The Feasibility of Japanese Histological Grade Classification for Predicting Renal Function Deterioration among Taiwanese Individuals with IgA Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Population
2.2. Other Data Collection
2.3. Outcome Definitions
2.4. Process of Taiwanese IgAN in Japanese Histological Grade Classification (JHGC)
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Taiwanese IgAN Cohort
3.2. Baseline Characteristics According to the Japanese Histological Grade Classification (JHGC)
3.2.1. Merged Japanese Histological and Clinical Grading System (Merged 3H and 3C)
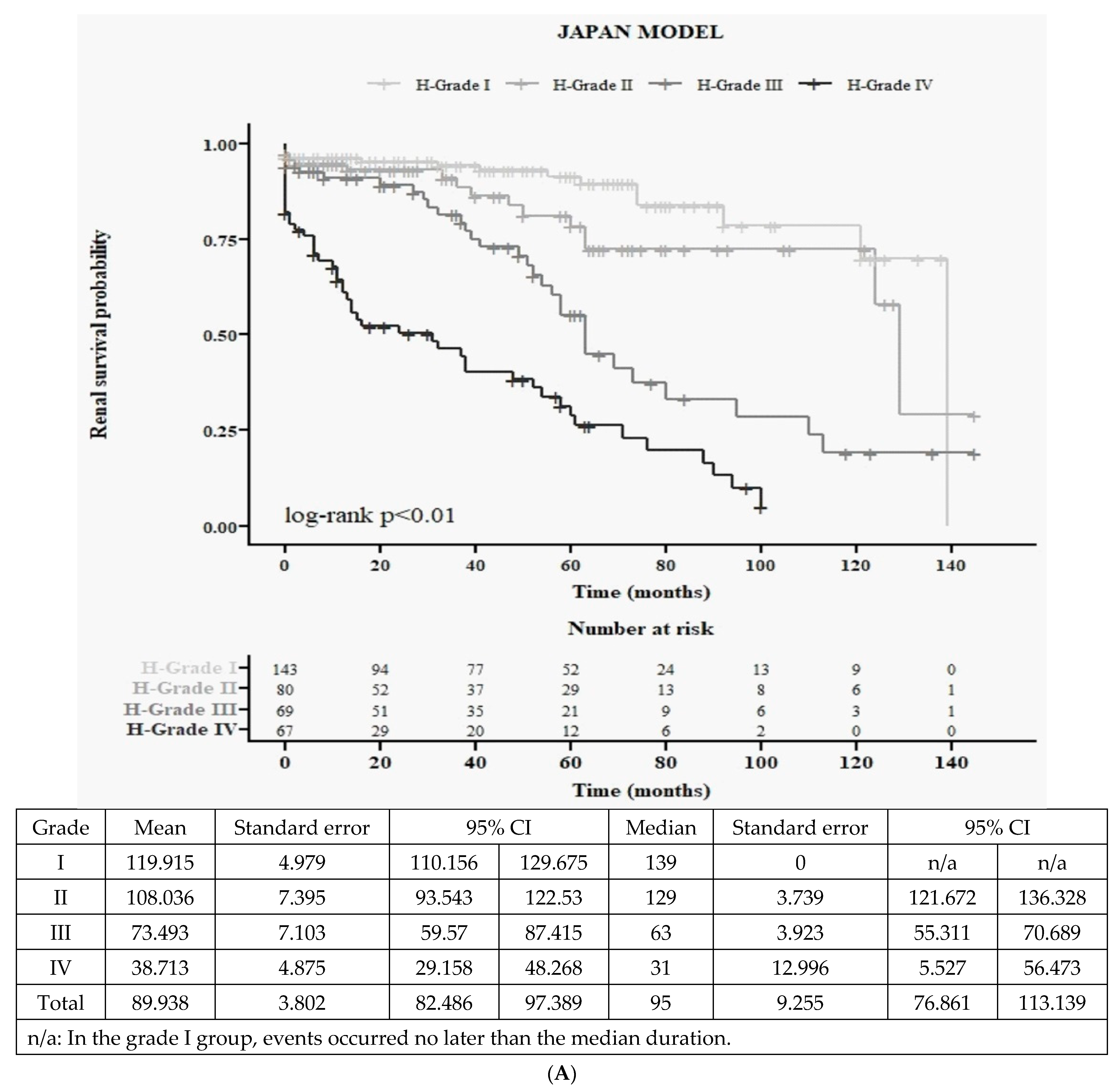
3.2.2. Japanese Histological Grading System (4H): Baseline Characteristics and Kaplan–Meier Survival Curves
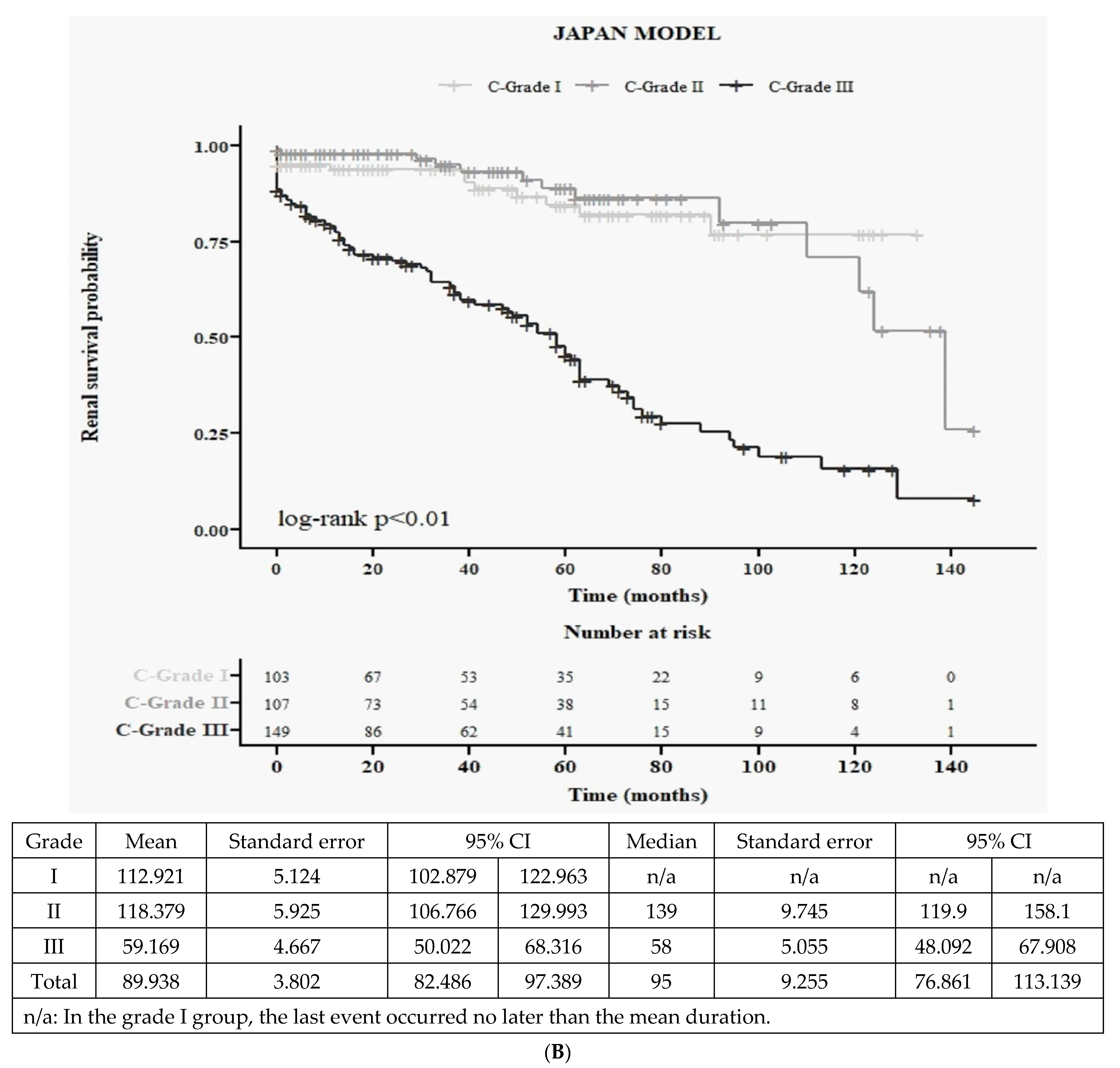
3.2.3. Japanese Clinical Grading System (3C): Baseline Characteristics and Kaplan–Meier Survival Curves
3.3. Renal Outcome According to Various Grading Groups
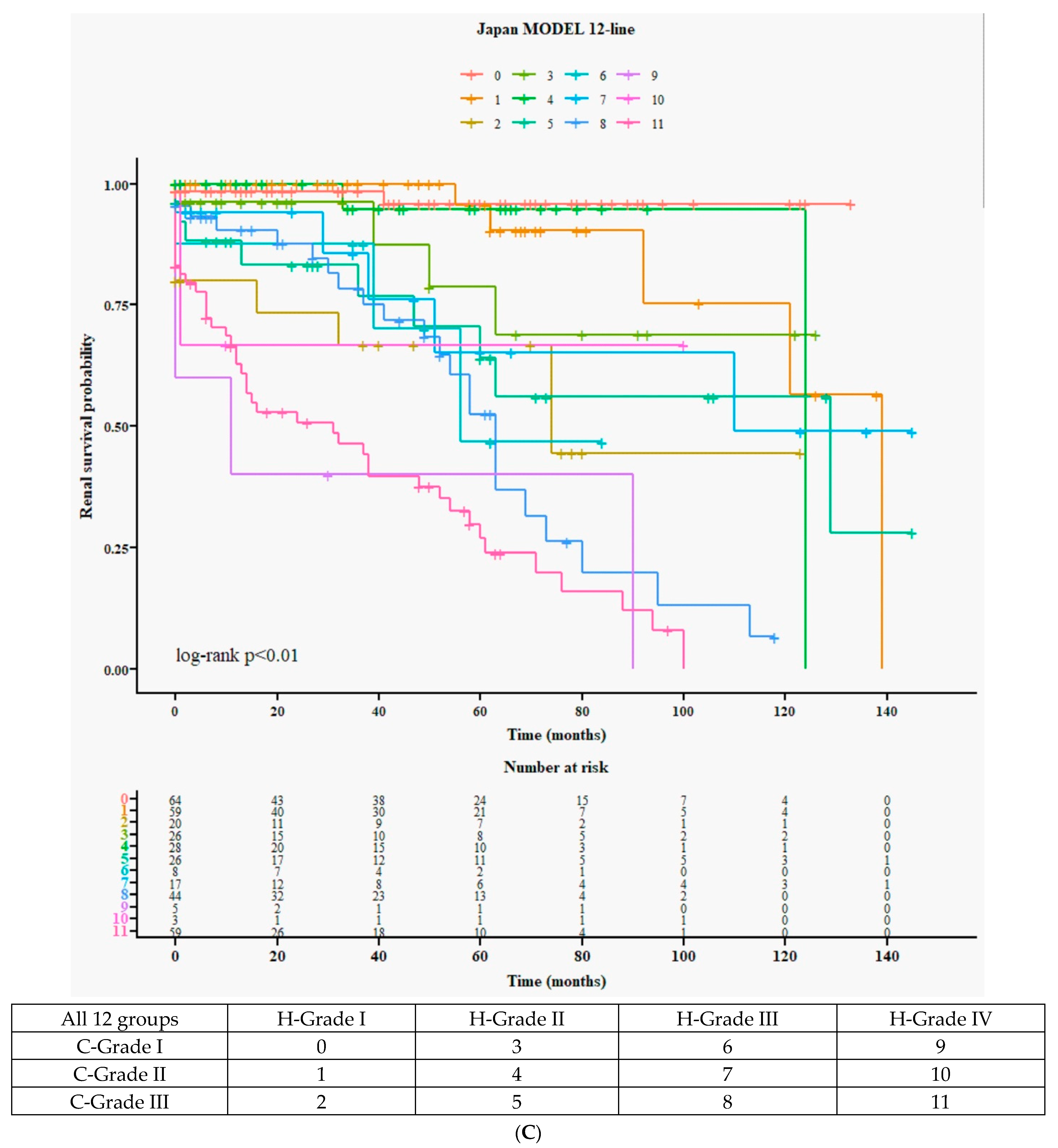
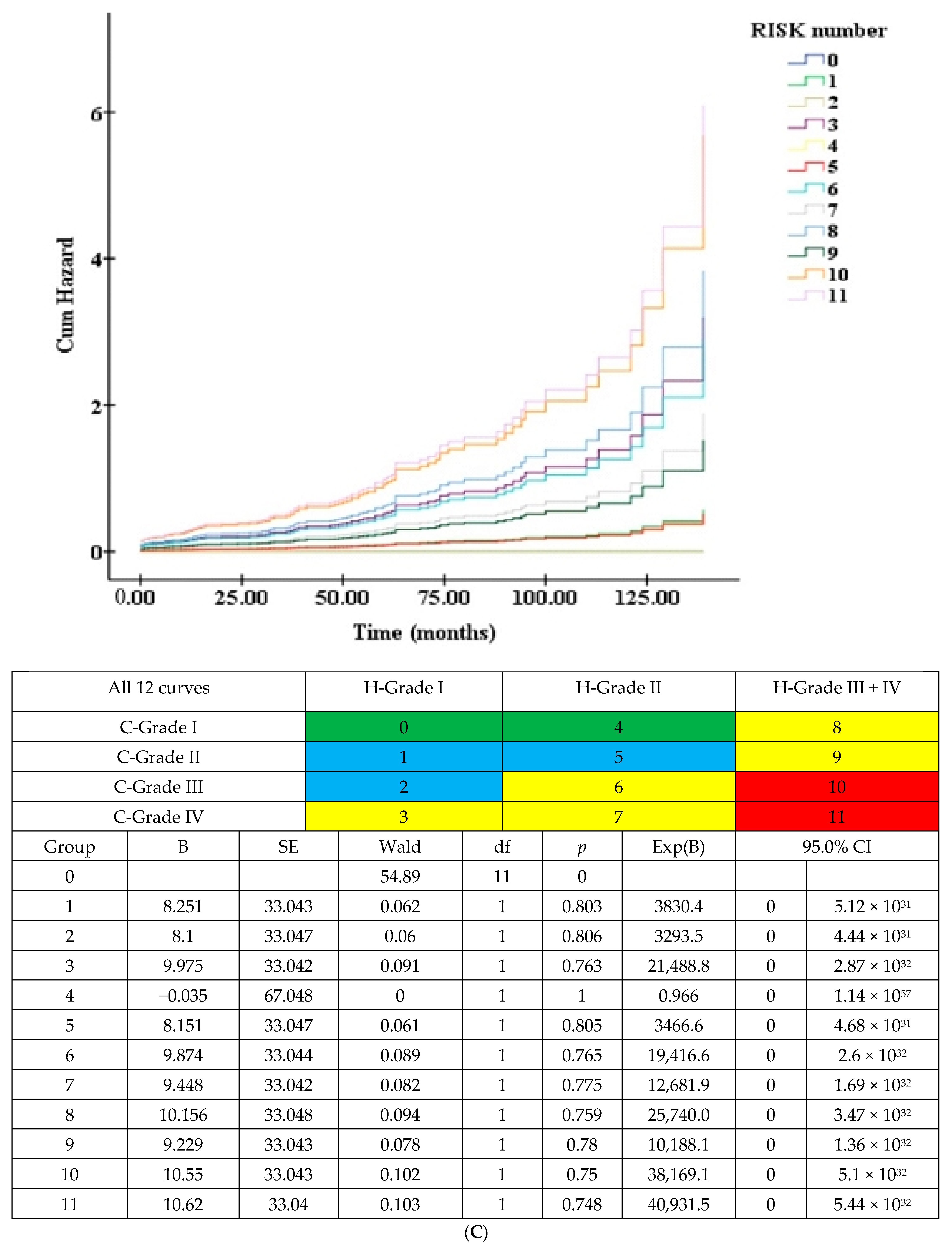
3.3.1. Detailed 12 Survival Curves (Figure 1C) and Cox Accumulative HR (Figure 1D) from 4H × 3C
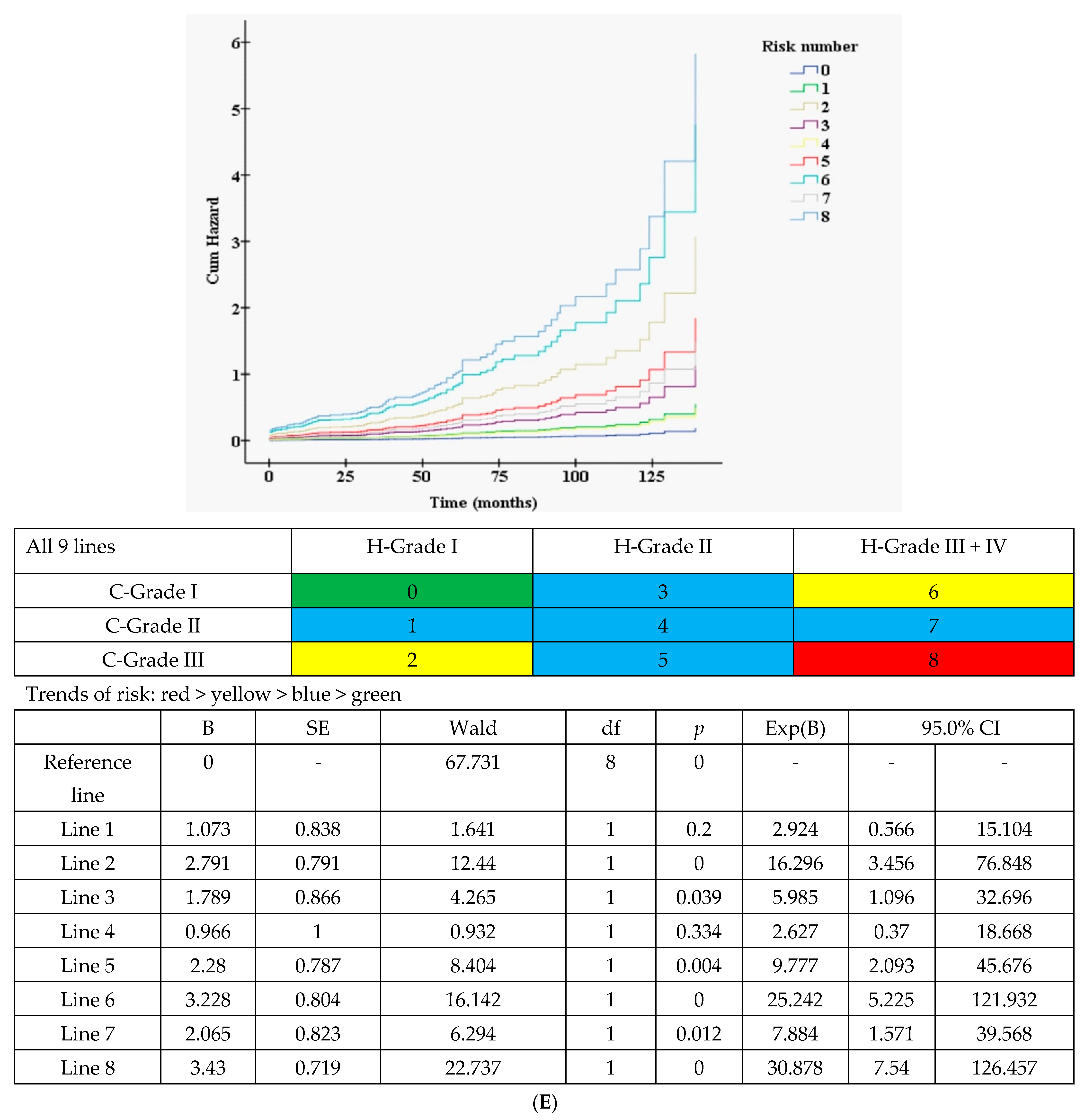
3.3.2. Nine Curves According to 3H (merged H-grade III + IV) × 3C (Figure 1E)
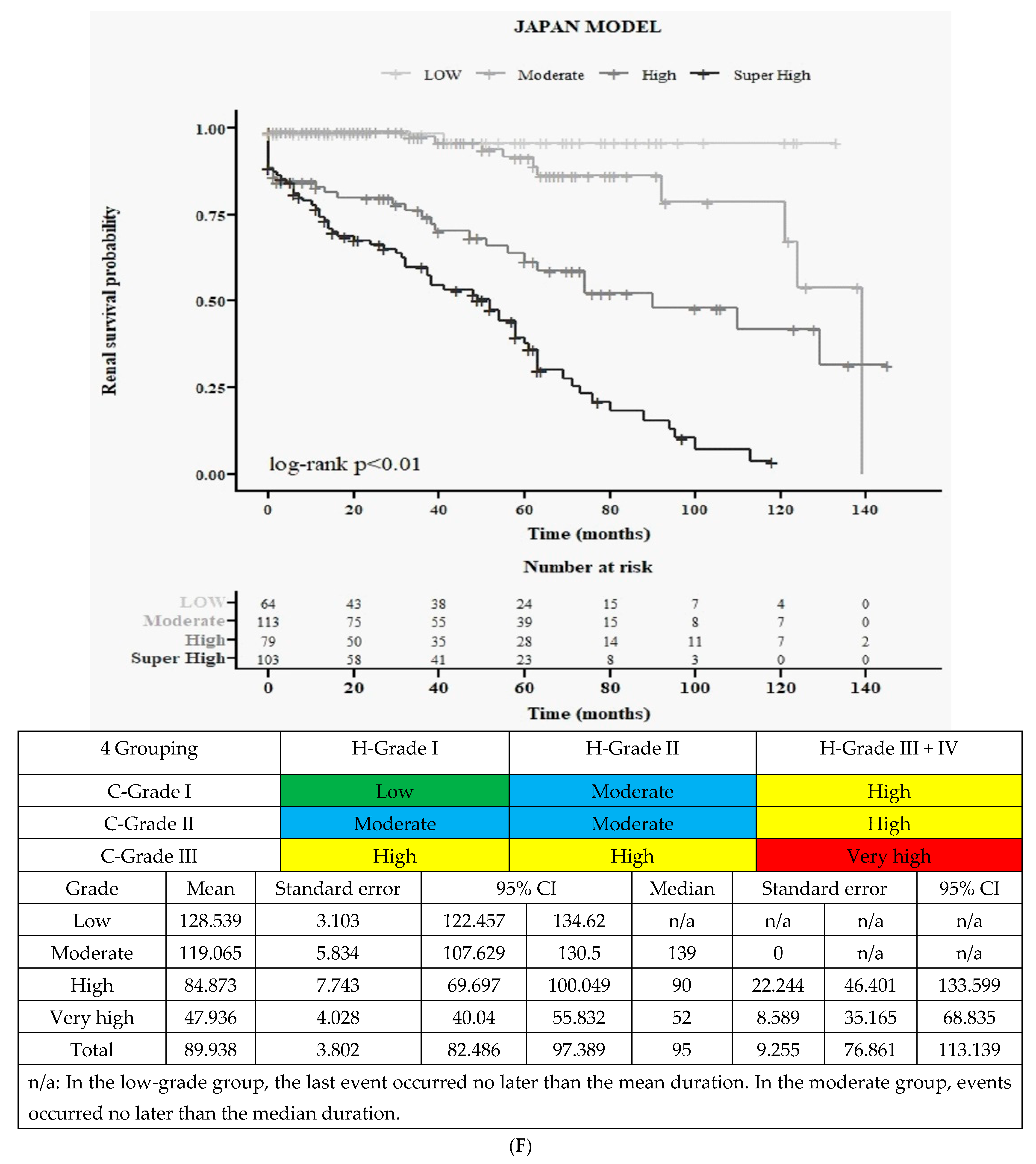
3.3.3. Final All Four Groups According to Merged 3H and 3C (Figure 1F)
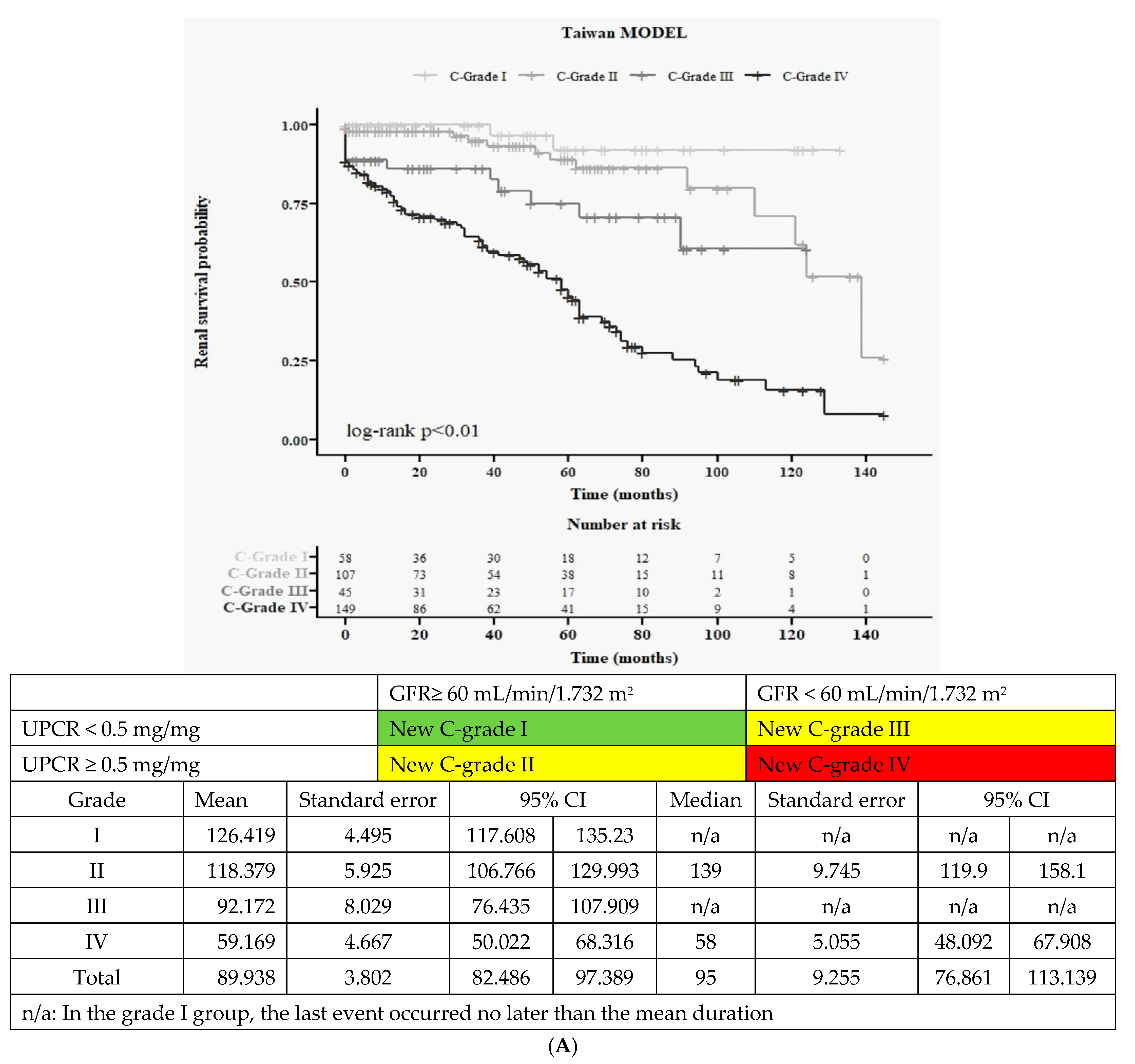
3.4. New Clinical Grading System (New 4C): Baseline Characteristics and Kaplan–Meier Survival Curves
3.4.1. Baseline Characteristics (Supplementary Table S3)
3.4.2. Kaplan–Meier Survival Curves for Renal Function (Figure 2A)
3.5. Renal Outcome Prediction According to Various Adjusted Grading Groups
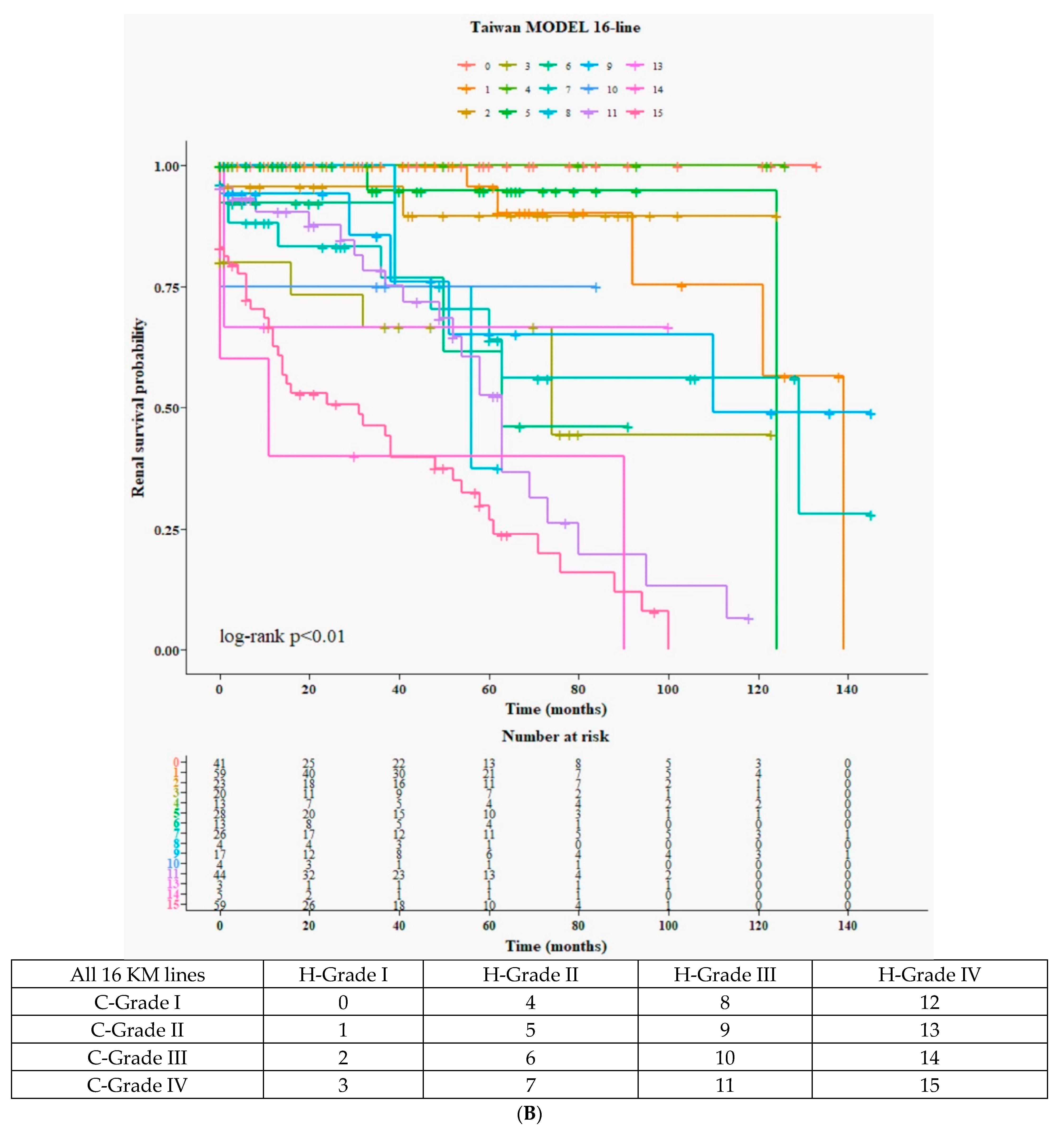
3.5.1. Detailed 16 Curves from 4H × 4C (Figure 2B)
3.5.2. Twelve Curves According to 3H (Merged H-Grade III + IV) × New 4C (Figure 2C)
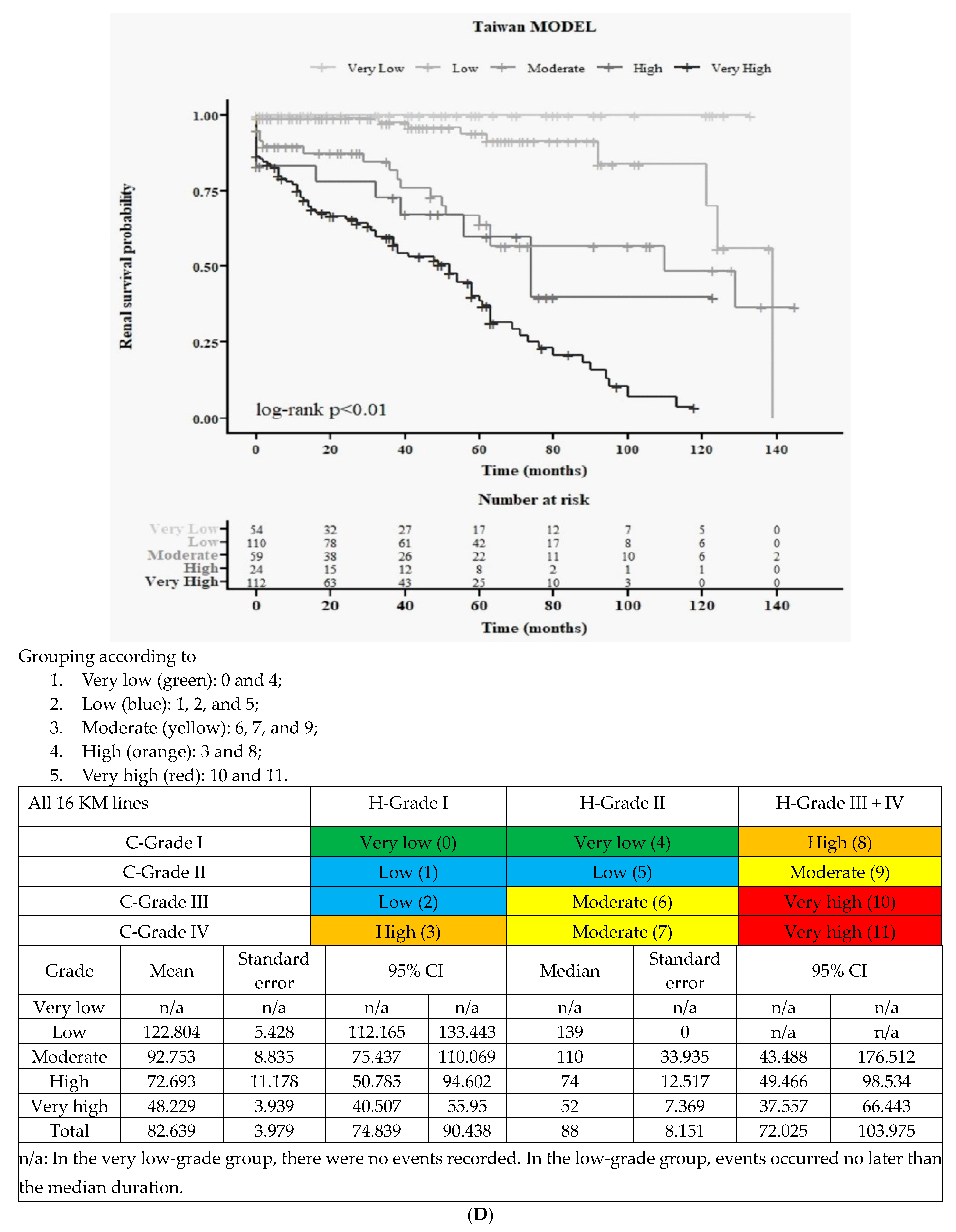
3.5.3. Final All Five Groups According to Merged 3H and New 4C (Figure 2D)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Galla, J.H. IgA nephropathy. Kidney Int. 1995, 47, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Liu, Z.H. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004, 66, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Kawamura, T.; Endoh, M.; Kojima, M.; Tomino, Y.; Tamakoshi, A.; Ohno, Y.; Inaba, Y.; Sakai, H. A scoring system to predict renal outcome in IgA nephropathy: From a nationwide prospective study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2006, 21, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Rekola, S.; Bergstrand, A.; Bucht, H. Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int. 1991, 40, 1050–1054. [Google Scholar] [CrossRef][Green Version]
