Aortic Valve Replacement: Totally Endoscopic versus Mini-Sternotomy
Abstract
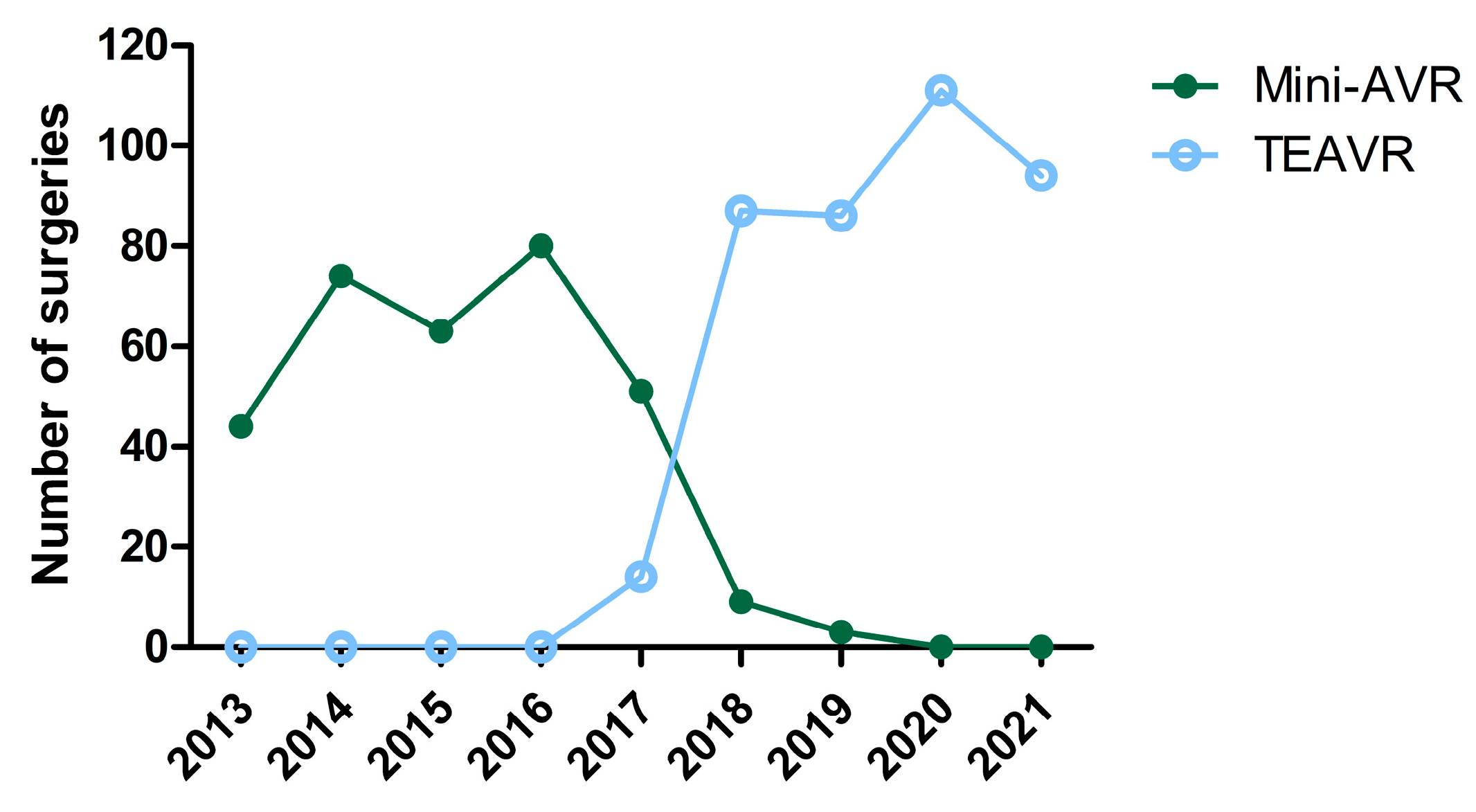
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Technique
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Perioperative Data
3.3. Postoperative Parameters
3.4. MACCE
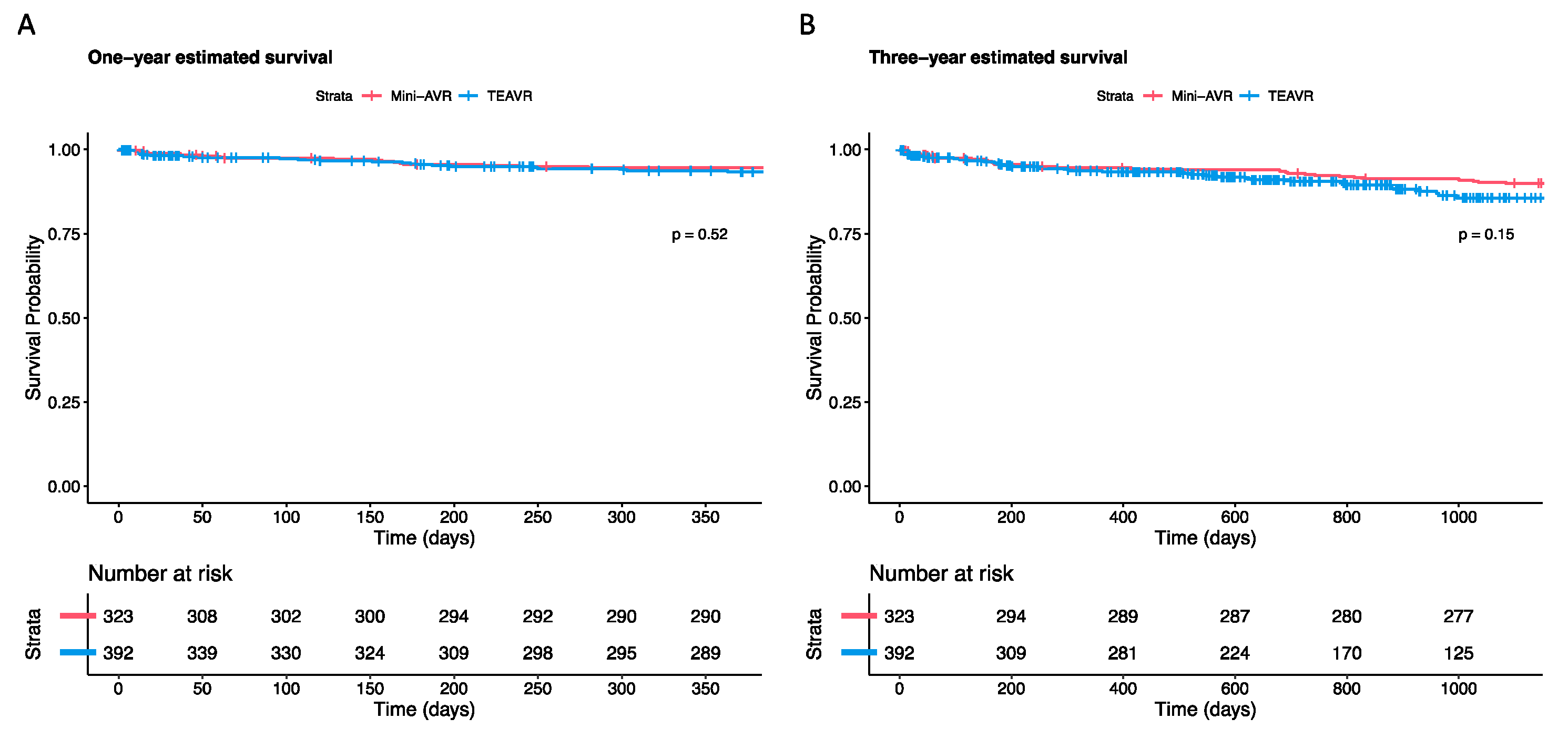
3.5. All-Cause Mortality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Praet, K.M.; Kampen, A.; Kofler, M.; Richter, G.; Sündermann, S.H.; Meyer, A.; Unbehaun, A.; Kurz, S.; Jacobs, S.; Falk, V.; et al. Minimally invasive surgical aortic valve replacement: The RALT approach. J. Card. Surg. 2020, 35, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Dokollari, A.; Parise, O.; Sani, G.; Prifti, E.; Bisleri, G.; Gelsomino, S. Ministernotomy compared with right anterior minithoracotomy for aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2021, 165, 1022–1032.e2. [Google Scholar] [CrossRef] [PubMed]
- Vola, M.; Fuzellier, J.F.; Chavent, B.; Duprey, A. First human totally endoscopic aortic valve replacement: An early report. J. Thorac. Cardiovasc. Surg. 2014, 147, 1091–1093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cresce, G.D.; Sella, M.; Hinna Danesi, T.; Favaro, A.; Salvador, L. Minimally Invasive Endoscopic Aortic Valve Replacement: Operative Results. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, M.; Sawaki, S.; Ozeki, T.; Orii, M.; Usui, A.; Ito, T. Totally endoscopic aortic valve replacement via an anterolateral approach using a standard prosthesis. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 424–430. [Google Scholar] [CrossRef]
- Pitsis, A.; Boudoulas, H.; Boudoulas, K.D. Operative steps of totally endoscopic aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yang, L.; He, B.C.; Ke, Y.J.; Yang, Y.C.; Yan, Q.; Chen, Z.R.; Huang, H.L. Total thoracoscopic repair of ventricular septal defect: A single-center experience. J. Card. Surg. 2021, 36, 2213–2218. [Google Scholar] [CrossRef]
- Yilmaz, A.; Rehman, A.; Sonker, U.; Kloppenburg, G.T. Minimal access aortic valve replacement using a minimal extracorporeal circulatory system. Ann. Thorac. Surg. 2009, 87, 720–725. [Google Scholar] [CrossRef]
