A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Follow-Up
2.2. Laboratory Methods
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimopoulos, K.; Tripodi, A.; Goetze, J.P. Laboratory investigation and diagnosis of thrombotic thrombocytopenic purpura. Crit. Rev. Clin. Lab. Sci. 2023, 60, 1–15. [Google Scholar] [CrossRef]
- Joly, B.S.; Coppo, P.; Veyradier, A. Thrombotic thrombocytopenic purpura. Blood 2017, 129, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, F.; Fakhfakh, Y.; Charfi, M.; Kassar, O.; Chaari, M.; Bouaoun, L.; Bahloul, M.; Frikha, I.; Kallel, F.; Ben Amor, I.; et al. Thrombotic Thrombocytopenic Purpura (TTP): A Single-Center Experience. HemaSphere 2022, 6, 2174–2175. [Google Scholar] [CrossRef]
- Chiasakul, T.; Cuker, A. Clinical and laboratory diagnosis of TTP: An integrated approach. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 30, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Mc Daniel, J.K.; Zheng, X.L. Thrombotic thrombocytopenic purpura: Pathogenesis, diagnosis and potential novel therapeutics. J. Thromb. Haemost. 2017, 15, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.; Poullin, P.; de Maistre, E.; Provôt, F.; Delmas, Y.; et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): A cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haetmatol. 2016, 3, e237–e245. [Google Scholar] [CrossRef] [PubMed]
- Radhwi, O.; Badawi, M.A.; Almarzouki, A.; Al–Ayoubi, F.; ElGohary, G.; Asfina, K.N.; Basendwah, A.M.; Alhazmi, I.A.; Almahasnah, E.A.; AlBahrani, A.; et al. A Saudi multicenter experience on therapeutic plasma exchange for patients with thrombotic thrombocytopenic purpura: A call for national registry. J. Clin. Apher. 2023, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Yan, M.; Wen, R.; Li, J. Sequential treatment of rituximab and belimumab in thrombotic thrombocytopenia purpura associated with systemic lupus erythematous: A respective case series and literature review. Int. J. Rheum. Dis. 2023, 26, 960–964. [Google Scholar] [CrossRef]
- Djulbegovic, M.; Tong, J.; Xu, A.; Yang, J.; Chen, Y.; Cuker, A.; Pishko, A.M. Adding caplacizumab to standard of care in thrombotic thrombocytopenic purpura: A systematic review and meta-analysis. Blood Adv. 2023, 7, 2132–2142. [Google Scholar] [CrossRef]
- Uemura, M.; Tatsumi, K.; Matsumoto, M.; Fujimoto, M.; Matsuyama, T.; Ishikawa, M.; Iwamoto, T.; Mori, T.; Wanaka, A.; Fukui, H.; et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood 2005, 106, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Furlan, M.; Robles, R.; Lämmle, B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996, 87, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.T.; de Groot, R.; Xiang, Y.; Luken, B.M.; Lane, D.A. Unraveling the scissile bond: How ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 2011, 118, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Joly, B.S.; Picod, A.; Veyradier, A.; Coppo, P. The Specificities of Thrombotic Thrombocytopenic Purpura at Extreme Ages: A Narrative Review. J. Clin. Med. 2023, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Newnham, M.; South, K.; Bleda, M.; Auger, W.R.; Barberà, J.A.; Bogaard, H.; Bunclark, K.; Cannon, J.E.; Delcroix, M.; Hadinnapola, C.; et al. The ADAMTS13-VWF axis is dysregulated in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801805. [Google Scholar] [CrossRef]
