Comparison of Remimazolam–Flumazenil versus Propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Search Strategy
2.3. Selection Process and Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Analysis
2.7. Heterogeneity Treatment and Meta-Regression
2.8. Sensitivity Analysis
2.9. Publication Bias
2.10. Certainty of Evidence
3. Results
3.1. Search and Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Meta-Analysis Results
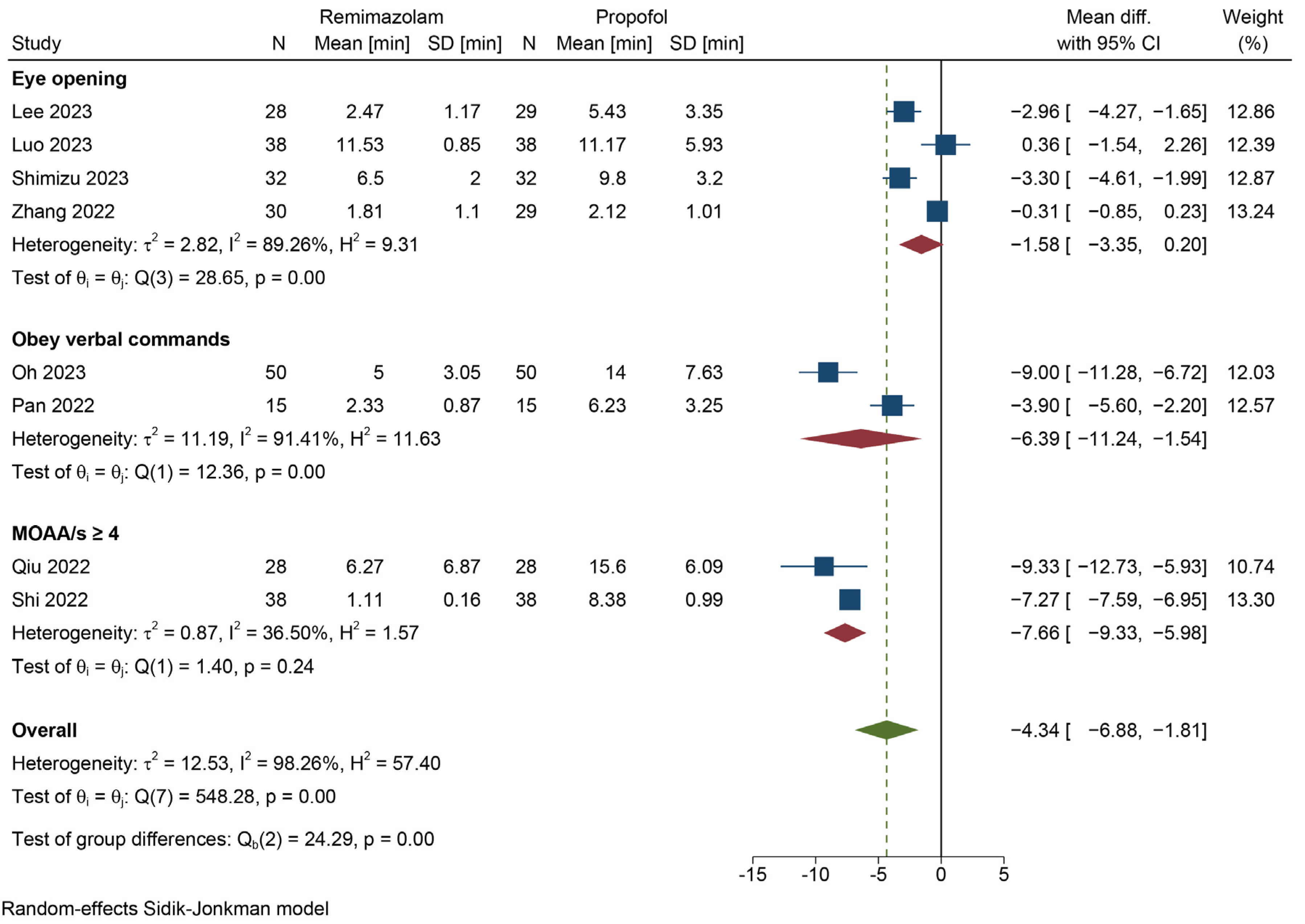
3.4.1. Emergence Time
3.4.2. Extubation Time
3.4.3. Length of PACU Stay
3.4.4. Postoperative Complications
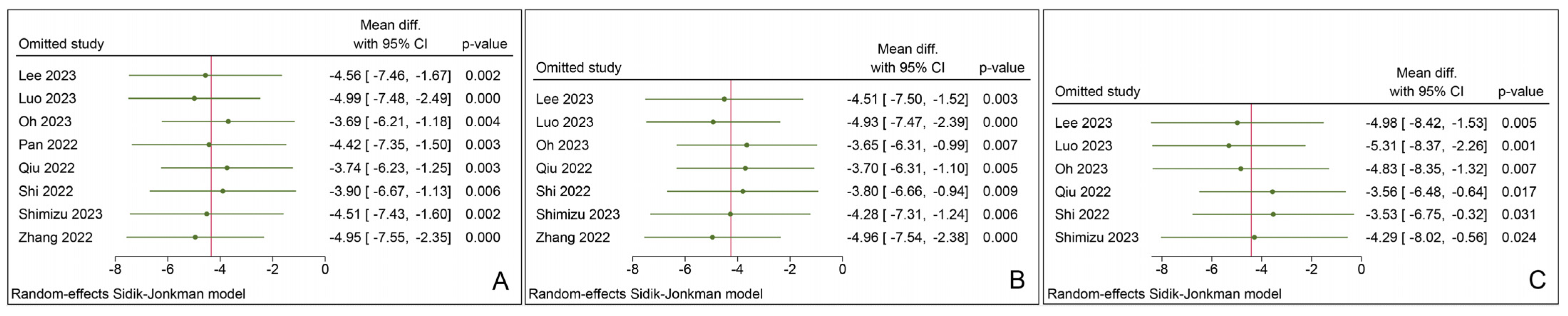
3.5. Sensitivity Analysis
3.6. Publication Bias
4. Discussion
4.1. Limitations
4.2. Strengths and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, C.T. Propofol: Milk of Amnesia. Cell 2018, 175, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.G.; Chung, C.K.E.; Ip, K.Y.; Wiles, M.D. Influence of propofol-based total intravenous anaesthesia on peri-operative outcome measures: A narrative review. Anaesthesia 2020, 75 (Suppl. S1), e90–e100. [Google Scholar] [CrossRef] [PubMed]
- Dankoski, E. The 2018 Lasker~DeBakey Clinical Medical Research Award recognizes John Baird Glen for the discovery of propofol. J. Clin. Investig. 2018, 128, 4198–4200. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Pruna, A.; Turi, S.; Borghi, G.; Lee, T.C.; Zangrillo, A.; Landoni, G.; Pasin, L. Propofol and survival: An updated meta-analysis of randomized clinical trials. Crit. Care 2023, 27, 139. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Shingu, K.; Ogawa, T.; Tomoda, K.; Shindo, K.; Tamai, S.; Mori, K. Flumazenil does not antagonize halothane, thiamylal or propofol anaesthesia in rats. Br. J. Anaesth. 1992, 69, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Saari, T.I.; Uusi-Oukari, M.; Ahonen, J.; Olkkola, K.T. Enhancement of GABAergic activity: Neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol. Rev. 2011, 63, 243–267. [Google Scholar] [CrossRef]
- Pastis, N.J.; Yarmus, L.B.; Schippers, F.; Ostroff, R.; Chen, A.; Akulian, J.; Wahidi, M.; Shojaee, S.; Tanner, N.T.; Callahan, S.P.; et al. Safety and Efficacy of Remimazolam Compared With Placebo and Midazolam for Moderate Sedation During Bronchoscopy. Chest 2019, 155, 137–146. [Google Scholar] [CrossRef]
- Rex, D.K.; Bhandari, R.; Desta, T.; DeMicco, M.P.; Schaeffer, C.; Etzkorn, K.; Barish, C.F.; Pruitt, R.; Cash, B.D.; Quirk, D.; et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 2018, 88, 427–437. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.S.; Kim, J.Y.; Han, D.W.; Yang, J.Y.; Kim, M.J.; Song, Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J. Clin. Anesth. 2022, 82, 110955. [Google Scholar] [CrossRef]
