Role of Genetic Polymorphisms in the Development of Complications in Patients with Implanted Left Ventricular Assist Devices: HeartWare, HeartMate II, and HeartMate 3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Selection of the Single-Nucleotide Polymorphisms
2.3. DNA Extraction and SNP Genotyping
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of HF Patients with Three Types of Implanted LVAD Devices: HW, HMII, HM3
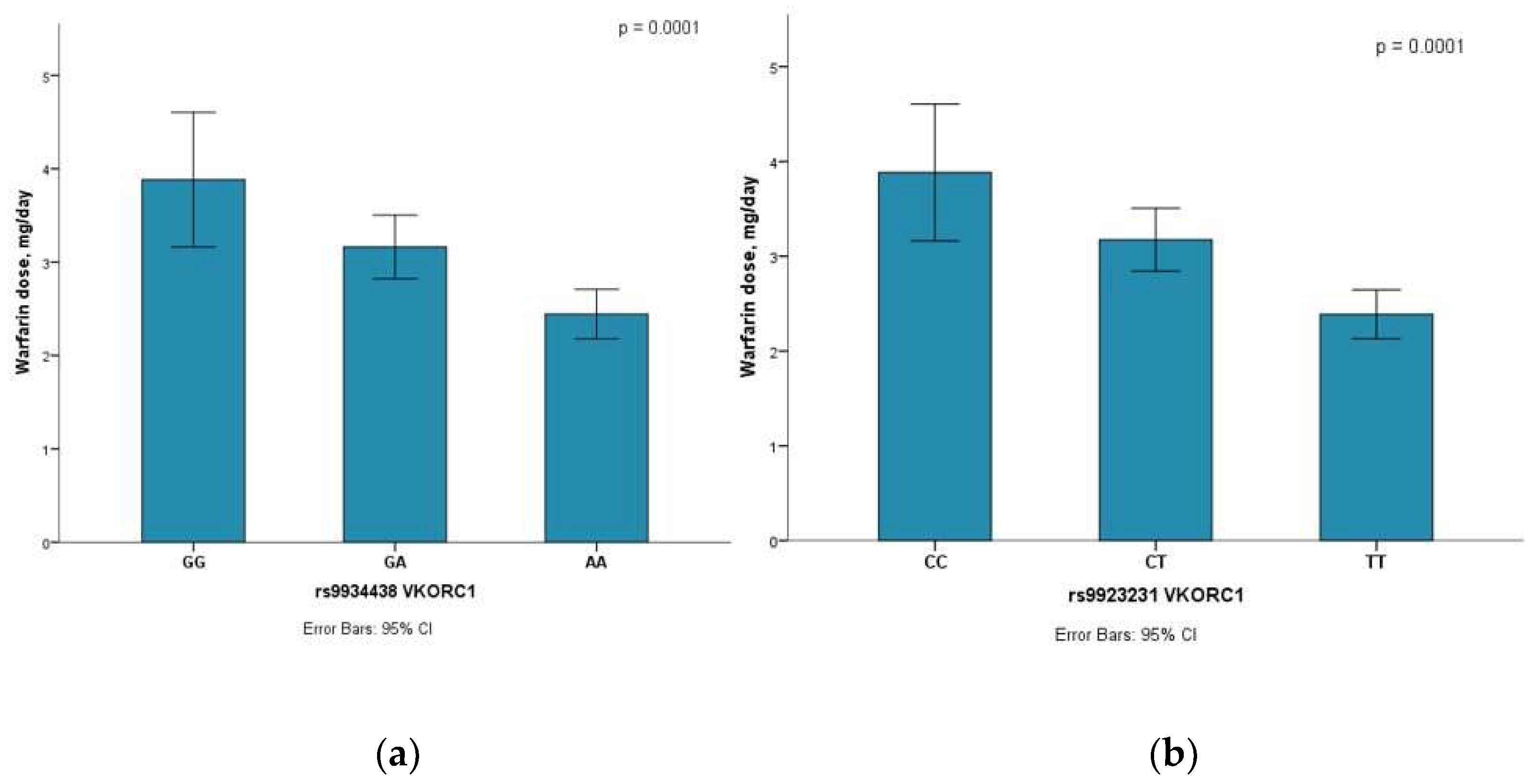
3.2. SNP Association with HF Patients
3.3. Correlation Analysis
3.4. Multiple Linear Regression Analysis
3.5. Multinomial Logistic Regression Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berardi, C.; Bravo, C.A.; Li, S.; Khorsandi, M.; Keenan, J.E.; Auld, J.; Rockom, S.; Beckman, J.A.; Mahr, C. The History of Durable Left Ventricular Assist Devices and Comparison of Outcomes: HeartWare, HeartMate II, HeartMate 3, and the Future of Mechanical Circulatory Support. J. Clin. Med. 2022, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Garbade, J.; Gustafsson, F.; Shaw, S.; Lavee, J.; Saeed, D.; Pya, Y.; Krabatsch, T.; Schmitto, J.D.; Morshuis, M.; Chuang, J.; et al. Postmarket Experience With HeartMate 3 Left Ventricular Assist Device: 30-Day Outcomes From the ELEVATE Registry. Ann. Thorac. Surg. 2019, 107, 33–39. [Google Scholar] [CrossRef]
- Kadakia, S.; Moore, R.; Ambur, V.; Toyoda, Y. Current status of the implantable LVAD. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Maynes, E.J.; Gadda, M.N.; O’Malley, T.J.; Morris, R.J.; Shah, M.K.; Pirlamarla, P.R.; Alvarez, R.J.; Entwistle, J.W.; Massey, H.T.; et al. Continuous-flow LVAD exchange to a different pump model: Systematic review and meta-analysis of the outcomes. Artif. Organs 2021, 45, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Eckman, P.M.; John, R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012, 125, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Kormos, R.L.; Pagani, F.D.; Myers, S.L.; Stevenson, L.W.; Acker, M.A.; Goldstein, D.L.; Silvestry, S.C.; Milano, C.A.; et al. Interagency registry for mechanically assisted circulatory support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J. Hear. Lung Transplant. 2014, 33, 12–22. [Google Scholar] [CrossRef]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Hear. Lung Transplant. 2019, 38, 114–126. [Google Scholar] [CrossRef]