- González-López, T.J.; Provan, D.; Bárez, A.; Bernardo-Gutiérrez, A.; Bernat, S.; Martínez-Carballeira, D.; Jarque-Ramos, I.; Soto, I.; Jiménez-Bárcenas, R.; Fernández-Fuertes, F. Primary and secondary immune thrombocytopenia (ITP): Time for a rethink. Blood Rev. 2023, 61, 101112. [Google Scholar] [CrossRef]
- Upshaw, J.D. Congenital deficiency of a factor in normal plasma that reverses microangiopathic hemolysis and thrombocytopenia. N. Eng. J. Med. 1978, 298, 1350–1352. [Google Scholar] [CrossRef]
- Schulman, I.; Pierce, M.; Lukens, A.; Currimbhoy, Z. Studies on thrombopoiesis. I. A factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency. Blood 1960, 16, 943–957. [Google Scholar] [CrossRef]
- Levy, G.; Nichols, W.; Lian, E.; Foroud, T.; McClintick, J.N.; McGee, B.M.; Yang, A.Y.; Siemieniak, D.R.; Stark, K.R.; Gruppo, R.; et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001, 413, 488–494. [Google Scholar] [CrossRef]
- Sakai, K.; Matsumoto, M. Clinical Manifestations, Current and Future Therapy, and Long-Term Outcomes in Congenital Thrombotic Thrombocytopenic Purpura. J. Clin. Med. 2023, 12, 3365. [Google Scholar] [CrossRef]
- Lancellotti, S.; Sacco, M.; Tardugno, M.; Ferretti, A.; De Cristofaro, R. Immune and Hereditary Thrombotic Thrombocytopenic Purpura: Can ADAMTS13 Deficiency Alone Explain the Different Clinical Phenotypes? J. Clin. Med. 2023, 12, 3111. [Google Scholar] [CrossRef]
- Denorme, F.; Vanhoorelbeke, K.; De Meyer, S.F. von Willebrand Factor and Platelet Glycoprotein Ib: A Thromboinflammatory Axis in Stroke. Front. Immunol. 2019, 10, 2884. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Fuchs, T.A.; Chauhan, A.K.; Yang, J.J.; De Meyer, S.F.; Kollnberger, M.; Wakefield, T.W.; Lammle, B.; Massberg, S.; Wagner, D.D. Von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2010, 117, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, M.S.; Hansen, E.S.; Ueland, T.; Aukrust, P.; Brækkan, S.K.; Morelli, V.M.; Hansen, J.B. Impact of the von Willebrand factor-ADAMTS-13 axis on the risk of future venous thromboembolism. J. Thromb. Haemost. 2023, 21, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, C.; Lenting, P.J.; Denis, C.V. von Willebrand factor and inflammation. J. Thromb. Haemost. 2017, 15, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.; Hathaway, L.S.; Collins, P.W.; Bowen, D.J. von Willebrand factor: Demographics of plasma protein level in a large blood donor cohort from South Wales in the United Kingdom. Haemophilia 2012, 18, e79–e81. [Google Scholar] [CrossRef]