- Johnston, P.A.; Brown, J.S.; Braumholtz, D.A.; Davision, A. Clinico-pathological correlations and long-term follow-up of 253 United Kingdom patients with IgA nephropathy. A report from the MRC Glomerulonephritis Registry. Q. J. Med. 1992, 84, 619–627. [Google Scholar]
- Alamartine, E.; Sabatier, J.C.; Guerin, C.; Berliet, J.-M.; Berthoux, F. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1991, 18, 12–19. [Google Scholar] [CrossRef]
- Chacko, B.; John, G.T.; Neelakantan, N.; Korula, A.; Balakrishnan, N.; Kirubakaran, M.G.; Jacob, C.K. Presentation, prognosis and outcome of IgA nephropathy in Indian adults. Nephrology 2005, 10, 496–503. [Google Scholar] [CrossRef]
- Yeo, S.C.; Goh, S.M.; Barratt, J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology 2019, 24, 885–895. [Google Scholar] [CrossRef]
- Tomino, Y.; Sakai, H. Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin. Exp. Nephrol. 2003, 7, 93–97. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Imai, H.; Kikuchi, S.; Hayashi, S.; Endoh, M.; Kawamura, T.; Tomino, Y.; Moriwaki, K.; Kiyomoto, H.; Kohagura, K.; et al. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: A nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin. Exp. Nephrol. 2009, 13, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Suzuki, Y.; Nakata, J.; Sakamoto, N.; Horikoshi, S.; Kawamura, T.; Matsuo, S.; Tomino, Y. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin. Exp. Nephrol. 2013, 17, 827–833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kondo, N.; Moriyama, T.; Tachikawa, M.; Tomita, E.; Hattori, A.; Yamamura, Y.; Nonaka, M. Tonsillectomy plus steroid pulse therapy is the most effective treatment in adult patients with C-Grade I IgA nephropathy, and the weight of the extracted palatine tonsils and Yamamoto scale have no significant correlation with the effects of this treatment. Auris Nasus Larynx 2019, 46, 764–771. [Google Scholar] [PubMed]
- A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef]
- Roberts, I.S.; A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Joh, K.; Okonogi, H.; Koike, K.; Utsunomiya, Y.; Miyazaki, Y.; Matsushima, M.; Yoshimura, M.; Horikoshi, S.; Suzuki, Y.; et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: Emphasis on end-stage renal disease. J. Nephrol. 2013, 26, 350–357. [Google Scholar] [CrossRef]
- Yuzawa, Y.; Yamamoto, R.; Takahashi, K.; Katafuchi, R.; Tomita, M.; Fujigaki, Y.; Kitamura, H.; Goto, M.; Yasuda, T.; Sato, M.; et al. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin. Exp. Nephrol. 2016, 20, 511–535. [Google Scholar] [CrossRef]