- Bravo, C.A.; Fried, J.A.; Willey, J.Z.; Javaid, A.; Mondellini, G.M.; Braghieri, L.; Lumish, H.; Topkara, V.K.; Kaku, Y.; Witer, L.; et al. Presence of Intracardiac Thrombus at the Time of Left Ventricular Assist Device Implantation Is Associated With an Increased Risk of Stroke and Death. J. Card. Fail. 2021, 27, 1367–1373. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A fully magnetically levitated left ventricular assist device—Final report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Wever-Pinzon, O.; Naka, Y.; Garan, A.R.; Takeda, K.; Pan, S.; Takayama, H.; Mancini, D.M.; Colombo, P.; Topkara, V.K. National trends and outcomes in device-related thromboembolic complications and malfunction among heart transplant candidates supported with continuous-flow left ventricular assist devices in the United States. J. Hear. Lung Transplant. 2016, 35, 884–892. [Google Scholar] [CrossRef]
- Zhalbinova, M.R.; Rakhimova, S.E.; Kozhamkulov, U.A.; Akilzhanova, G.A.; Kaussova, G.K.; Akilzhanov, K.R.; Pya, Y.V.; Lee, J.H.; Bekbossynova, M.S.; Akilzhanova, A.R. Association of genetic polymorphisms with complications of implanted LVAD devices in patients with congestive heart failure: A Kazakhstani study. J. Pers. Med. 2022, 12, 744. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Topkara, V.K.; Knotts, R.J.; Jennings, D.L.; Garan, A.R.; Levin, A.P.; Breskin, A.; Castagna, F.; Cagliostro, B.; Yuzefpolskaya, M.; Takeda, K.; et al. Effect of CYP2C9 and VKORC1 gene variants on warfarin response in patients with continuous-flow left ventricular assist devices. ASAIO J. 2016, 62, 558–564. [Google Scholar] [CrossRef]
- Starling, R.C.; Moazami, N.; Silvestry, S.C.; Ewald, G.; Rogers, J.G.; Milano, C.A.; Rame, J.E.; Acker, M.A.; Blackstone, E.H.; Ehrlinger, J.; et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N. Engl. J. Med. 2014, 370, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Topkara, V.K.; O’Neill, J.K.; Carlisle, A.; Novak, E.; Silvestry, S.C.; Ewald, G.A. HeartWare and HeartMate II Left ventricular assist devices as bridge to transplantation: A comparative analysis. Ann. Thorac. Surg. 2014, 97, 506–512. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year outcomes with a magnetically levitated cardiac pump in heart failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Williamitis, C.A.; Slaughter, M.S. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: Is there an advantage to pulsatility? Ann. Cardiothorac. Surg. 2014, 3, 573–581. [Google Scholar] [CrossRef]