- Alavi, P.; Rathod, A.M.; Jahroudi, N. Age-associated increase in thrombogenicity and its correlation with von Willebrand factor. J. Clin. Med. 2021, 10, 4190. [Google Scholar] [CrossRef]
- Wieland, I.; Wermes, C.; Welte, K.; Sykora, K.W. Two Examples of the Influence of Psychological Stress on the von Willebrand Factor Activity. In 37th Hemophilia Symposium Hamburg 2006; Scharrer, I., Schramm, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 142–144. [Google Scholar]
- Malan, N.T.; von Känel, R.; Schutte, A.E.; Huisman, H.W.; Schutte, R.; Smith, W.; Mels, C.M.; Kruger, R.; Meiring, M.; van Rooyen, J.M.; et al. Testosterone and acute stress are associated with fibrinogen and von Willebrand factor in African men: The SABPA study. Int. J. Cardiol. 2013, 168, 4638–4642. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Patmore, S.; Dhami, S.P.S.; O’Sullivan, J.M. Von Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemost. 2020, 18, 2444–2456. [Google Scholar] [CrossRef]
- Pinsky, D.J.; Naka, Y.; Liao, H.; Oz, M.C.; Wagner, D.D.; Mayadas, T.N.; Johnson, R.C.; Hynes, R.O.; Heath, M.; Lawson, C.A.; et al. Hypoxia-induced exocytosis of endothelial cell Weibel Palade bodies. A mechanism for rapid neuthophil recruitment after cardiac preservation. J. Clin. Investig. 1996, 97, 495–500. [Google Scholar] [CrossRef]
- Gómez-Seguí, I.; Pascual Izquierdo, C.; Mingot Castellano, M.E.; De la Rubia Comos, J. An update on the pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert. Rev. Hematol. 2023, 16, 17–32. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, J.Y. Thrombotic thrombocytopenic purpura with neurological impairment: A Review. Medicine 2022, 101, e31851. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Scully, M. Management of thrombotic thrombocytopenic purpura: Current perspectives. J. Blood Med. 2014, 11, 15–23. [Google Scholar] [CrossRef]
- Coppo, P.; Schwarzinger, M.; Buffet, M.; Wynckel, A.; Clabault, K.; Presne, C.; Poullin, P.; Malot, S.; Vanhille, P.; Azoulay, E.; et al. French Reference Center for Thrombotic Microangiopathies. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: The French TMA reference center experience. PLoS ONE 2010, 5, e10208. [Google Scholar] [CrossRef] [PubMed]
- Bendapudi, P.K.; Hurwitz, S.; Fry, A.; Marques, M.B.; Waldo, S.W.; Li, A.; Sun, L.; Upadhyay, V.; Hamdan, A.; Brunner, A.M.; et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: A cohort study. Lancet Haematol. 2017, 4, e157–e164. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Khalighi, P.R.; Wu, Q.; Garcia, D.A. External validation of the PLASMIC score: A clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J. Thromb. Haemost. 2018, 16, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Simmons, S.C.; Williams, L.A.; Staley, E.M.; Zheng, X.L.; Pham, H.P. ADAMTS13 test and/or PLASMIC clinical score in management of acquired thrombotic thrombocytopenic purpura: A cost-effective analysis. Transfusion 2017, 57, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Staley, E.M.; Cao, W.; Pham, H.P.; Kim, C.H.; Kocher, N.K.; Zheng, L.; Gangaraju, R.; Lorenz, R.G.; Williams, L.A.; Marques, M.B.; et al. Clinical factors and biomarkers predict outcome in patients with immune-mediated thrombotic thrombocytopenic purpura. Haematologica 2019, 104, 166–175. [Google Scholar] [CrossRef]