- Okonogi, H.; The Special IgA Nephropathy Study Group; Kawamura, T.; Joh, K.; Koike, K.; Miyazaki, Y.; Ogura, M.; Tsuboi, N.; Hirano, K.; Matsushima, M.; et al. A grading system that predicts the risk of dialysis induction in IgA nephropathy patients based on the combination of the clinical and histological severity. Clin. Exp. Nephrol. 2019, 23, 16–25. [Google Scholar] [CrossRef]
- Sato, R.; Joh, K.; Komatsuda, A.; Ohtani, H.; Okuyama, S.; Togashi, M.; Omokawa, A.; Nara, M.; Nagata, D.; Kusano, E.; et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin. Exp. Nephrol. 2015, 19, 411–418. [Google Scholar] [CrossRef]
- Moriyama, T.; Nitta, K. Secondary Publication: Clinical Grade of IgA Nephropathy. Nippon Rinsho 2019, 77, 8. [Google Scholar]
- Tsai, S.F.; Wu, M.J.; Wen, M.C.; Chen, C.-H. Serologic and Histologic Predictors of Long-Term Renal Outcome in Biopsy-Confirmed IgA Nephropathy (Haas Classification): An Observational Study. J. Clin. Med. 2019, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Agarwal, R.; Herrington, W.G.; Heerspink, H.L.; Mann, J.F.; Shahinfar, S.; Tuttle, K.R.; Donner, J.-A.; Jha, V.; Nangaku, M.; et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020, 98, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.-H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Er, L.; Reich, H.N.; Barratt, J.; Cattran, D.C.; et al. Application of the International IgA Nephropathy Prediction Tool one or two years post-biopsy. Kidney Int. 2022, 102, 160–172. [Google Scholar] [CrossRef]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Er, L.; Espino-Hernandez, G.; Kim, S.J.; et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern. Med. 2019, 179, 942–952. [Google Scholar] [CrossRef]
- Barbour, S.J.; Canney, M.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Induruwage, D.; Er, L.; et al. Improving treatment decisions using personalized risk assessment from the International IgA Nephropathy Prediction Tool. Kidney Int. 2020, 98, 1009–1019. [Google Scholar] [CrossRef]
- Thompson, A.; Carroll, K.; Inker, L.A.; Floege, J.; Perkovic, V.; Boyer-Suavet, S.; Major, R.W.; Schimpf, J.I.; Barratt, J.; Cattran, D.C.; et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clin. J. Am. Soc. Nephrol. CJASN 2019, 14, 469–481. [Google Scholar] [CrossRef]
- Itami, S.; Moriyama, T.; Miyabe, Y.; Karasawa, K.; Nitta, K. A Novel Scoring System Based on Oxford Classification Indicating Steroid Therapy Use for IgA Nephropathy. Kidney Int. Rep. 2022, 7, 99–107. [Google Scholar] [CrossRef]