- Chen, Z.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. Quantitative Characterization of Shear-Induced Platelet Receptor Shedding: Glycoprotein Ibα, Glycoprotein VI, and Glycoprotein IIb/IIIa. ASAIO J. 2018, 64, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Kareem, K.; Tran, D.; Conway, R.G.; Arias, K.; Griffith, B.P.; Wu, Z.J. Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif. Organs 2019, 43, 745–755. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kuehl, G.E.; Bigler, J.; Rimorin, C.F.; Schwarz, Y.; Shen, D.D.; Lampe, J.W. UGT1A6 polymorphism and salicylic acid glucuronidation following aspirin. Pharmacogenetics Genom. 2007, 17, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Varnado, S.; Graviss, E.A.; Nguyen, D.T.; Cruz-Solbes, A.; Guha, A.; Krisl, J.C. Role of thromboelastography in predicting and defining pump thrombosis in left ventricular assist device patients. Thromb. Res. 2020, 192, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Volod, O.; Lam, L.D.; Lin, G.; Kam, C.; Kolyouthapong, K.; Mac, J.; Mirocha, J.; Ambrose, P.J.; Czer, L.S.C.; Arabia, F.A. Role of thromboelastography platelet mapping and international normalized ratio in defining “normocoagulability” during anticoagulation for mechanical circulatory support devices: A pilot retrospective study. ASAIO J. 2017, 63, 24–31. [Google Scholar] [CrossRef]
- Boyle, A.J.; Jorde, U.P.; Sun, B.; Park, S.J.; Milano, C.A.; Frazier, O.H.; Sundareswaran, K.S.; Farrar, D.J.; Russell, S.D. Pre-Operative risk factors of bleeding and stroke during left ventricular assist device support. J. Am. Coll. Cardiol. 2014, 63, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Piche, S.L.; Nei, S.D.; Frazee, E.; Schettle, S.D.; Boilson, B.A.; Plevak, M.F.; Dierkhising, R.A.; Stulak, J.M. Baseline thromboelastogram as a predictor of left ventricular assist device thrombosis. ASAIO J. 2019, 65, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Tang, X.-F.; Zhang, Y.; Wang, J.; Yao, Y.; Ma, Y.-L.; Xu, B.; Gao, R.