- Dong, S.A.; Guo, Y.; Liu, S.S.; Wu, L.L.; Wu, L.N.; Song, K.; Wang, J.H.; Chen, H.R.; Li, W.Z.; Li, H.X.; et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J. Clin. Anesth. 2023, 86, 111077. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Li, J.; Zhang, L.; Li, Q.; Li, Y.; Lu, Y.; Sun, J.; Zhang, Y.; Cheng, Y.; et al. Safety and effectiveness of the combination of remimazolam tosilate and propofol in gastroscopy: A multicenter, randomized controlled, single-blind clinical trial. Front. Pharmacol. 2023, 14, 1124667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.J.; Hu, H.F.; Li, X.L.; Li, X.M.; Wang, D.C.; Kuang, M.J. The safety and efficacy between remimazolam and propofol in intravenous anaesthesia of endoscopy operation: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 3566–3577. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sang, N.; Song, K.; Zhong, W.; Wang, H.; Jiang, J.; Huang, Y.; Hu, P. Psychomotor Recovery Following Remimazolam-induced Sedation and the Effectiveness of Flumazenil as an Antidote. Clin. Ther. 2020, 42, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Worthington, M.T.; Antonik, L.J.; Goldwater, D.R.; Lees, J.P.; Wilhelm-Ogunbiyi, K.; Borkett, K.M.; Mitchell, M.C. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth. Analg. 2013, 117, 1093–1100. [Google Scholar] [CrossRef]
- Brogden, R.N.; Goa, K.L. Flumazenil. A reappraisal of its pharmacological properties and therapeutic efficacy as a benzodiazepine antagonist. Drugs 1991, 42, 1061–1089. [Google Scholar] [CrossRef]
- Hood, S.D.; Norman, A.; Hince, D.A.; Melichar, J.K.; Hulse, G.K. Benzodiazepine dependence and its treatment with low dose flumazenil. Br. J. Clin. Pharmacol. 2014, 77, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guan, J.; Liu, L.; Fu, B.; Chen, L.; Zheng, X. Discharge readiness after remimazolam versus propofol for colonoscopy: A randomised, double-blind trial. Eur. J. Anaesthesiol. 2022, 39, 911–917. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, S.T.; Duan, K.M.; Bai, Z.H.; Feng, Y.F.; Guo, Q.L.; Cheng, Z.G.; Wu, H.; Shangguan, W.N.; Wu, X.M.; et al. Efficacy and safety of remimazolam besylate in bronchoscopy for adults: A multicenter, randomized, double-blind, positive-controlled clinical study. Front. Pharmacol. 2022, 13, 1005367. [Google Scholar] [CrossRef]