- Haaskjold, Y.L.; Bjørneklett, R.; Bostad, L.; Bostad, L.S.; Lura, N.G.; Knoop, T. Utilizing the MEST score for prognostic staging in IgA nephropathy. BMC Nephrol. 2022, 23, 26. [Google Scholar] [CrossRef]
- Jaugey, A.; Maréchal, E.; Tarris, G.; Paindavoine, M.; Martin, L.; Chabannes, M.; Funes de la Vega, M.; Chaintreuil, M.; Robier, C.; Ducloux, D.; et al. Deep learning automation of MEST-C classification in IgA nephropathy. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2023, 38, 1741–1751. [Google Scholar] [CrossRef]
- Barbour, S.J.; Espino-Hernandez, G.; Reich, H.N.; Coppo, R.; Roberts, I.S.; Feehally, J.; Herzenberg, A.M.; Cattran, D.C.; Bavbek, N.; Cook, T.; et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016, 89, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.; Reich, H. An update on predicting renal progression in IgA nephropathy. Curr. Opin. Nephrol. Hypertens. 2018, 27, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lv, J.; Wang, W.; Li, G.; Liu, Z.; Chen, H.; Xu, F.; Sun, J.; Ouyang, Y.; Zhang, X.; et al. Kidney Failure Risk Prediction Equations in IgA Nephropathy: A Multicenter Risk Assessment Study in Chinese Patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.V.; Bergstralh, E.J.; Grande, J.P.; Rademcher, D.M. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2002, 17, 1197–1203. [Google Scholar] [CrossRef]
- Izzi, C.; Ravani, P.; Torres, D.; Prati, E.; Viola, B.F.; Guerini, S.; Foramitti, M.; Frascà, G.; Amoroso, A.; Ghiggeri, G.M.; et al. IgA nephropathy: The presence of familial disease does not confer an increased risk for progression. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 47, 761–769. [Google Scholar] [CrossRef]
- D’Amico, G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1992, 20, 315–323. [Google Scholar] [CrossRef]
- Ikee, R.; Kobayashi, S.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Hemmi, N.; Imakiire, T.; Kikuchi, Y.; Suzuki, S.; Miura, S. Impact of hypertension and hypertension-related vascular lesions in IgA nephropathy. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2006, 29, 15–22. [Google Scholar] [CrossRef]
- Frisch, G.; Lin, J.; Rosenstock, J.; Markowitz, G.; D’Agati, V.; Radhakrishnan, J.; Preddie, D.; Crew, J.; Valeri, A.; Appel, G. Mycophenolate mofetil (MMF) vs. placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2005, 20, 2139–2145. [Google Scholar] [CrossRef]
- Coppo, R.; Troyanov, S.; Bellur, S.; Cattran, D.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Morando, L.; Camilla, R.; Tesar, R.; et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014, 86, 828–836. [Google Scholar] [CrossRef]
