Upregulation of Platelet-Activating Factor Receptor Expression and Lyso-Platelet-Activating Factor Isoforms in Human Nasal Polyp Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Tissue Handling
- PAFR immunohistochemistry. Pieces of tissues (about 5 mm diameter) were immersed in 4% neutral-buffered formalin for 24 h and then dehydrated and cleared using increasing ethanol concentrations. Finally, they were embedded into immunohistochemistry grade paraffin and stored at room temperature until microtome sections were performed.
- PAFR protein (Western blot) and mRNA (reverse transcription-quantitative PCR (RT-qPCR)). Pieces of tissues (about 5 mm diameter) were placed in cryogenic tubes and quickly frozen via immersion in liquid nitrogen. Frozen samples were stored at −80 °C until mRNA or protein isolation procedures were performed.
- PAFR immunofluorescence. Pieces of tissues (up to 0.5 cm diameter) were embedded in OCT compound (Tissue-Tek; Sakura Finetek, Torrance, CA, USA) in cryomolds and quickly frozen by immersing it in liquid nitrogen. Samples were stored at −80 °C until frozen sectioning on a microtome cryostat.
- Lyso-PAF analysis via gas–liquid chromatography–mass spectrometry (GLC/MS). Pieces of tissues (1 g of tissue) were placed in cryogenic tubes and quickly frozen via immersion in liquid nitrogen. Frozen samples were stored at −80 °C until Lyso-PAF analysis was performed.
2.3. Tissue Analyses
- Protein location via immunohistochemistry. Paraffin-embedded tissue was cut in sections of 5–10 µm in a microtome and mounted on poly-L-lysine-coated glass slides. A protocol for the deparaffinization of paraffin-embedded sections was performed and, after permeabilization with sodium citrate (0.01 M; pH 6), the staining protocol was started. Samples were blocked with 4% hydrogen peroxide to reduce background staining before reducing nonspecific binding with 1:10 goat serum in PBS (Sigma-Aldrich Co., St. Louis, MO, USA) for 60 min at room temperature. Antibodies against PAFR (rabbit polyclonal anti-human PAFR, Novusbio NBP1-90346) at a dilution of 1:400 were incubated overnight at 4 °C. Immunoreactivity of PAFR was detected with the EnVision+ System-HRP using an Olympus microscope. Controls using samples treated with either primary or secondary antibodies alone were performed with no specific immunoreactivity.
- Protein expression via Western blot analysis. Total proteins were isolated from frozen tissues using RIPA lysis buffer. Tissue samples were placed in tubes containing ice-cold RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40 (IGEPAL), 1 mg/mL leupeptin, 1 mg/mL aprotinin, 0.1 mM Na3VO4, 1 mM NaF, 1 mM dithiothreitol, 0.5 mg/mL Pefabloc, and 5 mg/mL sodium deoxycholate (Sigma)). Samples were kept on ice and sonicated twice for 15 s in a sonifier (Branson, Danbury, Conn) before centrifugation at 12,000× g for 10 min at 4 °C. Twenty micrograms of protein were denatured in a thermocycler (70 °C for 10 min) in loading buffer (NuPAGE LDS sample buffer), loaded onto 7% SDS–polyacrylamide gels, and electrophoresed at 125 V for 90 min in a Novex XCell II Mini-Cell (Invitro-gen). Finally, proteins were transferred to nitrocellulose membranes, and nonspecific binding sites were blocked with blocking buffer (5% non-fat dry milk and 0.1% Tween-20 in 10 nM PBS) for 1 h at room temperature in an orbital shaker. The membranes were then incubated in blocking buffer overnight at 4 °C with primary antibodies against PAFR (rabbit polyclonal anti-PAFR antibody, Abcam, ab104162) at a 1:500 dilution or incubated for 2 h at room temperature with primary antibodies against β-actin (mouse monoclonal to β-actin HRP conjugated, abcam, ab49900) at a dilution of 1:10,000. After this period, the blots were washed in 0.05% Tween-20 in 10 nM PBS. In the case of membranes incubated with anti-PAFR antibody, a second incubation with a goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Inc. Dallas, TX, USA, dilution 1:3000) at room temperature for 2 h was performed. After repeated washes, the blots were incubated with an enhanced chemiluminescent Immobilon® Forte Western HRP substrate (Cat No WBLUF0100, Millipore, Burlington, MA, USA), and light emissions were detected using a CCD Camera System LAS 4000 (Fujifilm, Tokyo, Japan). Band intensities were quantified with ImageQuantTL Software v8.2.0. Protein signals were normalized to those of β-actin.