- Doi, M.; Morita, K.; Takeda, J.; Sakamoto, A.; Yamakage, M.; Suzuki, T. Efficacy and safety of remimazolam versus propofol for general anesthesia: A multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J. Anesth. 2020, 34, 543–553. [Google Scholar] [CrossRef]
- Zhang, J.; Cairen, Z.; Shi, L.; Pang, S.; Shao, Y.; Wang, Y.; Lu, Z. Remimazolam versus propofol for procedural sedation and anesthesia: A systemic review and meta-analysis. Minerva. Anestesiol. 2022, 88, 1035–1042. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, Y.T.; Chi, K.Y.; Huang, Y.T. Remimazolam versus propofol for procedural sedation: A meta-analysis of randomized controlled trials. PeerJ 2023, 11, e15495. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Z.; Liu, C.C.; Yu, H.Y.; Chao, C.C.; Lin, S.M. Lack of effect of flumazenil on the reversal of propofol anaesthesia. Acta Anaesthesiol. Scand. 1995, 39, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Q.; Xu, Y. Postoperative Discharge Scoring Criteria After Outpatient Anesthesia: A Review of the Literature. J. Perianesth. Nurs. 2023, 38, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Chung, F. Are discharge criteria changing? J. Clin. Anesth. 1993, 5, 64S–68S. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kawashima, S.; Makino, H.; Doi, M.; Nakajima, Y. Comparison of postoperative nausea and vomiting between remimazolam and propofol: A propensity score-matched, retrospective, observational, single-center cohort study. Korean J. Anesthesiol. 2023, 76, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nishiwaki, K. Comparison of remimazolam and propofol in anesthetic management for awake craniotomy: A retrospective study. J. Anesth. 2022, 36, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Morimoto, T.; Ichinomiya, T.; Murata, H.; Yoshitomi, O.; Hara, T. Effect of remimazolam on the incidence of delirium after transcatheter aortic valve implantation under general anesthesia: A retrospective exploratory study. J. Anesth. 2023, 37, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Mimuro, S.; Kurita, T.; Kobayashi, M.; Doi, M.; Katoh, T.; Nakajima, Y. Recall of extubation after remimazolam anesthesia with flumazenil antagonism during emergence: A retrospective clinical study. J. Anesth. 2022, 36, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Watanabe, T.; Kido, K.; Kamiya, S.; Otsuki, S.; Narasaki, S.; Toyota, Y.; Kondo, T.; Horikawa, Y.T.; Saeki, N.; et al. Remimazolam Requires Less Vasopressor Support during Induction and Maintenance of General Anesthesia in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: A Retrospective Analysis from a Single Center. BioMed Res. Int. 2022, 2022, 6386606. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kato, H.; Oshima, S.; Aoki, Y.; Obata, Y.; Mimuro, S.; Doi, M.; Nakajima, Y. Sedation using remimazolam does not reduce the duration of mechanical ventilation after elective cardiovascular surgery. Intensive Care Med. Exp. 2022, 10, 1–236. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.Y.; Ahn, Y.N.; You, A.H. Comparison of the Incidence of Postoperative Acute Kidney Injury Following the Administration of Remimazolam or Sevoflurane in Elderly Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Trial. J. Pers. Med. 2023, 13, 789. [Google Scholar] [CrossRef]
- Doi, M.; Hirata, N.; Suzuki, T.; Morisaki, H.; Morimatsu, H.; Sakamoto, A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): Results of a multicenter, randomized, double-blind, parallel-group comparative trial. J. Anesth. 2020, 34, 491–501. [Google Scholar] [CrossRef]
- Matsumoto, A.; Satomi, S.; Kakuta, N.; Narasaki, S.; Toyota, Y.; Miyoshi, H.; Horikawa, Y.T.; Saeki, N.; Tanaka, K.; Tsutsumi, Y.M. Remimazolam’s Effects on Postoperative Nausea and Vomiting Are Similar to Those of Propofol after Laparoscopic Gynecological Surgery: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 5402. [Google Scholar] [CrossRef]
- Zhang, F.; Chang, H.; Qing, W.; Yu, R.; Liao, Q.; Tong, J. Remimazolam Tosylate Combined with Low-Dose Propofol Improves Sedation and Safety in Hysteroscopy. Drug Des. Dev. Ther. 2022, 16, 4101–4108. [Google Scholar] [CrossRef]
- Tan, Y.; Ouyang, W.; Tang, Y.; Fang, N.; Fang, C.; Quan, C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J. Gastroenterol. Hepatol. 2022, 37, 576–583. [Google Scholar] [CrossRef]
- Nobukuni, K.; Shirozu, K.; Maeda, A.; Funakoshi, K.; Higashi, M.; Yamaura, K. Recovery of memory retention after anesthesia with remimazolam: An exploratory, randomized, open, propofol-controlled, single-center clinical trial. JA Clin. Rep. 2023, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, C.H.; Yoon, J.Y.; Byeon, G.J.; Kim, H.Y.; Choi, E.J. Comparison of postoperative nausea and vomiting between Remimazolam and Propofol in Patients undergoing oral and maxillofacial surgery: A prospective Randomized Controlled Trial. BMC Anesthesiol. 2023, 23, 132. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.B.; Kim, Y.J.; Cho, H.Y.; Kim, W.H.; Seo, J.H. Comparison of the recovery profile of remimazolam with flumazenil and propofol anesthesia for open thyroidectomy. BMC Anesthesiol. 2023, 23, 147. [Google Scholar] [CrossRef]