- Yilmaz, A.; Van Genechten, S.; Claessens, J.; Packlé, L.; Maessen, J.; Kaya, A. A totally endoscopic approach for aortic valve surgery. Eur. J. Cardiothorac. Surg. 2022, 62, ezac467. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Mourad, F.; Abd Al Jawad, M. Mini Sternotomy and Mini Thoracotomy for Aortic Valve Replacement: Is There a Difference? Heart Surg. Forum 2021, 24, e855–e859. [Google Scholar] [CrossRef]
- Tokoro, M.; Ito, T.; Maekawa, A.; Sawaki, S.; Yanagisawa, J.; Ozeki, T.; Orii, M. Trans-right axillary aortic valve replacement: Propensity-matched comparison with standard sternotomy approach†. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 521–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vo, A.T.; Nguyen, D.H.; Van Hoang, S.; Le, K.M.; Nguyen, T.T.; Nguyen, V.L.; Nguyen, B.H.; Truong, B.Q. Learning curve in minimally invasive mitral valve surgery: A single-center experience. J. Cardiothorac. Surg. 2019, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Nakamura, Y.; Ito, Y.; Kuroda, M.; Nishijima, S.; Okuzono, Y.; Hirano, T.; Hori, T. The learning curve of minimally invasive aortic valve replacement for aortic valve stenosis. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Gilmanov, D.; Farneti, P.A.; Kallushi, E.; Miceli, A.; Chiaramonti, F.; Murzi, M.; Solinas, M. Right anterior minithoracotomy for aortic valve replacement: 10-year experience of a single center. J. Thorac. Cardiovasc. Surg. 2015, 150, 548–556.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hancock, H.C.; Maier, R.H.; Kasim, A.S.; Mason, J.M.; Murphy, G.J.; Goodwin, A.T.; Owens, W.A.; Kirmani, B.H.; Akowuah, E.F. Mini-Sternotomy Versus Conventional Sternotomy for Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2491–2492. [Google Scholar] [CrossRef]