-L.; Gao, Z.; Chen, J.; et al. Relationship between ABCB1 polymorphisms, thromboelastography and risk of bleeding events in clopidogrel-treated patients with ST-elevation myocardial infarction. Thromb. Res. 2014, 134, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Hammad, J.S.; Rollini, F.; Tello-Montoliu, A.; Patel, R.; Darlington, A.; Kraemer, D.F.; Cho, J.R.; DeGroat, C.; Bhatti, M.; et al. Role of thromboelastography and rapid thromboelastography to assess the pharmacodynamic effects of vitamin K antagonists. J. Thromb. Thrombolysis 2015, 40, 118–125. [Google Scholar] [CrossRef]
- Dunham, C.M.; Rabel, C.; Hileman, B.M.; Schiraldi, J.; Chance, E.A.; Shima, M.T.; Molinar, A.A.; Hoffman, D.A. TEG® and RapidTEG® are unreliable for detecting warfarin-coagulopathy: A prospective cohort study. Thromb. J. 2014, 12, 4. [Google Scholar] [CrossRef]
- Awad, M.; Czer, L.S.C.; Soliman, C.; Mirocha, J.; Ruzza, A.; Pinzas, J.; Rihbany, K.; Chang, D.; Moriguchi, J.; Ramzy, D.; et al. Prevalence of Warfarin Genotype Polymorphisms in Patients with Mechanical Circulatory Support. ASAIO J. 2015, 61, 391–396. [Google Scholar] [CrossRef]
- Hu, J.; Mondal, N.K.; Sorensen, E.N.; Cai, L.; Fang, H.-B.; Griffith, B.P.; Wu, Z.J. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2014, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol. Cell. Biochem. 2015, 409, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Grinshtein, Y.I.; Kosinova, A.A.; Grinshtein, I.Y.; Subbotina, T.N.; Savchenko, A.A. The Prognostic Value of Combinations of Genetic Polymorphisms in the ITGB3, ITGA2, and CYP2C19*2 Genes in Predicting Cardiovascular Outcomes After Coronary Bypass Grafting. Genet. Test. Mol. Biomark. 2018, 22, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kucharska-Newton, A.M.; Monda, K.L.; Campbell, S.; Bradshaw, P.T.; Wagenknecht, L.E.; Boerwinkle, E.; Wasserman, B.A.; Heiss, G. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2011, 216, 151–156. [Google Scholar] [CrossRef]
- Potapov, E.V.; Ignatenko, S.; Nasseri, B.A.; Loebe, M.; Harke, C.; Bettmann, M.; Doller, A.; Regitz-Zagrosek, V.; Hetzer, R. Clinical significance of PlA polymorphism of platelet GP IIb/IIIa receptors during long-term VAD support. Ann. Thorac. Surg. 2004, 77, 869–874. [Google Scholar] [CrossRef]
| Characteristic | HF Patients, n = 98 | HW (n = 18) | HMII (n = 34) | HM3 (n = 46) | p Value |
|---|---|---|---|---|---|
| Age (years) | 52.7 ± 11.0 | 53.1 ±11.7 | 53.3 ± 10.9 | 52.0 ± 10.9 | 0.87 |