- George, J.N.; Nester, C.M. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 2014, 371, 654–666. [Google Scholar] [CrossRef]
- Rieger, M.; Mannucci, P.M.; Kremer Hovinga, J.A.; Herzog, A.; Gerstenbauer, G.; Konetschny, C.; Zimmermann, K.; Scharrer, I.; Peyvandi, F.; Galbusera, M.; et al. ADAMTS 13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated disease. Blood 2005, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Cao, W.; Halkidis, K.; Abdelgawwad, M.S.; Kocher, N.K.; Guillory, B.; Williams, L.A.; Gangaraju, R.; Marques, M.B.; Zheng, X.L. Longitudinal assessments of plasma ADAMTS13 biomarkers predict recurrence of immune thrombotic thrombocytopenic purpura. Blood Adv. 2019, 3, 4177–4186. [Google Scholar] [CrossRef]
- Sui, J.; Zheng, L.; Zheng, L. ADAMTS13 Biomarkers in Management of Immune Thrombotic Thrombocytopenic Purpura. Arch. Pathol. Lab. Med. 2023, 147, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Joly, B.S.; Darmon, M.; Dekimpe, C.; Dupont, T.; Dumas, G.; Yvin, E.; Beranger, N.; Vanhoorelbeke, K.; Azoulay, E.; Veyradier, A. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. J. Thromb. Haemost. 2021, 19, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, S.W.; Santos de Oliveira, M.H.; Lippi, G.; Favaloro, E.J.; Benoit, J.L. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: Evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy. Int. J. Lab. Hematol. 2021, 43, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; Tardugno, M.; Lancellotti, S.; Ferretti, A.; Ponziani, F.R.; Riccardi, L.; Zocco, M.A.; De Magistris, A.; Santopaolo, F.; Pompili, M.; et al. ADAMTS-13/von Willebrand factor ratio: A prognostic biomarker for portal vein thrombosis in compensated cirrhosis. A prospective observational study. Dig. Liver Dis. 2022, 54, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J. British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef]
- Cuker, A.; Cataland, S.R.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; George, J.N.; Knoebl, P.N.; Kremer Hovinga, J.A.; Lämmle, B.; Matsumoto, M.; et al. Redefining outcomes in immune TTP: An international working group consensus report. Blood 2021, 137, 1855–1861. [Google Scholar] [CrossRef]
- Sukumar, S.; Lämmle, B.; Cataland, S.R. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J. Clin. Med. 2021, 10, 536. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; Kremer Hovinga, J.; Lämmle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. International Working Group for Thrombotic Thrombocytopenic Purpura. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2017, 15, 312–322. [Google Scholar] [CrossRef]