- Fischlein, T.; Folliguet, T.; Meuris, B.; Shrestha, M.L.; Roselli, E.E.; McGlothlin, A.; Kappert, U.; Pfeiffer, S.; Corbi, P.; Lorusso, R. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2021, 161, 920–932. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Young Lee, M.; Chilakamarri Yeshwant, S.; Chava, S.; Lawrence Lustgarten, D. Mechanisms of Heart Block after Transcatheter Aortic Valve Replacement—Cardiac Anatomy, Clinical Predictors and Mechanical Factors that Contribute to Permanent Pacemaker Implantation. Arrhythmia Electrophysiol. Rev. 2015, 4, 81–85. [Google Scholar] [CrossRef]
- Glauber, M.; Ferrarini, M.; Miceli, A. Minimally invasive aortic valve surgery: State of the art and future directions. Ann. Cardiothorac. Surg. 2015, 4, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Claessens, J.; Goris, P.; Yilmaz, A.; Van Genechten, S.; Claes, M.; Packlé, L.; Pierson, M.; Vandenbrande, J.; Kaya, A.; Stessel, B. Patient-Centred Outcomes after Totally Endoscopic Cardiac Surgery: One-Year Follow-Up. J. Clin. Med. 2023, 12, 4406. [Google Scholar] [CrossRef] [PubMed]
- Zoni, D.; Cresce, G.D.; Hinna-Danesi, T.; Benvegnù, L.; Poddi, S.; Gallo, M.; Sella, M.; Salvador, L. Endoscopic aortic valve surgery in isolated and concomitant procedures. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad101. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhou, K.; Wang, Z.; Zang, X.; Guo, H.; Gao, Q.; Teng, Y.; Liu, J.; He, B.; Guo, H.; et al. Totally endoscopic aortic valve replacement: Techniques and early results. Front. Cardiovasc. Med. 2022, 9, 1106845. [Google Scholar] [CrossRef]

| Mini-AVR (N = 323) | TEAVR (N = 392) | p-Value | |
|---|---|---|---|
| Age (years) | 72.65 ± 9.93 | 71.20 ± 10.85) | 0.063 |
| Octogenarians | 86 (26.63) | 96 (24.49) | 0.514 |
| Gender (male) | 179 (55.42) | 234 (59.69) | 0.249 |
| BMI (kg/m2) | 27.89 ± 4.68 | 27.57 ± 4.74 | 0.373 |
| EuroSCORE II | 2.54 ± 2.22 | 2.33 ± 2.24 | 0.251 |
| NYHA score | 0.015 | ||
| 98 (30.34) 157 (48.61) 62 (19.20) 2 (0.62) | 124 (31.63) 220 (56.12) 43 (10.97) 4 (1.02) | |
Comorbidities
| 45 (13.93) 105 (32.51) 4 (1.24) 78 (24.15) | 78 (19.90) 60 (15.31) 3 (0.77) 84 (21.43) | <0.001 0.544 |