| Gender | |||||
| Male | 92 (93.9) | 16 (17.4) | 31 (33.7) | 45 (48.9) | 0.23 |
| Female | 6 (6.1) | 2 (33.3) | 3 (50.0) | 1 (16.7) | |
| Ethnicity | |||||
| Asian | 77 (78.6) | 17 (22.1) | 25 (32.5) | 35 (45.5) | 0.20 |
| Caucasian | 21 (21.4) | 1 (4.8) | 9 (42.9) | 11 (52.4) | |
| Body weight (kg) | 79.8 ± 13.9 | 70.7 ± 12.9 | 85.9 ± 15.3 | 78.9 ± 11.1 | 0.001 * |
| Height (cm) | 169.8 ± 6.36 | 169.4 ± 5.1 | 169.9 ± 7.96 | 169.8 ± 5.56 | 0.97 |
| BMI (kg/m) | 27.7 ± 4.5 | 24.6 ± 4.74 | 29.7 ± 4.65 | 27.3 ± 3.53 | 0.001 ** |
| SBP | 104.8 ± 15.5 | 105.9 ± 19.4 | 106.2 ± 16.0 | 103.3 ± 13.5 | 0.51 |
| DBP | 71.2 ± 10.3 | 71.2 ± 10.8 | 71.2 ± 10.4 | 71.3 ± 10.3 | 0.98 |
| History of smoking | |||||
| Smokers | 58 (59.2) | 10 (17.2) | 17 (29.3) | 31 (53.4) | 0.28 |
| Non-smokers | 40 (40.8) | 8 (20.0) | 17 (42.5) | 15 (37.5) | |
| Diagnosis | |||||
| ICM | 44 (44.9) | 9 (20.5) | 16 (36.4) | 19 (43.2) | 0.78 |
| DCM | 40 (40.8) | 7 (17.5) | 15 (37.5) | 18 (45.0) | |
| HCM | 11 (11.2) | 1 (9.1) | 3 (27.3) | 7 (63.6) | |
| VHD | 3 (3.1) | 1 (33.3) | 0 | 2 (66.7) | |
| NYHA (before 14 days) | |||||
| I | 1 (1.0) | 0 | 1 (100) | 0 | 0.48 |
| II | 1 (1.0) | 0 | 1 (100) | 0 | |
| III | 2 (2.0) | 0 | 1 (50.0) | 1 (50.0) | |
| IV | 26 (26.5) | 6 (23.1) | 9 (34.6) | 11 (42.3) | |
| IIIA | 34 (34.7) | 8 (23.5) | 13 (38.2) | 13 (38.2) | |
| IIIB | 34 (34.7) | 4 (11.8) | 9 (26.5) | 21 (61.8) | |
| HF type | |||||
| HFrEF | 97 (99.0) | 18 (18.6) | 33 (34.0) | 46 (47.4) | 0.53 |
| HFmrEF | 1 (1.0) | 0 | 1 (100.0) | 0 | |
| INR | |||||
| Basic INR | 1.21 ± 0.36 | 1.33 ± 0.46 | 1.16 ± 0.21 | 1.20 ± 0.40 | 0.35 |
| Target INR | 2.39 ± 0.26 | 2.83 ± 0.26 | 2.30 ± 0.12 | 2.27 ± 0.12 | 0.001 ** |
| Device strategy | |||||
| BTT | 10 (10.2) | 0 | 7 (70.0) | 3 (30.0) | 0.05 * |
| DT | 88 (89.8) | 18 (20.5) | 27 (30.7) | 43 (48.9) | |
| Warfarin dose (mg/day) | 2.99 ± 1.15 | 2.99 ± 1.42 | 2.82 ± 1.02 | 3.11 ± 1.13 | 0.21 |
| Duration of LVAD support till outcome, from 2011 until 2016, n = 36 (in months) | 29.6 ± 17.3 | 26.0 ± 10.2 | 38.6 ± 16.0 | 10.9 ± 6.73 | 0.001 * |
| Duration of LVAD support till outcome in all HF patients, n = 98 (in months) | 24.1 ± 15.8 | 24.4 ±13.4 | 38.3 ± 14.1 | 13.5 ± 7.56 | 0.001 ** |
| Patients’ achieved outcomes till 2017 | |||||
| Survived | 71 (72.4) | 10 (14.1) | 20 (28.2) | 41 (57.7) | 0.001 * |