- Graça, N.A.G.; Joly, B.S.; Voorberg, J.; Vanhoorelbeke, K.; Béranger, N.; Veyradier, A.; Coppo, P. TTP: From empiricism for an enigmatic disease to targeted molecular therapies. Br. J. Haematol. 2022, 197, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Amin Asnafi, A.; Jalali, M.T.; Pezeshki, S.M.S.; Jaseb, K.; Saki, N. The Association Between Human Leukocyte Antigens and ITP, TTP and HIT. J. Pediatr. Hematol. Oncol. 2019, 41, 81–86. [Google Scholar] [CrossRef]
- Scully, M.; Brown, J.; Patel, R.; McDonald, V.; Brown, C.J.; Machin, S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: Evidence for an immunogenetic link. J. Thromb. Haemost. 2010, 8, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hrdinová, J.; D’Angelo, S.; Graça, N.A.; Ercig, B.; Vanhoorelbeke, K.; Veyradier, A.; Voorberg, J.; Coppo, P. Dissecting the pathophysiology of immune thrombotic thrombocytopenic purpura: Interplay between genes and environmental triggers. Haematologica 2021, 103, 924. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Kokame, K.; Sadler, J.E. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J. Biol. Chem. 2006, 281, 850–857. [Google Scholar] [CrossRef]
- Beattie, J.H.; Kwun, I.S. Is zinc deficiency a risk factor for atherosclerosis? Br. J. Nutr. 2004, 91, 177–181. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Demetrios, A.; Aaseth, J.; Tsatsakis, A.; et al. Zinc and respiratory tract infection: Perspectives for COVID-19. Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Sharma, S.; Tyagi, T.; Antoniak, S. Platelet in thrombo-inflammation: Unraveling new therapeutic targets. Front. Immunol. 2022, 13, 1039843. [Google Scholar] [CrossRef]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef]
- Naß, J.; Terglane, J.; Gerke, V. Weibel Palade bodies: Unique secretory organelles of endothelial cells that control blood vessel homeostasis. Front. Cell. Dev. Biol. 2021, 9, 813995. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.; Dragunaite, B.; Scully, M.; De Groot, R. Calprotectin levels are elevated in congenital TTP and immune TTP at ADAMTS13 relapse. HemaSphere 2023, 7, e2092072. [Google Scholar] [CrossRef]
- Rondaij, M.G.; Bierings, R.; Kragt, A.; van Mourik, J.A.; Voorberg, J. Dynamics and plasticity of Weibel-Palade bodies in en-dothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Kappers-Klunne, M.C.; van Asten, J.G.; van Vliet, H.H. ADAMTS-13 and Von Willebrand factor in relation to platelet re-sponse during plasma exchange in thrombotic thrombocytopenic purpura: A clue for disease mechanism? Ann. Hematol. 2009, 88, 1025–1028. [Google Scholar] [CrossRef][Green Version]
- Page, E.E.; Kremer Hovinga, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Thrombotic thrombocytopenic purpura: Diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017, 6, 590–600. [Google Scholar] [CrossRef]