| 223 (69.04) 204 (63.16) 95 (29.41) 72 (22.29) 11 (3.41) 38 (11.76) 58 (17.96) | 238 (60.71) 257 (65.56) 76 (19.39) 61 (15.56) 13 (3.32) 26 (6.63) 270 (68.88) | 0.019 0.504 0.002 0.021 0.661 0.018 <0.001 |
| History of: | 0.013 | ||
| 20 (6.19) 8 (2.48) | 12 (3.06) 12 (3.06) | |
| LVEF (%) | 58.12 ± 11.92 | 58.83 ± 35.82 | 0.9187 |
TTE
| 80.07 ± 23.48 50.86 ± 16.13 0.74 ± 0.21 | 73.2 ± 21.95 48.04 ± 15.78 0.78 ± 0.21 | 0.002 0.090 0.050 |
| Bicuspid valve | 30 (9.29) | 72 (18.37) | <0.001 |
| Mini-AVR (N = 323) | TEAVR (N = 392) | p-Value | |
|---|---|---|---|
| Indication for surgery | 0.202 | ||
| 289 (89.47) 10 (3.10) 24 (7.43) | 357 (91.07) 17 (4.34) 18 (4.59) | |
| Cross-clamping time (h) | 43.74 ± 13.73 | 61.93 ± 16.76 | <0.001 |
| CPB time (h) | 64.86 ± 23.02 | 93.23 ± 23.67 | <0.001 |
| Unplanned mechanical circulatory support | 0 (0) | 3 (0.77) | 0.115 |
| Perioperative bleeding (mL) | 706.40 ± 542.77 | 444.50 ± 515.84 | <0.001 |
Transfusion
| 111 (34.37) 2.03 ± 1.19 16 (4.95) 2.94 ± 1.12) 28 (8.67) 1.21 ± 0.42 | 57 (14.54) 1.84 ± 0.90 1 (0.26) 3 ± 0 1 (0.26) 1 ± 0 | <0.001 <0.001 <0.001 |
| Aortic prosthesis | <0.001 | ||
| 254 (78.64) 50 (15.48) 0 (0) 9 (2.79) 6 (1.86) 2 (0.62) 2 (0.62) | 218 (55.61) 70 (17.86) 9 (2.30) 1 (0.26) 72 (18.37) 14 (3.57) 7 (1.79) | |
| Conversion | 0.288 | ||
| 0 (0) / | 0 (0) 1 (0.26) |
| Continuous Data | |||||
|---|---|---|---|---|---|
| Mini-AVR (N = 323) | TEAVR (N = 392) | p-Value | Estimate | 95% CI | |
| Ventilation time (h) | 10.51 ± 18.93 | 12.85 ± 64.55 | 0.409 | 3.90 | −5.35–13.16 |
| Bleeding 24 h (mL) | 230.60 ± 159.04 | 297.30 ± 291.52 | <0.001 | 78.38 | 33.23–123.53 |
| ICU LOS (h) | 69.64 ± 68.68 | 60.41 ± 133.41 | 0.853 | −1.90 | −22.08–18.27 |
| Hospital LOS (days) | 10.12 ± 7.63 | 7.09 ± 10.96 | 0.002 | −2.78 | −4.56 to −0.99 |
LVEF (%)
| 56.39 ± 9.64 55.67 ± 11.37 | 55.88 ± 10.66 57.90 ± 10.39 | 0.883 0.295 | −0.17 1.64 | −2.47–2.12 −1.42–4.69 |
| TTE In-hospital
| 22.81 ± 8.31 13.05 ± 4.85 1.76 ± 0.40 | 20.21 ± 9.27 12.05 ± 6.10 1.93 ± 0.48 | 0.057 0.371 0.022 | −2.38 −0.85 0.22 | −4.83–0.07 −2.70–1.00 0.03–0.41 |
Follow-up