- Tanaka, S.; Ninomiya, T.; Katafuchi, R.; Masutani, K.; Tsuchimoto, A.; Noguchi, H.; Hirakata, H.; Tsuruya, K.; Kitazono, T. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 2082–2090. [Google Scholar] [CrossRef]
- Shirai, S.; Yasuda, T.; Kumagai, H.; Matsunobu, H.; Ichikawa, D.; Shibagaki, Y.; Yasuda, Y.; Matsuzaki, K.; Hirano, K.; Kawamura, T.; et al. Prognostic factors of IgA nephropathy presenting with mild proteinuria at the time of diagnosis (a multicenter cohort study). Clin. Exp. Nephrol. 2023, 27, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Matsuzaki, K.; Yasuda, T.; Nishikawa, M.; Yasuda, Y.; Koike, K.; Maruyama, S.; Yokoo, T.; Matsuo, S.; Kawamura, T.; et al. Association Between Tonsillectomy and Outcomes in Patients With Immunoglobulin A Nephropathy. JAMA Netw. Open 2019, 2, e194772. [Google Scholar] [CrossRef] [PubMed]

| Risk Classification | Total (n = 359) | Low (n = 64) | Moderate (n = 113) | High (n = 79) | Very High (n = 103) | p |
|---|---|---|---|---|---|---|
| Variables | Median (Q1, Q3) for continuous variables; n (%) for categorical variables | |||||
| Demographic data | ||||||
| Age (years old) | 46 (37, 59) | 46 (34.5, 59) | 43 (32, 55) | 50 (36, 63) | 51 (42, 60) | <0.001 |
| Body mass index (kg/m2) | 23.47 (20.70, 27.45) | 23.07 (20.96, 27.22) | 23.96 (20.69, 27.75) | 23.44 (20.70, 27.47) | 22.60 (20.17, 26.07) | 0.901 |
| Male | 188 (52.4%) | 34 (53.1%) | 56 (49.6%) | 45 (57%) | 53 (51.5%) | 0.784 |
| Blood data | ||||||
| Blood WBC (/cumm) | 7630 (6100, 9230) | 6770 (5630, 8290) | 7870 (6100, 9220) | 7320 (6100, 9880) | 7820 (6390, 9390) | 0.26 |
| Hemoglobin (g/dL) | 12.7 (10.9, 14.1) | 13.1 (12, 14.5) | 13.3 (12.1, 14.6) | 12.5 (10.5, 14.1) | 11.2 (9.8, 13.1) | <0.001 |
| Platelet (×103/cumm) | 247 (202, 293) | 239 (202, 292.5) | 252 (215, 324) | 249 (196, 286) | 238 (196, 277) | 0.001 |
| Fasting glucose (mg/dL) | 94 (86, 107) | 93 (85, 99) | 94 (87, 108) | 95.5 (86, 105) | 95 (86, 110) | 0.209 |
| Glycated hemoglobin (%) | 5.5 (5.3, 5.8) | 5.5 (5.2, 5.8) | 5.5 (5.3, 5.8) | 5.6 (5.4, 6) | 5.6 (5.2, 5.9) | 0.4 |
| Albumin (g/dL) | 4 (3.6, 4.2) | 4.2 (4, 4.5) | 4 (3.7, 4.3) | 3.8 (3.4, 4) | 3.7 (3.5, 4) | <0.001 |
| Total cholesterol (mg/dL) | 187.5 (164, 219) | 172.5 (154, 191) | 191 (165, 219) | 186 (162, 221) | 196.5 (170, 228) | 0.07 |
| Triglyceride (mg/dL) | 122 (83.5, 184.5) | 102 (66, 138) | 118 (84, 185) | 126.5 (87, 180) | 143 (95, 195) | 0.003 |
| Low-density lipoprotein (mg/dL) | 110.5 (90, 142) | 99 (85, 122) | 108.5 (90, 141) | 110.5 (91, 140) | 123.5 (92, 147) | 0.095 |
| High-density lipoprotein (mg/dL) | 47.4 (31.2, 64) | 47.4 (35.8, 59.8) | 48.8 (34.2, 66) | 47.3 (31.7, 69) | 42.4 (29, 64.4) | 0.379 |