- Luo, W.; Sun, M.; Wan, J.; Zhang, Z.; Huang, J.; Zhang, J.; Xiong, W.; Xia, L.; Xu, P.; Miao, C.; et al. Efficacy and safety of remimazolam tosilate versus propofol in patients undergoing day surgery: A prospective randomized controlled trial. BMC Anesthesiol. 2023, 23, 182. [Google Scholar] [CrossRef]
- Oh, E.J.; Chung, Y.J.; Lee, J.H.; Kwon, E.J.; Choi, E.A.; On, Y.K.; Min, J.J. Comparison of propofol vs. remimazolam on emergence profiles after general anesthesia: A randomized clinical trial. J. Clin. Anesth. 2023, 90, 111223. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, M.; Gu, F.; Chen, J.; Zhang, W.; Huang, Z.; Zhu, D.; Song, J.; Fang, J.; Yu, W.; et al. Comparison of Remimazolam-Flumazenil versus Propofol for Rigid Bronchoscopy: A Prospective Randomized Controlled Trial. J. Clin. Med. 2022, 12, 257. [Google Scholar] [CrossRef]
- Qiu, Y.; Gu, W.; Zhao, M.; Zhang, Y.; Wu, J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: A randomized trial. Front. Med. 2022, 9, 938940. [Google Scholar] [CrossRef]
- Shi, F.; Chen, Y.; Li, H.; Zhang, Y.; Zhao, T. Efficacy and Safety of Remimazolam Tosilate versus Propofol for General Anesthesia in Cirrhotic Patients Undergoing Endoscopic Variceal Ligation. Int. J. Gen. Med. 2022, 15, 583–591. [Google Scholar] [CrossRef]
- Shimizu, T.; Takasusuki, T.; Yamaguchi, S. Remimazolam Compared to Propofol for Total Intravenous Anesthesia with Remifentanil on the Recovery of Psychomotor Function: A Randomized Controlled Trial. Adv. Ther. 2023, 40, 4395–4404. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, Q.; Wang, Z.; Zhu, S. Application effects of remimazolam and propofol on elderly patients undergoing hip replacement. BMC Anesthesiol. 2022, 22, 118. [Google Scholar] [CrossRef]
- Yue, L.; Ma, X.; Li, N.; Chen, J.; Wang, J.; Wan, Z.; Yang, L. Remimazolam versus propofol in combination with esketamine for surgical abortion: A double-blind randomized controlled trial. Clin. Transl. Sci. 2023, 16, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jiang, K.; Shi, W.; Xiao, S.; Zhang, S.; Zhang, Y.; Zhou, Y.; Tan, C.; Tan, S.; Zou, X. Effect of Remimazolam Tosilate on Respiratory Depression in Elderly Patients Undergoing Gastroscopy: A Multicentered, Prospective, and Randomized Study. Drug Des. Devel. Ther. 2022, 16, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, J.; Hao, R.; Wang, S.; Chen, M.; Liu, H.; Qi, L.; Hao, Z. The Efficacy and Safety of Remimazolam Besylate Combined with Esketamine for Outpatient Colonoscopy: A Prospective, Randomized, Controlled Clinical Trial. Drug Des. Devel. Ther. 2023, 17, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S.; Lee, D.H.; Park, J.S. Finding the ideal sedative: A non-inferiority study of remimazolam vs propofol in endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Masui, K. Caution!! Reappearance of remimazolam effect after a flumazenil bolus: A larger bolus of flumazenil and a lower total remimazolam clearance are higher risks. J. Anesth. 