| Not-survived | 27 (27.6) | 8 (29.6) | 14 (51.9) | 5 (18.5) | |
| Thrombosis | |||||
| Yes | 13 (13.3) | 4 (30.8) | 8 (61.5) | 1 (7.7) | 0.005 * |
| No | 85 (86.7) | 14 (16.5) | 26 (30.6) | 45 (52.9) | |
| Bleeding | |||||
| Yes | 14 (14.3) | 4 (28.6) | 6 (42.9) | 4 (28.6) | 0.27 |
| No | 84 (85.7) | 14 (16.7) | 28 (33.3) | 42 (50.0) | |
| Infections | |||||
| Yes | 39 (39.8) | 12 (30.8) | 18 (46.2) | 9 (23.1) | 0.0001 * |
| No | 59 (60.2) | 6 (10.2) | 16 (27.1) | 37 (62.7) | |
| Stroke | |||||
| No Stroke | 78 (79.6) | 12 (15.4) | 25 (32.1) | 41 (52.6) | 0.18 |
| Hemorrhagic stroke | 8 (8.2) | 2 (25.0) | 4 (50.0) | 2 (25.0) | |
| Ischemic stroke | 12 (12.2) | 4 (33.3) | 5 (41.7) | 3 (25.0) | |
| Myocardial infarction | |||||
| Yes | 44 (44.9) | 9 (20.5) | 16 (36.4) | 19 (43.2) | 0.77 |
| No | 54 (55.1) | 9 (16.7) | 18 (33.3) | 27 (50.0) | |
| Study Groups | Parameters | Before 14 Days | p Value | After 3–6 Months | p Value | After 12–18 Months | p Value |
|---|---|---|---|---|---|---|---|
| HW | D—Dimer, mcg/mL | 1.34 ± 1.23 | 0.30 | 2.67 ± 3.20 | 0.28 | 1.38 ± 0.63 | 0.01 ** |
| HMII | 1.32 ± 1.85 | 0.98 ± 0.49 | 0.65 ± 0.30 | ||||
| HM3 | 1.08 ± 1.74 | 1.97 ± 1.13 | 0.49 ± 0.26 | ||||
| HW | Hemoglobin, g/L | 132.8 ± 19.3 | 0.39 | 111.9 ± 23.0 | 0.005 ** | 102.9 ± 22.4 | 0.001 * |
| HMII | 138.9 ± 22.2 | 134.2 ± 12.6 | 136.2 ± 13.8 | ||||
| HM3 | 140.6 ± 17.0 | 127.2 ± 18.3 | 106.1 ± 18.5 | ||||
| HW | Hematocrit, % | 39.6 ± 5.14 | 0.55 | 34.2 ± 6.66 | 0.02 ** | 31.5 ± 6.54 | 0.001 * |
| HMII | 40.9 ± 8.22 | 39.5 ± 3.85 | 40.1 ± 4.50 | ||||
| HM3 | 41.7 ± 6.08 | 36.5 ± 5.80 | 32.0 ± 5.50 | ||||
| HW | Leukocytes, ×109/L | 5.99 ± 1.10 | 0.25 | 5.98 ± 1.28 | 0.04 * | 7.08 ± 1.93 | 0.92 |
| HMII | 6.44 ± 1.46 | 7.47 ± 1.49 | 6.94 ± 1.74 | ||||
| HM3 | 6.68 ± 1.54 | 7.14 ± 1.94 | 6.73 ± 1.68 | ||||
| HW | Erythrocytes, ×1012/L | 4.89 ± 0.54 | 0.93 | 4.19 ± 0.36 | 0.03 * | 3.93 ± 0.96 | 0.03 ** |
| HMII | 4.98 ± 0.76 | 4.69 ± 0.65 | 4.72 ± 0.49 | ||||
| HM3 | 4.97 ± 0.63 | 4.22 ± 0.72 | 3.73 ± 0.83 | ||||
| HW | INR | 1.35 ± 0.49 | 0.35 | 2.76 ± 0.87 | 0.15 | 2.90 ± 1.04 | 0.03 * |
| HMII | 1.29 ± 0.52 | 2.30 ± 0.77 | 2.21 ± 0.48 | ||||
| HM3 | 1.20 ± 0.40 | 2.21 ± 0.50 | 2.32 ± 0.84 | ||||
| HW | APTT, s | 42.2 ± 8.86 | 0.02 ** | 50.7 ± 10.2 | 0.53 | 60.9 ±12.9 | 0.01 * |