| 22.63 ± 11.19 14.09 ± 8.12 1.63 ± 0.51 1766 ± 1011.46 | 20.13 ± 12.19 12.01 ± 5.27 1.77 ± 0.42 605.5 ± 507.95 | 0.034 0.081 0.605 <0.001 | −3.27 −2.16 0.07 −1227.70 | −6.27 to −0.26 −4.57–0.25 −0.18–0.31 −1383.26 to −1072.14 |
| Clinical follow-up time (days) | 2 081 ± 895.43 | 742.9 ± 519.11 | <0.001 | −1381.11 | −1511.71 to −1250.50 |
| Clinical follow-up index | 0.91 ± 0.21 | 0.75 ± 0.34 | <0.001 | −0.20 | −0.25 to −0.15 |
| Categorical Data | |||||
| Mini-AVR (N = 323) | TEAVR (N = 392) | p-Value | OR | 95% CI | |
| Unplanned mechanical circulatory support | 3 (0.93) | 4 (1.02) | 0.077 | 0.08 | 0.002–1.03 |
Transfusions
| 75 (23.15) 3.05 ± 2.33 17 (5.25) 212.12 ± 0.78 21 (6.48) 1.33 ± 0.48 | 64 (16.33) 3.80 ± 3.05 13 (3.32) 1.85 ± 0.55 20 (5.10) 1.2 ± 0.41 | <0.001 0.002 <0.001 | 0.21 0.05 0.04 | 0.13–0.36 0.006–0.28 0.005–0.15 |
| Reoperation (<48 h) | 10 (3.09) | 16 (4.08) | 0.206 | 1.93 | 0.005–0.15 |
| 4 (1.23) 6 (1.85) 0 (0) | 13 (3.32) 5 (1.28) 0 (0) | |||
| Reoperation (>48 h) | 3 (0.93) | 2 (0.51) | 0.102 | 0.14 | 0.010–1.39 |
| 0 (0) 1 (0.31) 0 (0) 1 (0.31) 1 (0.31) 0 (0) | 0 (0) 1 (0.26) 0 (0) 0 (0) 0 (0) 1 (0.26) | |||
| Reoperation (>7 days) | 10 (3.09) | 7 (1.79) | 0.204 | 0.46 | 0.13–1.49 |
| 1 (0.31) 7 (2.16) 1 (0.31) 1 (0.31) 0 (0) | 2 (0.51) 3 (0.77) 0 (0) 1 (0.26) 1 (0.26) | |||
Paravalvular leakage
| 6 (1.85) 10 (3.09) | 2 (0.51) 11 (2.81) | 0.624 0.105 | 0.57 0.44 | 0.05–4.77 0.16–1.19 |
| Endocarditis | 10 (3.09) | 6 (1.53) | 0.357 | 0.57 | 0.16–1.84 |
| Valve mispositioning | 0 (0) | 0 (0) | - | - | - |
| Mediastinitis | 1 (0.31) | 0 (0) | - * | - * | - * |
| New-onset AF Electric cardioversion | 104 (32.10) 48 (14.81) | 97 (24.74) 22 (5.61) | 0.030 0.118 | 0.61 0.58 | 0.39–0.95 0.29–1.14 |
| Conduction disturbance | 44 (13.58) | 72 (18.37) | 0.028 | 1.79 | 1.07–3.04 |
| 30-day PPM | 12 (3.70) | 32 (8.16) | 0.001 | 4.29 | 1.80–10.80 |
| Vascular complications | 0.090 | 0.38 | 0.12–1.13 | ||
| 15 (4.63) 0 (0) | 7 (1.79) 1 (0.26) | |||
| Neurological complications | 30 (9.26) | 20 (5.10) | 0.026 | 0.44 | 0.21–0.90 |
| 13 (4.01) 4 (1.23) 13 (4.01) | 11 (2.81) 2 (0.51) 7 (1.79) | |||
| Neurological timing | 0.246 | 0.63 | 0.29–1.41 | ||