| Blood urea nitrogen (mg/dL) | 20 (14, 31) | 15 (13, 22) | 15 (12, 19) | 22 (17, 29) | 38 (26, 50) | <0.001 |
| Creatinine (mg/dL) | 1.3 (0.9, 2.1) | 1 (0.7, 1.4) | 1 (0.7, 1.2) | 1.5 (1.2, 1.8) | 2.7 (1.8, 4.2) | <0.001 |
| Estimated glomerular filtrate rate (min/min.1.732 m2) | 54.04 (32.18, 86.29) | 84.22 (48.1, 106.59) | 83.06 (66.81, 101.4) | 47.2 (37.96, 69.39) | 25.08 (14.97, 36.9) | <0.001 |
| Uric acid (mg/dL) | 6.6 (5.3, 8.1) | 6.3 (5, 7.3) | 6 (4.9, 7) | 7 (5.9, 8.1) | 7.5 (6.3, 9.1) | <0.001 |
| C3 (mg/dL) | 107.8 (93.3, 120.9) | 110.2 (95.9, 130.2) | 114.5 (98.6, 124.7) | 105 (94.9, 118.6) | 97.4 (78.2, 118.1) | 0.008 |
| C4 (mg/dL) | 31 (24, 37) | 28 (22, 36) | 29 (24, 35) | 31 (24, 36) | 34 (28, 41) | <0.001 |
| IgG (mg/dL) | 1061 (899, 1266) | 1184 (1016, 1298) | 1065 (908, 1266) | 1037 (730, 1251) | 1012 (823, 1231) | 0.072 |
| IgA (mg/dL) | 328 (256, 418) | 318 (258, 399) | 336 (257, 441) | 323 (251, 422) | 329 (252, 393) | 0.112 |
| IgM (mg/dL) | 98 (72, 132) | 112 (86, 138) | 107 (80, 138) | 96 (66, 127) | 88 (57, 126) | <0.001 |
| IgE (mg/dL) | 69.3 (13.6, 95.3) | 95.3 (30.4, 654) | 69.9 (69.3, 70.5) | 13.6 (13.6, 13.6) | 4.7 (4.7, 4.7) | 0.258 |
| Urinary data | ||||||
| Urine protein–creatinine ratio (mg/g) | 1154.5 (420.6, 2612.09) | 190 (96.94, 320) | 1060 (520, 2020) | 1431.5 (684.41, 3240.12) | 2070 (1237.64, 4464) | <0.001 |
| Hematuria | 310 (86.4%) | 55 (85.9%) | 93 (82.3%) | 71 (89.9%) | 91 (88.3%) | 0.448 |
| Pyuria | 205 (57.1%) | 31 (48.4%) | 72 (63.7%) | 43 (54.4%) | 59 (57.3%) | 0.241 |
| Pathological data based on Oxford classification | ||||||
| M | 205 (58.4%) | 17 (27.4%) | 62 (54.9%) | 52 (67.5%) | 74 (74.7%) | <0.001 |
| E | 104 (29.6%) | 5 (8.1%) | 26 (23%) | 27 (35.1%) | 46 (46.5%) | <0.001 |
| S | 219 (62.4%) | 11 (17.7%) | 70 (61.9%) | 53 (68.8%) | 85 (85.9%) | <0.001 |
| T1 | 72 (20.5%) | 1 (1.6%) | 6 (5.3%) | 18 (23.4%) | 47 (47.5%) | <0.001 |
| T2 | 31 (8.8%) | 1 (1.6%) | 0 (0%) | 4 (5.2%) | 26 (26.3%) | <0.001 |
| C1 | 38 (10.7%) | 0 (0%) | 13 (11.5%) | 13 (16.7%) | 12 (11.9%) | 0.046 |
| C2 | 11 (3.1%) | 0 (0%) | 1 (0.9%) | 3 (3.8%) | 7 (6.9%) | 0.123 |
| Risk Classification | Total (n = 359) | Very Low (n = 54) | Low (n = 110) | Moderate (n = 59) | High (n = 24) | Very High (n = 112) | p |
|---|---|---|---|---|---|---|---|
| Variables | Median (Q1, Q3) for continuous variables; n (%) for categorical variables | ||||||
| Demographic data | |||||||
| Age (years old) | 46 (37, 59) | 42.5 (32, 53) | 44 (32, 58) | 49 (40, 60) | 59.5 (36.5, 66.5) | 51 (42, 60) | <0.001 |