2023, 37, 1–5. [Google Scholar] [CrossRef]
- Takemori, T.; Oyama, Y.; Makino, T.; Hidaka, S.; Kitano, T. Long-term delayed emergence after remimazolam-based general anesthesia: A case report. JA Clin. Rep. 2022, 8, 86. [Google Scholar] [CrossRef]
- Kempenaers, S.; Hansen, T.G.; Van de Velde, M. Remimazolam and serious adverse events: A scoping review. Eur. J. Anaesthesiol. 2023, 40, 841–853. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Ljungqvist, O.; de Boer, H.D.; Balfour, A.; Fawcett, W.J.; Lobo, D.N.; Nelson, G.; Scott, M.J.; Wainwright, T.W.; Demartines, N. Opportunities and Challenges for the Next Phase of Enhanced Recovery After Surgery: A Review. JAMA Surg. 2021, 156, 775–784. [Google Scholar] [CrossRef] [PubMed]



| Study Year | Group-ing | Age (Years) | Gender (F/M) | ASA Class | Duration of Surgery (Min) | Duration of Anesthesia (Min) |
|---|---|---|---|---|---|---|
| Kim [44] 2023 | RF | 41.7 ± 12.2 | 33/61 | 1-2 | 42.8 ± 24.5 | 71.8 ± 26.1 |
| P | 43.3 ± 13.2 | 36/59 | 1-2 | 46.4 ± 22.9 | 75 ± 22.4 | |
| Lee [45] 2023 | RF | 45 ± 13.4 | 21/7 | 1-2 | 85 (70, 98) | NR |
| P | 51 ± 12.1 | 19/10 | 1-2 | 85 (75, 105) | NR | |
| Luo [46] 2023 | RF | 44.7 ± 16.8 | 16/22 | 1-2 | 38.6 ± 29.1 | NR |
| R | 43.5 ± 15.6 | 24/14 | 1-2 | 42.2 ± 27.7 | NR | |
| P | 44.3 ± 18.1 | 19/19 | 1-2 | 38.7 ± 25.1 | NR | |
| Oh [47] 2023 | RF | 60 (54, 65) | 8/42 | 2-3 | NR | 124 (112, 142) |
| P | 60 (52, 64) | 8/42 | 2-3 | NR | 123 (116, 140) | |
| Pan [48] 2022 | RF | 61.13 ± 8.62 | 3/12 | 2-4 | 36.67 ± 19.85 | 41.47 ± 19.31 |
| P | 60.13 ± 7.24 | 0/15 | 2-3 | 44.40 ± 22.72 | 49.60 ± 22.86 | |
| Qiu [49] 2022 | RF | 62.8 ± 7.1 | 7/21 | 1-3 | 53 (27.5, 81) | 92.5 (66.3, 120.8) |
| PF | 64.7 ± 8.9 | 9/19 | 1-3 | 55 (35.5, 77.3) | 87 (71.5, 114.8) | |
| Shi [50] 2022 | RF | 52.74 ± 4.93 | 22/16 | 2-3 | 27 ± 2.72 | NR |
| P | 51.61 ± 5.48 | 20/18 | 2-3 | 26.88 ± 2.88 | NR | |
| Shimizu [51] 2023 | RF | 43.5 ± 10.4 | 10/22 | 1-2 | 107 ± 38 | 157 ± 42 |
| P | 44.4 ± 10.1 | 10/22 | 1-2 | 121 ± 57 | 178 ± 60 | |
| Zhang [52] 2022 | RF | 74.31 ± 10.6 | 19/11 | 2-3 | NR | 130.16 ± 43.01 |
| PF | 75.04 ± 9.98 | 17/12 | 2-3 | NR | 131.64 ± 45.63 |