| 2 (0.62) 15 (4.63) 4 (1.23) 21 (6.48) | 3 (0.77) 11 (2.81) 3 (0.77) 9 (2.30) | |||
| AKI Renal replacement therapy | 29 (8.95) 2 (0.62) | 12 (3.06) 8 (2.04) | 0.464 0.365 | 0.73 2.48 | 0.30–1.67 0.41–22.97 |
| Cardiac related readmissions | 73 (22.53) | 72 (18.37) | 0.200 | 0.74 | 0.46–1.18 |
| Mini-AVR (N = 323) | TEAVR (N = 392) | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|
| Periprocedural mortality | 0 (0) | 1 (0.26) | 0.999 | 2.12 × 10 24 | 0.00–Inf |
Causes of death
| 0 (0) 0 (0) 0 (0) 0 (0) | 1 (0.26) 0 (0) 0 (0) 0 (0) | |||
| 30-day mortality rate | 4 (1.24) | 7 (1.79) | 0.826 | 1.99 | −0.54–8.12 |
Causes of death
| 4 (1.24) 0 (0) 0 (0) 0 (0) | 4 (1.03) 0 (0) 3 (0.77) 0 (0) | |||
| One-year survival (%) | 94.5 | 93.3 | 0.520 * | ||
Causes of death
| 6 (1.86) 0 (0) 3 (0.93) 8 (2.48) | 5 (1.28) 0 (0) 11 (2.81) 7 (1.79) | |||
| Three-year survival (%) | 89.9 | 85.6 | 0.150 * | ||
Causes of death
| 8 (2.48) 0 (0) 8 (2.48) 15 (4.65) | 9 (2.30) 0 (0) 17 (4.34) 12 (3.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Claessens, J.; Packlé, L.; Van Genechten, S.; Dönmez, K.; Awouters, C.; Herbots, L. Aortic Valve Replacement: Totally Endoscopic versus Mini-Sternotomy. J. Clin. Med. 2023, 12, 7300. https://doi.org/10.3390/jcm12237300
Yilmaz A, Claessens J, Packlé L, Van Genechten S, Dönmez K, Awouters C, Herbots L. Aortic Valve Replacement: Totally Endoscopic versus Mini-Sternotomy. Journal of Clinical Medicine. 2023; 12(23):7300. https://doi.org/10.3390/jcm12237300
Chicago/Turabian StyleYilmaz, Alaaddin, Jade Claessens, Loren Packlé, Silke Van Genechten, Kübra Dönmez, Camille Awouters, and Lieven Herbots. 2023. "Aortic Valve Replacement: Totally Endoscopic versus Mini-Sternotomy" Journal of Clinical Medicine 12, no. 23: 7300. https://doi.org/10.3390/jcm12237300
APA StyleYilmaz, A., Claessens, J., Packlé, L., Van Genechten, S., Dönmez, K., Awouters, C., & Herbots, L. (2023). Aortic Valve Replacement: Totally Endoscopic versus Mini-Sternotomy. Journal of Clinical Medicine, 12(23), 7300. https://doi.org/10.3390/jcm12237300