| Body mass index (kg/m2) | 23.47 (20.70, 27.45) | 23.33 (20.87, 27.29) | 23.50 (20.69, 27.85) | 23.35 (20.70, 27.47) | 23.92 (20.31, 27.17) | 22.78 (21.01, 27.01) | 0.966 |
| Male | 188 (52.4%) | 31 (57.4%) | 52 (47.3%) | 33 (55.9%) | 14 (58.3%) | 58 (51.8%) | 0.679 |
| Blood data | |||||||
| Blood white blood cell (/cumm) | 7630 (6100, 9230) | 7300 (5800, 8400) | 7720 (5840, 8965) | 7735 (6330, 9810) | 7170 (6010, 9810) | 7700 (6365, 9490) | 0.23 |
| Hemoglobin (g/dL) | 12.7 (10.9, 14.1) | 13.6 (12.4, 14.7) | 13.1 (11.9, 14.4) | 12.6 (11, 14.2) | 12.1 (10.1, 13.2) | 11.3 (9.8, 13.1) | <0.001 |
| Platelet (×103/cumm) | 247 (202, 293) | 249 (203, 313) | 250 (203, 310) | 249 (204, 286) | 240.5 (189, 263) | 236 (195.5, 278.5) | 0.155 |
| Fasting glucose (mg/dL) | 94 (86, 107) | 88.5 (81.5, 98.5) | 95 (87, 108) | 93 (86, 102) | 98 (84, 105.5) | 95.5 (86.5, 110) | 0.1 |
| Glycated hemoglobin (%) | 5.5 (5.3, 5.8) | 5.4 (5.3, 5.8) | 5.5 (5.3, 5.8) | 5.6 (5.4, 5.9) | 5.5 (5.3, 6.1) | 5.6 (5.2, 5.8) | 0.525 |
| Albumin (g/dL) | 4 (3.6, 4.2) | 4.3 (4, 4.5) | 4 (3.7, 4.3) | 4 (3.5, 4.2) | 3.8 (3.5, 4.2) | 3.8 (3.5, 4) | <0.001 |
| Total cholesterol (mg/dL) | 187.5 (164, 219) | 175 (154, 208) | 186 (164.5, 215.5) | 193.5 (167, 217) | 191.5 (167, 225) | 195 (164, 225) | 0.254 |
| Triglyceride (mg/dL) | 122 (83.5, 184.5) | 103 (67, 140) | 113 (75, 186) | 121.5 (92, 192) | 124 (78.5, 166) | 145.5 (99, 192) | 0.008 |
| Low-density lipoprotein (mg/dL) | 110.5 (90, 142) | 99 (85, 133) | 105.5 (91, 141) | 110 (90, 139) | 109 (95.5, 129) | 120 (91, 147) | 0.438 |
| High-density lipoprotein (mg/dL) | 47.4 (31.2, 64) | 52.2 (36.2, 61.6) | 47.6 (30.2, 66) | 45.4 (33.4, 68.8) | 53.7 (46.6, 72.2) | 40.4 (28.6, 63.8) | 0.259 |
| Blood urea nitrogen (mg/dL) | 20 (14, 31) | 14 (12, 17) | 15.5 (13, 20) | 21 (16, 29) | 25.5 (19, 31.5) | 37 (26, 50) | <0.001 |
| Creatinine (mg/dL) | 1.3 (0.9, 2.1) | 0.9 (0.7, 1) | 1 (0.7, 1.2) | 1.5 (1.1, 1.7) | 1.6 (1.4, 2.4) | 2.6 (1.8, 4.6) | <0.001 |
| Estimated glomerular filtrate rate (min/min.1.732 m2) | 54.04 (32.18, 86.29) | 93.91 (79.59, 111.48) | 80.76 (60.73, 101.4) | 49.36 (40.37, 70.7) | 42.54 (29.27, 55.27) | 26.25 (14.84, 37.54) | <0.001 |
| Uric acid (mg/dL) | 6.6 (5.3, 8.1) | 6.1 (5, 7.2) | 6.1 (4.9, 7.2) | 6.5 (5.8, 7.6) | 7.7 (5.7, 9.6) | 7.5 (6.2, 9) | <0.001 |
| C3 (mg/dL) | 107.8 (93.3, 120.9) | 113.9 (101.1, 130.2) | 108.3 (95.4, 122.9) | 105.9 (90.8, 118.6) | 105.3 (94.9, 112.7) | 97.9 (78.9, 120) | 0.077 |
| C4 (mg/dL) | 31 (24, 37) | 31 (22, 38) | 28 (23, 33) | 32 (25, 35) | 30 (23, 37) | 34 (28, 41) | <0.001 |
| IgG (mg/dL) | 1061 (899, 1266) | 1166 (999, 1303) | 1068 (920, 1253) | 1079 (820, 1327) | 1036 (730, 1251) | 1007 (807, 1231) | 0.119 |