- Protein location via Immunofluorescence. Frozen tissues embedded in OCT were cut in sections of 5–10 µm in a cryostat and mounted on gelatin-coated histological slides. Sections were air dried for 30 min at room temperature and fixed for 8 min by adding 50 µL of ice-cold fixation buffer. Tissues were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich Co.) in PBS (Sigma-Aldrich Co.), pH 7.4, at room temperature, and then blocked with 6% goat serum (Sigma-Aldrich Co.) for 60 min to prevent non-specific binding. Primary antibodies (anti-PRG2 antibody-BMK13, Abcam, ab14462) at a dilution of 1:100 and rabbit polyclonal anti-human PAFR (Novusbio, NBP1-90346) at a dilution of 1:300 were incubated overnight at 4 °C. Negative controls were performed by omitting the primary antibody and using a negative control serum of the same isotype. Alexa Fluor® 488-conjugated AffiniPure donkey anti-rabbit IgG (H + L, green channel, cat number 149712, Jackson ImmunoResearch, West Grove, PA, USA) at a dilution of 1:300 and Alexa Fluor 555-conjugated Donkey anti-Mouse IgG (H + L, red channel) (Invitrogen, A-31570) at a dilution of 1:500 were used as the secondary antibodies. The samples were counterstained with DAPI to identify cell nuclei (blue channel), and the slides were cover-slipped with ProLong Gold Antifade reagent (Invitrogen, Waltham, MA, USA).
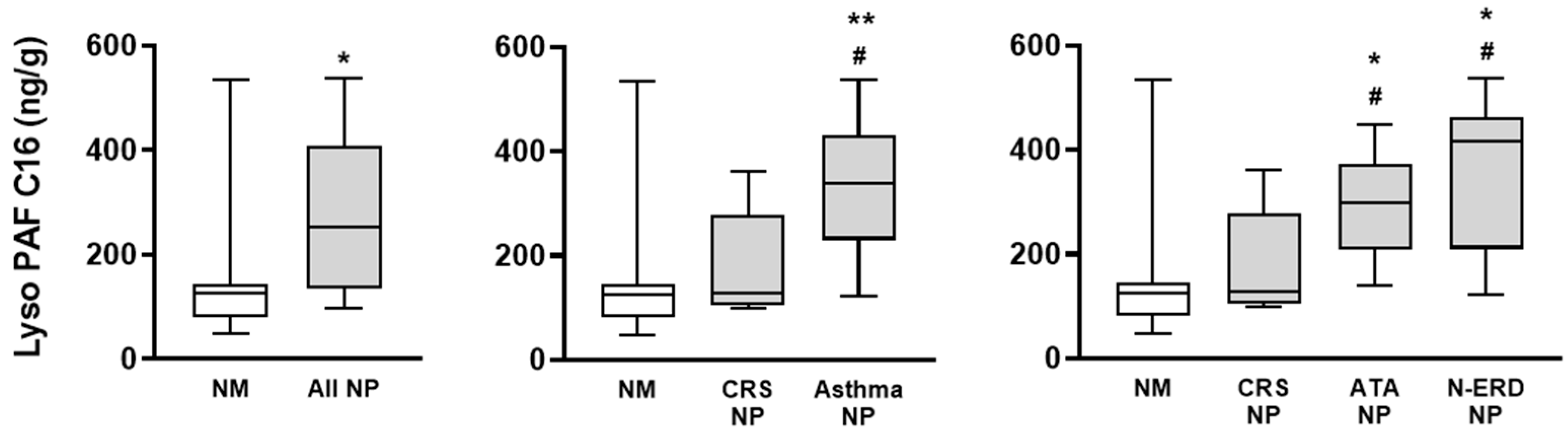
- Lyso-PAF via LC-MS/MS analysis. A LC-MS/MS method for the determination of Lyso-PAF C16 (Cayman Chemical, Ann Arbor, MI, USA, 60906), Lyso-PAF C18 (Cayman Chemical, 60916), and Lyso-PAF C18:1 (Avanti Polar Lipids, Birmingham, AL, USA, 878126C) in the nasal mucosa and nasal polyp samples was developed in the range of 0.2–20 ng/mL for the three components. Samples (approximately 0.5 g of tissue) were collected in 1 mL of methanol (Scharlab, Quezon City, Philippines, ME0306) and, after sample homogenization, 100 µL were processed with a liquid–liquid extraction method with chloroform (Scharlab, CL0207). The curves and QCs for the quantification of Lyso-PAF C16, C18, and C18:1 were prepared in H2O in the range of 0.2–20 ng/mL due to the lack of selectivity and the presence of interferents in matrices. The Lyso-PAF compound C18-d4 (Cayman Chemical, 10010228) was used as an internal standard. The HPLC separation of Lyso-PAF C16, C18, and C18:1 was carried out with a gradient and reverse phase chromatography with an XSelect CSH C18 column (130 Å; 3.5 µm; 2.1 × 100 mm) (Waters, 186005256) and 5 mM