| HMII | 41.3 ± 9.19 | 56.7 ± 19.1 | 49.9 ± 6.27 | ||||
| HM3 | 37.7 ± 6.62 | 53.1 ± 15.7 | 50.7 ± 12.5 | ||||
| HW | LDH, U/L | 360.2 ± 210.3 | 0.15 | 234.8 ± 105.6 | 0.002 ** | 271.1 ± 118.4 | 0.02 ** |
| HMII | 330.5 ± 178.8 | 351.5 ± 92.1 | 356.8 ± 181.3 | ||||
| HM3 | 248.1 ± 110.7 | 251.1 ± 88.0 | 213.8 ± 55.2 | ||||
| HW | Creatinine, mg/dL | 0.97 ± 0.45 | 0.75 | 0.78 ± 0.40 | 0.04 ** | 1.18 ± 0.51 | 0.84 |
| HMII | 1.13 ± 0.44 | 0.95 ± 0.53 | 1.03 ± 0.31 | ||||
| HM3 | 3.05 ± 14.0 | 1.02 ± 0.21 | 1.08 ± 0.28 | ||||
| HW | CRP, mg/dL | 1.95 ± 3.01 | 0.62 | 0.11 ± 0.07 | 0.05 ** | 2.08 ± 2.90 | 0.58 |
| HMII | 1.64 ± 2.16 | 0.73 ± 1.06 | 1.14 ± 1.72 | ||||
| HM3 | 1.19 ± 2.07 | 1.09 ± 1.58 | 0.66 ± 0.78 | ||||
| HW | LVEF, % | 21.3 ± 5.01 | 0.36 | 23.2 ± 4.82 | 0.02 * | 24.7 ± 4.04 | 0.74 |
| HMII | 23.1 ± 6.41 | 31.0 ± 6.69 | 28.5 ± 7.46 | ||||
| HM3 | 21.4 ± 4.67 | 25.1 ± 4.32 | 25.5 ± 3.70 |
| Characteristics | Unstandardized Coefficients | Standardized Coefficients | t | p Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| B | Std.Error | Beta | Lower Bound | Upper Bound | |||
| (Constant) | 2.01 | 3.56 | 0.56 | 0.58 | −5.21 | 9.22 | |
| Age | −0.001 | 0.03 | −0.005 | −0.04 | 0.97 | −0.07 | 0.07 |
| BMI | 0.03 | 0.08 | 0.06 | 0.38 | 0.70 | −0.12 | 0.18 |
| Device type | 0.57 | 0.46 | 0.18 | 1.23 | 0.23 | −0.37 | 1.51 |
| Warfarin dose | −1.03 | 0.31 | −0.49 | −3.33 | 0.002 * | −1.66 | −0.40 |
| Aspirin dose | 0.02 | 0.01 | 0.27 | 1.86 | 0.07 | −0.002 | 0.05 |
| VKORC1, rs8050894 | 0.79 | 1.02 | 0.15 | 0.78 | 0.44 | −1.27 | 2.86 |
| VKORC1, rs9923231 | −3.25 | 1.49 | −0.47 | −2.17 | 0.04 * | −6.28 | −0.22 |
| ITGB3, rs5918 | 2.18 | 1.08 | 0.41 | 2.03 | 0.05 * | 0.003 | 4.36 |
| UGT1A6, rs2070959 | −0.55 | 0.72 | −0.10 | −0.76 | 0.45 | −2.02 | 0.92 |
| Device Type | Characteristics | B | Std.Error | p Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| HW | Intercept | 3.63 | 2.52 | 0.15 | |||
| Age | 0.02 | 0.03 | 0.40 | 1.02 | 0.97 | 1.08 | |
| BMI | −0.23 | 0.09 | 0.01 * | 0.79 | 0.66 | 0.95 | |
| Warfarin dose, mg/day | 0.19 | 0.27 | 0.46 | 1.22 | 0.72 | 2.07 | |
| VKORC1 rs9923231, CC genotype | −1.66 | 1.22 | 0.17 | 0.19 | 0.02 | 2.07 | |
| VKORC1 rs9923231, CT/TT genotype | 0 b | ||||||
| ITGB3 rs5918, TT genotype | −0.46 | 0.62 | 0.46 | 0.63 | 0.19 | 2.14 | |
| ITGB3 rs5918, TC/CC | 0 b | ||||||