| Study Year | Country | Grouping | Sample Size | RB/RT | Anesthetic Dose | Flumazenil Dose | FDT | Surgery | Airway | NMBRA | ORA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction | Maintenance | |||||||||||
| Kim [44] 2023 | Korea | RF | 94 | NR | 12 mg/kg/h | 1–2 mg/kg/h | 0.2 mg | ➃ | OMS | Intubation | Yes | No |
| P | 95 | TCI 3–5 μg/mL | TCI 3–5 μg/mL | ― | ||||||||
| Lee [45] 2023 | Korea | RF | 28 | RB | 6 mg/kg/h | 1–2 mg/kg/h | 0.2–1 mg | ➀ | TT | Intubation | Yes | No |
| P | 29 | TCI 3 ng/mL | TCI ≥ 2 ng/mL | ― | ||||||||
| Luo [46] 2023 | China | RF | 38 | RT | 0.3 mg/kg | 1–3 mg/kg/h | 0.2–1 mg | ➁ | DS | LMA | Yes | Yes |
| R | 38 | 0.3 mg/kg | 1–3 mg/kg/h | ― | ||||||||
| P | 38 | 2–2.5 mg/kg | 6–12 mg/kg/h | ― | ||||||||
| Oh [47] 2023 | Korea | RF | 50 | RB | 6 mg/kg/h | 1–2 mg/kg/h | 0.2–1 mg | ➀ | CA | LMA | Yes | No |
| P | 50 | TCI 5 μg/mL | TCI 3–5 μg/mL | ― | ||||||||
| Pan [48] 2022 | China | RF | 15 | RB | 0.4 mg/kg | 1 mg/kg/h | 0.5 mg | ➂ | RBS | LMA | Yes | No |
| P | 15 | 1.5 mg/kg | 4–8 mg/kg/h | ― | ||||||||
| Qiu [49] 2022 | China | RF | 28 | RT | 0.3 mg/kg | 1–3 mg/kg/h | 0.5 mg | ➀ | ESD | Intubation | Yes | No |
| PF | 28 | 2 mg/kg | 5 mg/kg/h | 0.5 mg | ||||||||
| Shi [50] 2022 | China | RF | 38 | RT | 0.2 mg/kg | 1–2 mg/kg/h | 0.5 mg | ➀ | EVL | Intubation | No | No |
| P | 38 | 2 mg/kg | 4–10 mg/kg/h | ― | ||||||||
| Shimizu [51] 2023 | Japan | RF | 32 | NR | 12 mg/kg/h | 1–2 mg/kg/h | 0.2–0.5 mg | ➀ | ESS | Intubation | Yes | No |
| P | 32 | TCI 3–4 μg/mL | TCI 2–5 μg/mL | ― | ||||||||
| Zhang [52] 2022 | China | RF | 30 | NR | 0.2–0.4 mg/kg | 0.3–0.5 mg/kg/h | 0.3 mg | ➀ | HR | Intubation | No | No |
| PF | 29 | 1.5–2 mg/kg | 4–8 mg/kg/h | 0.3 mg | ||||||||
| Variable | β | z | p Value | 95% CI | R2 (%) |
|---|---|---|---|---|---|
| Individual-level variables | |||||
| Mean age | −0.04 | −0.31 | 0.76 | −0.31, 0.22 | 0 |
| ASA class 1–2 (%) | 0.01 | 0.15 | 0.88 | −0.12, 0.14 | 0 |
| Surgery duration | 0.01 | 0.28 | 0.78 | −0.06, 0.08 | 0 |
| Female (%) | 0.08 | 1.34 | 0.18 | −0.04, 0.19 | 10.47 |
| Mean BMI | −0.52 | −0.43 | 0.66 | −2.87, 1.82 | 0 |
| Study-level variables | |||||
| Country | 0 | ||||
| China/others | −0.67 | −0.15 | 0.88 | −9.48, 8.13 | |
| Korea/others | −2.6 | −0.52 | 0.6 | −12.47, 7.27 | |
| Control (P/PF) | 0.07 | 0.02 | 0.98 | −6.3, 6.44 | 0 |
| RB/RT | 0.08 | 0.02 | 0.98 | −6.63, 6.78 | 0 |
| Flumazenil dose | −10.83 | −1.19 | 0.23 | −28.65, 6.99 | 5.68 |
| FDT (➀/others) | −3.40 | −1.17 | 0.24 | −9.11, 2.31 | 4.94 |
| Airway (LMA/intubation) | 0.35 | 0.12 | 0.9 | −5.28, 5.98 | 0 |