ammonium acetate (Supelco, Washington, DC, USA, 73594)/methanol (LC-MS, Scharlab ME0326) mobile phases, with retention times of 5.7 min, 6.3 min, and 8.3 min for Lyso-PAF C16, C18:1, and C18, respectively. MS/MS detection was performed using a Sciex 6500+ triple quadrupole mass spectrometer (ionization in positive mode) to monitor the most abundant MRM fragments m/z 482→m/z 104, m/z 508→m/z 104, and m/z 510→m/z 104 for Lyso-PAF C16, C18:1, and C18, respectively. The LC-MS/MS method provided sufficient sensitivity, precision, and accuracy for the determination of the compounds Lyso-PAF C16, C18:1, and C18 in samples of the NM and NP.
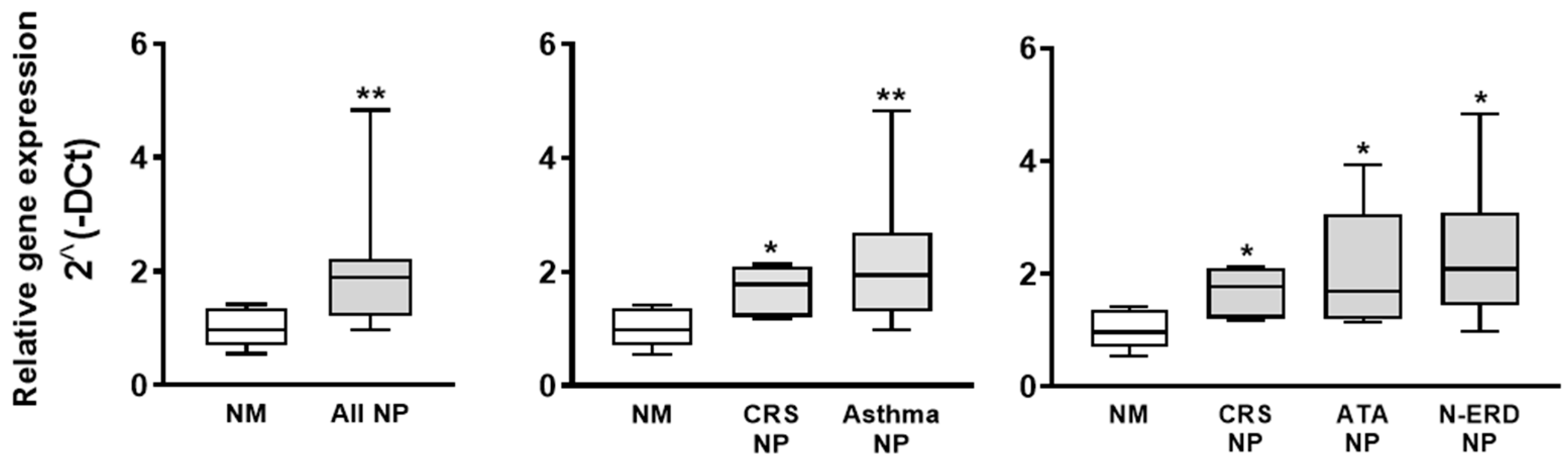
- mRNA expression via RT-qPCR analysis. Total RNA was isolated from frozen tissues using TRIzol reagent (Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s protocol. RNA was reverse transcribed to cDNA using the High-Capacity cDNA Reverse Transcription kit, according to the manufacturer’s instructions (Thermo Fisher, Waltham, MA, USA). PAFR mRNA expression was analyzed via real-time qPCR using TaqMan gene expression assays (Life Technologies) in the RT 7900 Real-Time PCR system (Thermo Fisher) with QuantStudioTM software v1.3. The TaqMan gene expression assays were glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs02758991_g1) and platelet-activating factor receptor (PAFR, Hs00265399_s1). The analysis of results was performed using the 2^(−DCt) method.
- Statistical data analysis: Data were given as the median and interquartile range. Whiskers represent the minimum and maximum values. Multiple comparisons were analyzed using the one-way analysis of variance statistical test with Tukey/Newman-Keuls post-hoc analysis or with Kruskal–Wallis with Dunn’s post-hoc analysis, as appropriate. Comparisons between two groups were made with the t-test or the Mann–Whitney U test, as appropriate. Statistical analyses were performed with GraphPad Prism software (GraphPad Prism Software v8.4.0, La Jolla, CA, USA). Statistical significance was set at p < 0.05.
3. Results
3.1. Analysis of PAFR Protein and Gene Expression
3.2. Lyso-PAF Analysis in NM and NP Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-Cano, R.M.; Casas-Saucedo, R.; Valero Santiago, A.; Bobolea, I.; Ribó, P.; Mullol, J. Platelet-Activating Factor (PAF) in Allergic Rhinitis: Clinical and Therapeutic Implications. J. Clin. Med. 2019, 8, 1338. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Alloatti, G.; Camussi, G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 2000, 80, 1669–1699. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.B.; Rohan, J.G.; Sahu, R.P. New Insights into the Pathologic Roles of the Platelet-Activating Factor System. Front. Endocrinol. 2021, 12, 624132. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kaneko, M.; Yoshioka, K.; Obara, K.; Tanaka, Y. Platelet-activating factor (PAF) strongly enhances contractile mechanical activities in guinea pig and mouse urinary bladder. Sci. Rep. 2022, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, H.; Seki, N.; Kikuchi, M.; Kanaizumi, E.; Watanabe, K.; Konno, N.; Himi, T. Expression and localization of platelet-activating factor receptor in human nasal mucosa. Ann. Allergy Asthma Immunol. 2005, 95, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Ogura, M.; Tsutsumi, T.; Tsuji, H.; Yamashita, T. Presence of platelet-activating factor in nasal polyps and eosinophils. Acta Otolaryngol. 2002, 122, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Castro Faria Neto, H.C.; Stafforini, D.M.; Prescott, S.M.; Zimmerman, G.A. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Memórias Do Inst. Oswaldo Cruz 2005, 100 (Suppl. S1), 83–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okumura, N.; Kojima, K.; Hashida, M.; Koura, Y.; Fukushima, A.; Ueno, H. Platelet-activating factor in human normal tears. Curr. Eye Res. 2005, 30, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, E.; Tedeschi, A.; Lorini, M.; Bianco, A.; Miadonna, A. Study of the effects of paf-acether on human nasal airways. Allergy 1991, 46, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; Valero, A.; Roca-Ferrer, J.; Bartra, J.; Sanchez-Lopez, J.; Mullol, J.; Picado, C. Platelet-activating factor nasal challenge induces nasal congestion and reduces nasal volume in both healthy volunteers and allergic rhinitis patients. Am. J. Rhinol. Allergy 2013, 27, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Iwamura, S. Platelet-activating factor (PAF)-induced rhinitis and involvement of PAF in allergic rhinitis in guinea pigs. Jpn. J. Pharmacol. 1990, 54, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Kim, J.P.; Kim, E.A.; Ahn, S.K.; Kim, B.G. Rat model of platelet-activating factor-induced rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2005, 114, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.E.; Foreman, J.C. The effect of platelet-activating factor on the responsiveness of the human nasal airway. Br. J. Pharmacol. 