| IgA (mg/dL) | 328 (256, 418) | 329 (257, 431) | 334 (260, 441) | 327 (234, 427) | 314 (261, 354) | 327 (250, 393) | 0.649 |
| IgM (mg/dL) | 98 (72, 132) | 117 (88, 141) | 108 (84, 138) | 96 (59, 127) | 93 (66, 115) | 87 (57, 121) | 0.001 |
| IgE (mg/dL) | 69.3 (13.6, 95.3) | 82.3 (49.8, 374.7) | 70.5 (70.5, 70.5) | 13.6 (13.6, 13.6) | - | 4.7 (4.7, 4.7) | 0.277 |
| Urinary data | |||||||
| Urine protein–creatinine ratio (mg/g) | 1154.5 (420.6, 2612.09) | 240 (140, 381.86) | 1116.47 (530, 2150) | 1330 (550, 2749.92) | 1160 (547.23, 3580) | 1937 (1080, 3852) | <0.001 |
| Hematuria | 310 (86.4%) | 48 (88.9%) | 89 (80.9%) | 51 (86.4%) | 22 (91.7%) | 100 (89.3%) | 0.408 |
| Pyuria | 205 (57.1%) | 22 (40.7%) | 72 (65.5%) | 31 (52.5%) | 16 (66.7%) | 64 (57.1%) | 0.035 |
| Pathological data based on Oxford classification | |||||||
| M | 205 (58.4%) | 20 (37%) | 50 (46.3%) | 41 (69.5%) | 14 (63.6%) | 80 (74.0%) | <0.001 |
| E | 104 (29.6%) | 6 (11.1%) | 24 (22.2%) | 19 (32.2%) | 7 (31.8%) | 48 (44.4%) | <0.001 |
| S | 219 (62.4%) | 20 (37%) | 54 (50%) | 44 (74.6%) | 11 (50%) | 90 (83.3%) | <0.001 |
| T1 | 72 (20.5%) | 0 (0%) | 2 (1.9%) | 18 (30.5%) | 3 (13.6%) | 49 (45.4%) | <0.001 |
| T2 | 31 (8.8%) | 0 (0%) | 1 (0.9%) | 2 (3.4%) | 0 (0%) | 28 (25.9%) | <0.001 |
| C1 | 38 (10.7%) | 2 (3.7%) | 9 (8.3%) | 11 (18.6%) | 4 (17.4%) | 12 (10.9%) | 0.069 |
| C2 | 11 (3.1%) | 0 (0%) | 1 (0.9%) | 3 (5.1%) | 0 (0%) | 7 (6.4%) | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Wu, M.-J.; Tsai, S.-F. The Feasibility of Japanese Histological Grade Classification for Predicting Renal Function Deterioration among Taiwanese Individuals with IgA Nephropathy. J. Clin. Med. 2023, 12, 7339. https://doi.org/10.3390/jcm12237339
Chen C-H, Wu M-J, Tsai S-F. The Feasibility of Japanese Histological Grade Classification for Predicting Renal Function Deterioration among Taiwanese Individuals with IgA Nephropathy. Journal of Clinical Medicine. 2023; 12(23):7339. https://doi.org/10.3390/jcm12237339
Chicago/Turabian StyleChen, Cheng-Hsu, Ming-Ju Wu, and Shang-Feng Tsai. 2023. "The Feasibility of Japanese Histological Grade Classification for Predicting Renal Function Deterioration among Taiwanese Individuals with IgA Nephropathy" Journal of Clinical Medicine 12, no. 23: 7339. https://doi.org/10.3390/jcm12237339
APA StyleChen, C.-H., Wu, M.-J., & Tsai, S.-F. (2023). The Feasibility of Japanese Histological Grade Classification for Predicting Renal Function Deterioration among Taiwanese Individuals with IgA Nephropathy. Journal of Clinical Medicine, 12(23), 7339. https://doi.org/10.3390/jcm12237339