| NMBRA (yes/no) | 0.76 | 0.24 | 0.8 | −5.39, 6.92 | 0 |
| ORA (yes/no) | −5.35 | −1.47 | 0.14 | −12.5, 1.8 | 14.43 |
| Primary outcome (yes/no) | −0.83 | −0.3 | 0.76 | −6.24, 4.58 | 0 |
| Sample size | −0.05 | −0.77 | 0.44 | −0.19, 0.08 | 0 |
| Emergence definition | 62.29 | ||||
| Eye opening/others | 6.48 | 3.15 | 0.002 | 2.45, 10.52 | |
| Obey verbal commands /others | 1.73 | 0.72 | 0.47 | −2.99, 6.46 | |
| Risk of bias (some concerns/high risk) | −1.02 | −0.32 | 0.75 | −7.23, 5.19 | 0 |
| Variable | β | z | p Value | 95% CI |
|---|---|---|---|---|
| Emergence definition | ||||
| Eye opening/others | 12.28 | 3.13 | 0.002 | 4.6, 19.96 |
| Obey verbal commands/others | 7.22 | 1.62 | 0.1 | −1.52, 15.96 |
| ORA (yes/no) | −5.96 | −1.08 | 0.28 | −16.73, 4.81 |
| Female (%) | 0.02 | 0.6 | 0.55 | −0.05, 0.1 |
| Flumazenil dose | 25.78 | 1.82 | 0.07 | −2.03, 53.6 |
| FDT (➀/others) | 2.49 | 0.52 | 0.6 | −6.89, 11.88 |
| Postoperative Complication | No of Studies | No of Patients | Effect Model | Method | I2 (%) | Results of Meta-Analyses | Certainty | |
|---|---|---|---|---|---|---|---|---|
| MD (95% CI) | p Value | |||||||
| Pain | 2 ! | 135 | Fixed | I-V | 0 | −0.01 (−0.08, 0.06) | 0.78 | High |
| RR (95% CI) | p value | |||||||
| PONV | 7 | 587 | Fixed | M-H | 0 | 0.87 (0.49, 1.56) | 0.64 | High |
| Respiratory depression | 4 | 311 | Fixed | M-H | 0 | 0.2 (0.04, 0.89) | 0.03 * | High |
| Emergence agitation | 4 | 291 | Fixed | M-H | 0 | 0.45 (0.1, 1.94) | 0.28 | Moderate |
| Re-sedation | 4 | 289 | Fixed | M-H | 35.07 | 4.15 (1.31, 13.13) | 0.01 * | Moderate |
| Delirium | 3 | 172 | Fixed | M-H | 0 | 0.67 (0.11, 3.87) | 0.65 | Low |
| Dizziness | 2 | 135 | Fixed | M-H | 0 | 0.42 (0.11, 1.57) | 0.2 | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Xu, F.; Wang, J.; Jiang, M. Comparison of Remimazolam–Flumazenil versus Propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7316. https://doi.org/10.3390/jcm12237316
Wu Q, Xu F, Wang J, Jiang M. Comparison of Remimazolam–Flumazenil versus Propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(23):7316. https://doi.org/10.3390/jcm12237316
Chicago/Turabian StyleWu, Quantong, Fuchao Xu, Jie Wang, and Ming Jiang. 2023. "Comparison of Remimazolam–Flumazenil versus Propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 23: 7316. https://doi.org/10.3390/jcm12237316
APA StyleWu, Q., Xu, F., Wang, J., & Jiang, M. (2023). Comparison of Remimazolam–Flumazenil versus Propofol for Recovery from General Anesthesia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(23), 7316. https://doi.org/10.3390/jcm12237316