1993, 110, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Klementsson, H.; Andersson, M. Eosinophil chemotactic activity of topical PAF on the human nasal mucosa. Eur. J. Clin. Pharmacol. 1992, 42, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Palumbo, G.; Milazzo, N.; Miadonna, A. Nasal neutrophilia and eosinophilia induced by challenge with platelet activating factor. J. Allergy Clin. Immunol. 1994, 93, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ganbo, T.; Hisamatsu, K.; Ishida, H. Study on the metabolism of lyso-platelet activating factor (lyso-PAF) in human paranasal sinus mucosa. The cultured ciliated epithelium can convert lyso-PAF to PAF. Life Sci. 1993, 52, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Dupuch, V.; Tridon, A.; Ughetto, S.; Walrand, S.; Bonnet, B.; Dubray, C.; Virlogeux, A.; Vasson, M.P.; Saroul, N.; Mom, T.; et al. Activation state of circulating eosinophils in nasal polyposis. Int. Forum Allergy Rhinol. 2018, 8, 584–591. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roca-Ferrer, J.; Pérez-González, M.; Alobid, I.; Tubita, V.; Fuentes, M.; Bantulà, M.; Muñoz-Cano, R.; Valero, A.; Izquierdo, I.; Mullol, J. Upregulation of Platelet-Activating Factor Receptor Expression and Lyso-Platelet-Activating Factor Isoforms in Human Nasal Polyp Tissues. J. Clin. Med. 2023, 12, 7357. https://doi.org/10.3390/jcm12237357
Roca-Ferrer J, Pérez-González M, Alobid I, Tubita V, Fuentes M, Bantulà M, Muñoz-Cano R, Valero A, Izquierdo I, Mullol J. Upregulation of Platelet-Activating Factor Receptor Expression and Lyso-Platelet-Activating Factor Isoforms in Human Nasal Polyp Tissues. Journal of Clinical Medicine. 2023; 12(23):7357. https://doi.org/10.3390/jcm12237357
Chicago/Turabian StyleRoca-Ferrer, Jordi, Maria Pérez-González, Isam Alobid, Valeria Tubita, Mireya Fuentes, Marina Bantulà, Rosa Muñoz-Cano, Antonio Valero, Iñaki Izquierdo, and Joaquim Mullol. 2023. "Upregulation of Platelet-Activating Factor Receptor Expression and Lyso-Platelet-Activating Factor Isoforms in Human Nasal Polyp Tissues" Journal of Clinical Medicine 12, no. 23: 7357. https://doi.org/10.3390/jcm12237357
APA StyleRoca-Ferrer, J., Pérez-González, M., Alobid, I., Tubita, V., Fuentes, M., Bantulà, M., Muñoz-Cano, R., Valero, A., Izquierdo, I., & Mullol, J. (2023). Upregulation of Platelet-Activating Factor Receptor Expression and Lyso-Platelet-Activating Factor Isoforms in Human Nasal Polyp Tissues. Journal of Clinical Medicine, 12(23), 7357. https://doi.org/10.3390/jcm12237357