- Westwood, J.P.; Webster, H.; McGuckin, S.; McDonald, V.; Machin, S.J.; Scully, M. Rituximab for thrombotic thrombocytopenic purpura: Benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J. Thromb. Haemost. 2013, 11, 481–490. [Google Scholar] [CrossRef]
- Falter, T.; Herold, S.; Weyer-Elberich, V.; Scheiner, C.; Schmitt, V.; von Auer, C.; Messmer, X.; Wild, P.; Lackner, K.J.; Lämmle, B.; et al. Relapse Rate in Survivors of Acute Autoimmune Thrombotic Thrombocytopenic Purpura Treated with or without Rituximab. Thromb. Haemost. 2018, 118, 1743–1751. [Google Scholar] [CrossRef]
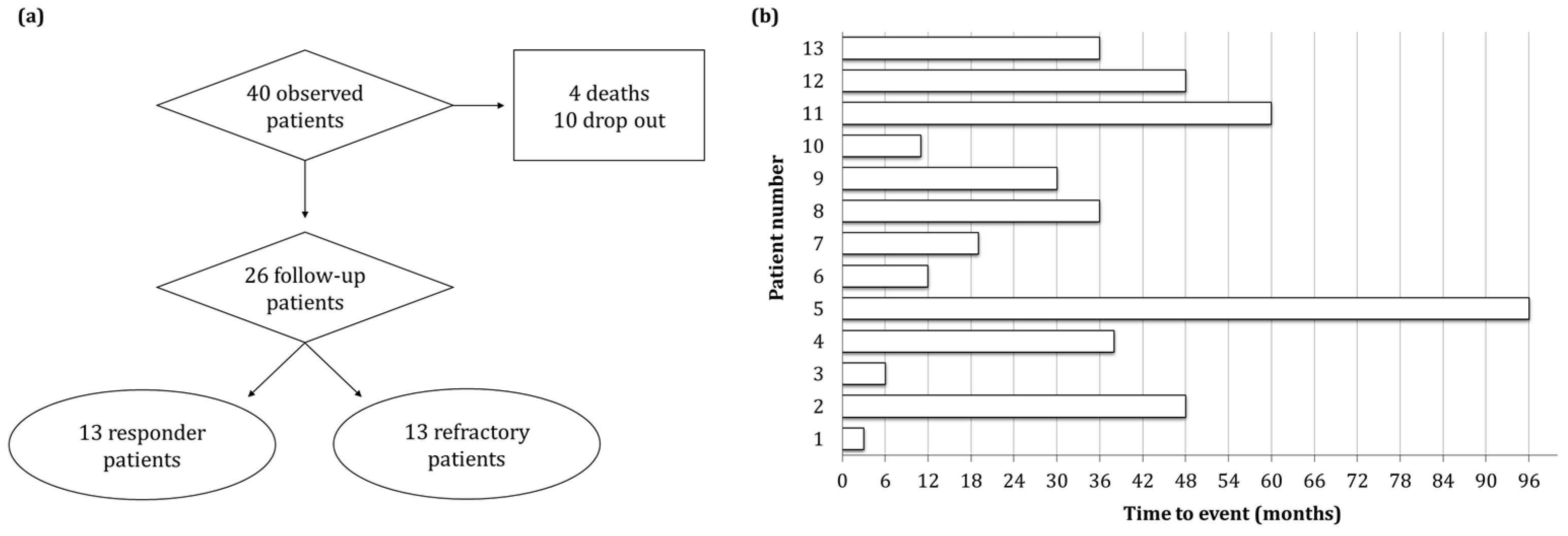
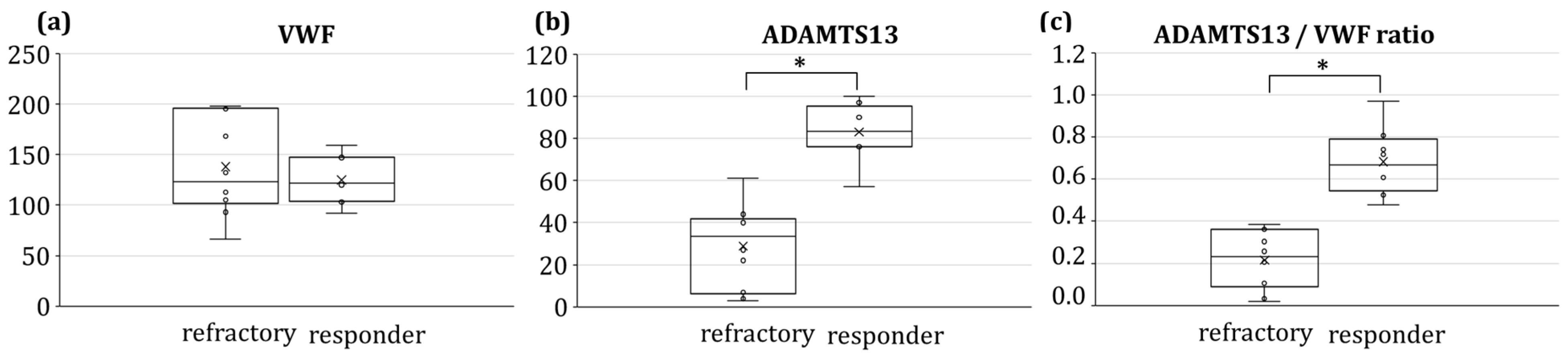
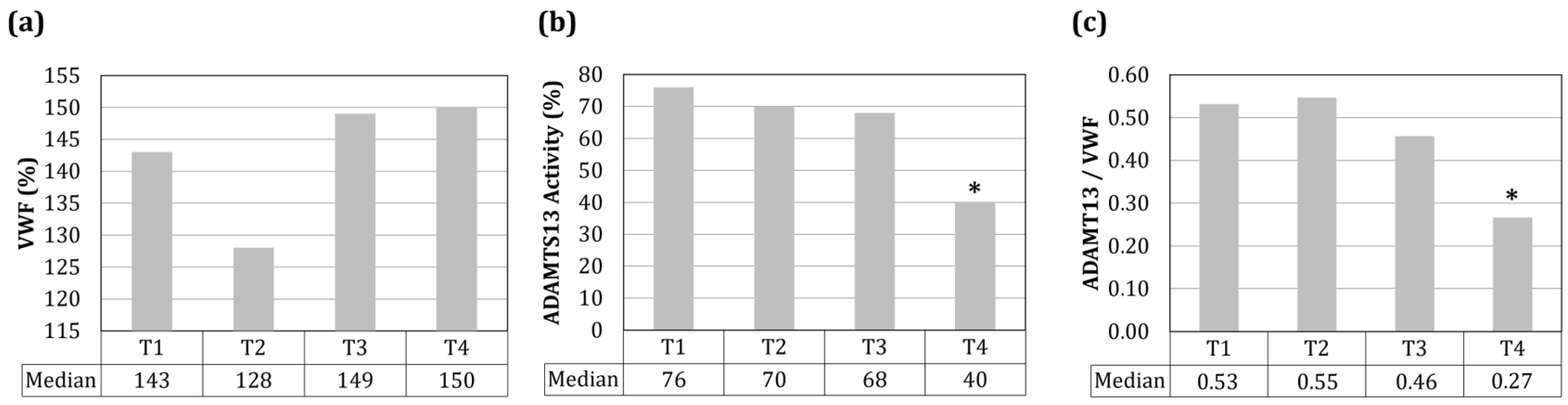
| Parameter | Characteristics | Percentage (%) |
|---|---|---|
| Median age (IQR) | 49 (29–59) | |
| Gender (M:F) | 9:31 | 23:77 |
| PLASMIC Score 1 | 7 (n = 10) | 38 |
| 6 (n = 5) | 19 | |
| n.a. (n = 11) | 42 | |
| Thrombosis | 9/26 | 35 |
| Bleeding | 17/26 | 65 |
| Therapy | Steroids (n = 10) | 38 |
| TPE (n = 20) | 77 | |
| Caplacizumab (n = 5) | 19 | |
| Vincristine (n = 3) | 11 | |
| Rituximab (refractory) 2 (n = 5) | 19 | |
| Rituximab (effective) (n = 12) | 46 | |
| Comorbidities | Infection (EBV, E. pylori, S. aureus, K. pneumoniae) (n = 4) | 15 |
| Cancer (prostate, breast, medulloblastoma) (n = 3) | 11 | |
| Diabetes mellitus type II (n = 1) | 4 | |
| Angina pectoris (n = 1) | 4 | |
| Autoimmune disorders (n = 5) | 23 | |
| Other parameters | Fever (n = 3) | 11 |
| Pregnancy (n = 1) | 4 | |
| Neurological symptoms (n = 13) | 50 | |
| Renal impairment (n = 3) | 11 | |
| HLA-DRB1*11 (n = 4) | 15 |
| Laboratory Parameter | Spearman’s r Coefficient | p-Value |
|---|---|---|
| ADAMTS13 | 0.84 | 1.3 × 10−5 |
| ADAMTS13/VWF ratio | 0.86 | 4.6 × 10−6 |
| VWF | −0.13 | 0.61 |
| ADAMTS13 inhibitor | −0.11 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonifacio, M.A.; Roselli, D.; Schifone, C.P.; Ricco, A.; Vitucci, A.; Aprile, L.; Mariggiò, M.A.; Ranieri, P. A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes? J. Clin. Med. 2023, 12, 7305. https://doi.org/10.3390/jcm12237305
Bonifacio MA, Roselli D, Schifone CP, Ricco A, Vitucci A, Aprile L, Mariggiò MA, Ranieri P. A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes? Journal of Clinical Medicine. 2023; 12(23):7305. https://doi.org/10.3390/jcm12237305
Chicago/Turabian StyleBonifacio, Maria Addolorata, Daniele Roselli, Claudia Pia Schifone, Alessandra Ricco, Angelantonio Vitucci, Lara Aprile, Maria Addolorata Mariggiò, and Prudenza Ranieri. 2023. "A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes?" Journal of Clinical Medicine 12, no. 23: 7305. https://doi.org/10.3390/jcm12237305
APA StyleBonifacio, M. A., Roselli, D., Schifone, C. P., Ricco, A., Vitucci, A., Aprile, L., Mariggiò, M. A., & Ranieri, P. (2023). A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes? Journal of Clinical Medicine, 12(23), 7305. https://doi.org/10.3390/jcm12237305








