harvey–Bradshaw index (Crohn’s disease) | 8 (6–10) | NA |
| Time since IBD diagnosis (months) | 106 (7–189) | 72 (2–162) (49) |
| IBD treatments at SVT diagnosis | ||
| None | 19 (39) | 17 (9) |
| Immunosuppressants | 15 (31) | 53 (29) |
| Biological agents | 12 (25) | 77 (42) |
| Systemic corticosteroids | 12 (25) | 107 (59) (183) |
| LMWH thromboprophylaxis | 11 (22) | 16 (24) (66) |
| C-reactive protein (mg/L) | 25 (14–64) | NA |
| ENEIDA Cohort (n = 49) | Systematic Review (n = 318) (available cases) | |
|---|---|---|
| Clinical presentation | 237 (75) | |
| Abdominal pain | 20 (45) | 125 (52) |
| Incidental finding | 13 (26) | 65 (27) |
| Ascites | 0 (0) | 4 (9) |
| Abdominal pain and fever | 8 (16) | 0 (0) |
| Fever | 3 (6) | 18 (8) |
| Nausea and vomiting | 0 (0) | 5 (2) |
| Variceal bleeding | 0 (0) | 2 (1) |
| Upper gastrointestinal bleeding | 2 (4) | 0 (0) |
| Intestinal ischemia | 1 (2) | 0 (0) |
| Location of thrombosis | 238 (75) | |
| Intrahepatic portal vein | 25 (51) | 18 (8) |
| Superior mesenteric vein | 23 (47) | 71 (30) |
| Main portal trunk | 18 (37) | 160 (67) |
| Splenic vein | 5 (11) | 34 (14) |
| Inferior mesenteric vein | 1 (2) | 3 (1) |
| Suprahepatic vein | 1 (2) | 2 (1) |
| Cavernomatous transformation at SVT diagnosis | 8 (16) | 14 (6) |
| Radiological examinations performed | 241 (76) | |
| Abdominal CT scan | 40 (82) | 214 (89) |
| Abdominal ultrasonography | 19 (39) | 36 (15) |
| MRI enterography | 4 (8) | 26 (11) |
| Previous abdominal surgery | 12 (25) |
| Digestive malignancies | 1 (2) |
| Extraintestinal malignancies | 7 (14) |
| Abdominal inflammatory disorder | 6 (12) |
| Immunological disorders | 2 (4) |
| Variable common immunodeficiency | 1 (2) |
| Haemolytic anaemia | 1 (2) |
| Haematological disorders | 6 (12) |
| Purpura idiopathic thrombocytopenic | 1 (2) |
| Other haematological disorders * | 5 (10) |
| Previous thrombotic episodes | 8 (16) |
| Deep thrombosis EEII | 6 (12) |
| Pulmonary thromboembolism | 0 |
| Thrombosis in other locations ** | 2 (4) |
| Previous pregnancy | 6 (12) |
| Previous miscarriage | 1 (2) |
| IBD-related intraabdominal inflammatory conditions | |
| Abscess | 9 (18) |
| Intestinal occlusion | 8 (16) |
| Intestinal perforation | 6 (12) |
| Megacolon | 1 (2) |
| Non-IBD-related intraabdominal inflammatory conditions | |
| Acute pancreatitis | 1 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig, M.; Masnou, H.; Mesonero, F.; Menchén, L.; Bujanda, L.; Castro, J.; González-Partida, I.; Vicente, R.; González-Muñoza, C.; Iborra, M.; et al. Splanchnic Vein Thrombosis in Inflammatory Bowel Disease: An Observational Study from the ENEIDA Registry and Systematic Review. J. Clin. Med. 2023, 12, 7366. https://doi.org/10.3390/jcm12237366
Puig M, Masnou H, Mesonero F, Menchén L, Bujanda L, Castro J, González-Partida I, Vicente R, González-Muñoza C, Iborra M, et al. Splanchnic Vein Thrombosis in Inflammatory Bowel Disease: An Observational Study from the ENEIDA Registry and Systematic Review. Journal of Clinical Medicine. 2023; 12(23):7366. https://doi.org/10.3390/jcm12237366
Chicago/Turabian StylePuig, Maria, Helena Masnou, Francisco Mesonero, Luís Menchén, Luís Bujanda, Jesús Castro, Irene González-Partida, Raquel Vicente, Carlos González-Muñoza, Marisa Iborra, and et al. 2023. "Splanchnic Vein Thrombosis in Inflammatory Bowel Disease: An Observational Study from the ENEIDA Registry and Systematic Review" Journal of Clinical Medicine 12, no. 23: 7366. https://doi.org/10.3390/jcm12237366
APA StylePuig, M., Masnou, H., Mesonero, F., Menchén, L., Bujanda, L., Castro, J., González-Partida, I., Vicente, R., González-Muñoza, C., Iborra, M., Sierra, M., Huguet, J. M., García, M. J., De Francisco, R., García-Alonso, F. J., Mañosa, M., Domènech, E., & on behalf of ENEIDA-GETECCU Registry. (2023). Splanchnic Vein Thrombosis in Inflammatory Bowel Disease: An Observational Study from the ENEIDA Registry and Systematic Review. Journal of Clinical Medicine, 12(23), 7366. https://doi.org/10.3390/jcm12237366