| HMII | Intercept | −3.30 | 2.25 | 0.14 | |||
| Age | 0.006 | 0.02 | 0.81 | 1.01 | 0.96 | 1.05 | |
| BMI | 0.14 | 0.06 | 0.02 * | 1.15 | 1.02 | 1.29 | |
| Warfarin dose, mg/day | −0.23 | 0.23 | 0.32 | 0.79 | 0.50 | 1.25 | |
| VKORC1 rs9923231, CC genotype | 0.24 | 0.78 | 0.76 | 1.27 | 0.27 | 5.86 | |
| VKORC1 rs9923231, CT/TT genotype | 0 b | ||||||
| ITGB3 rs5918, TT genotype | −1.09 | 0.53 | 0.04 * | 0.33 | 0.12 | 0.94 | |
| ITGB3 rs5918, TC/CC genotype | 0 b | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhalbinova, M.R.; Rakhimova, S.E.; Kozhamkulov, U.A.; Akilzhanova, G.A.; Chinybayeva, A.A.; Akilzhanov, K.R.; Shaimardanov, N.K.; Kuanysheva, A.G.; Lee, J.H.; Kairov, U.Y.; et al. Role of Genetic Polymorphisms in the Development of Complications in Patients with Implanted Left Ventricular Assist Devices: HeartWare, HeartMate II, and HeartMate 3. J. Clin. Med. 2023, 12, 7235. https://doi.org/10.3390/jcm12237235
Zhalbinova MR, Rakhimova SE, Kozhamkulov UA, Akilzhanova GA, Chinybayeva AA, Akilzhanov KR, Shaimardanov NK, Kuanysheva AG, Lee JH, Kairov UY, et al. Role of Genetic Polymorphisms in the Development of Complications in Patients with Implanted Left Ventricular Assist Devices: HeartWare, HeartMate II, and HeartMate 3. Journal of Clinical Medicine. 2023; 12(23):7235. https://doi.org/10.3390/jcm12237235
Chicago/Turabian StyleZhalbinova, Madina R., Saule E. Rakhimova, Ulan A. Kozhamkulov, Gulbanu A. Akilzhanova, Assel A. Chinybayeva, Kenes R. Akilzhanov, Nurlan K. Shaimardanov, Anargul G. Kuanysheva, Joseph H. Lee, Ulykbek Y. Kairov, and et al. 2023. "Role of Genetic Polymorphisms in the Development of Complications in Patients with Implanted Left Ventricular Assist Devices: HeartWare, HeartMate II, and HeartMate 3" Journal of Clinical Medicine 12, no. 23: 7235. https://doi.org/10.3390/jcm12237235
APA StyleZhalbinova, M. R., Rakhimova, S. E., Kozhamkulov, U. A., Akilzhanova, G. A., Chinybayeva, A. A., Akilzhanov, K. R., Shaimardanov, N. K., Kuanysheva, A. G., Lee, J. H., Kairov, U. Y., Bekbossynova, M. S., & Akilzhanova, A. R. (2023). Role of Genetic Polymorphisms in the Development of Complications in Patients with Implanted Left Ventricular Assist Devices: HeartWare, HeartMate II, and HeartMate 3. Journal of Clinical Medicine, 12(23), 7235. https://doi.org/10.3390/jcm12237235









