Myocardial, Valvular, and Vascular Structural and Functional Properties in Acromegaly
Abstract
1. Acromegaly and Cardiovascular Abnormalities
2. Acromegaly and Cardiovascular Imaging
3. The Left Heart and the Aorta
3.1. Left Ventricle
3.1.1. Under Healthy Circumstances
3.1.2. In Acromegaly
LV Structure, Volumes and Hypertrophy
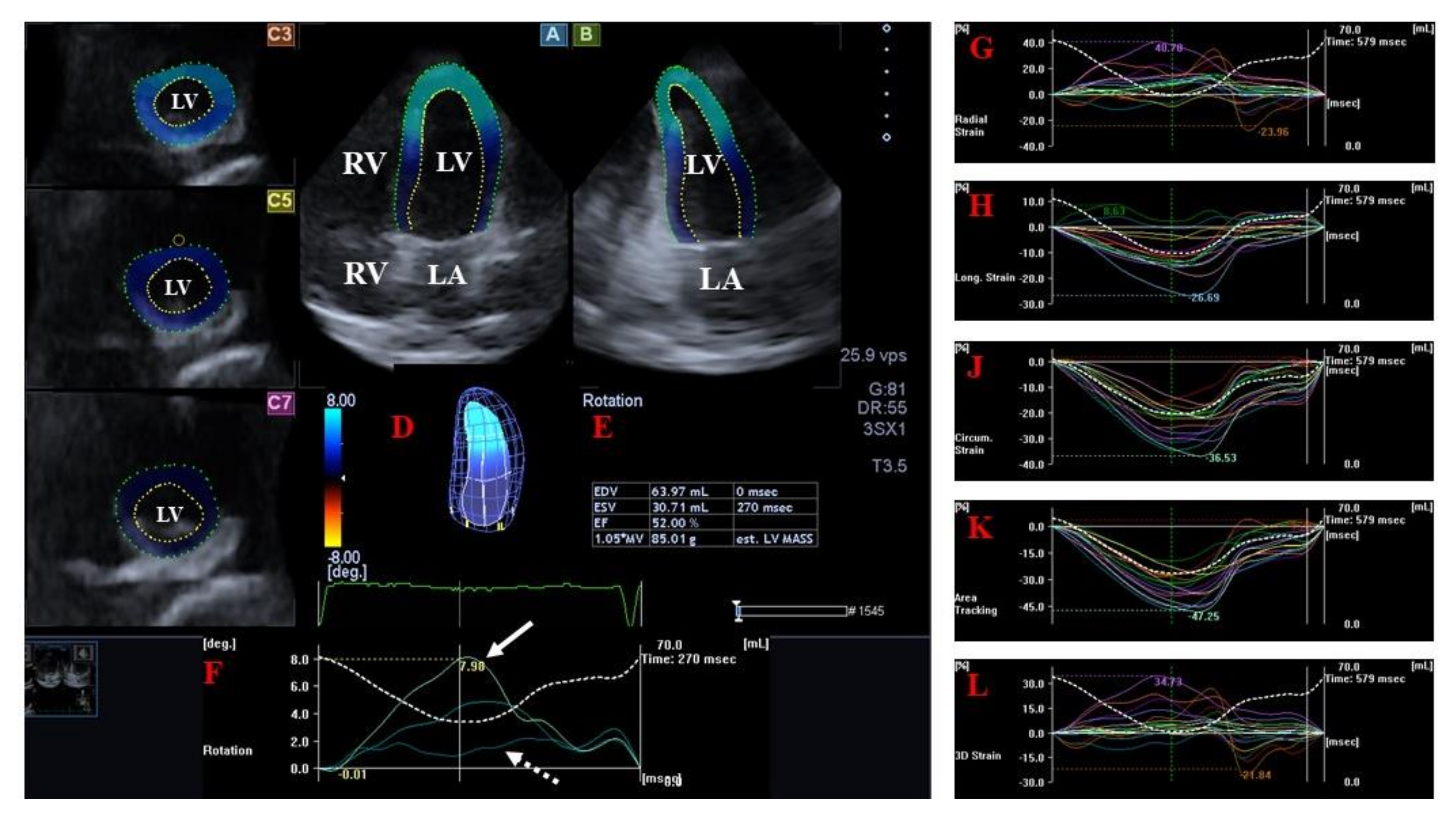
LV Strains and Rotational Parameters
3.2. Left Atrium
3.2.1. Under Healthy Circumstances
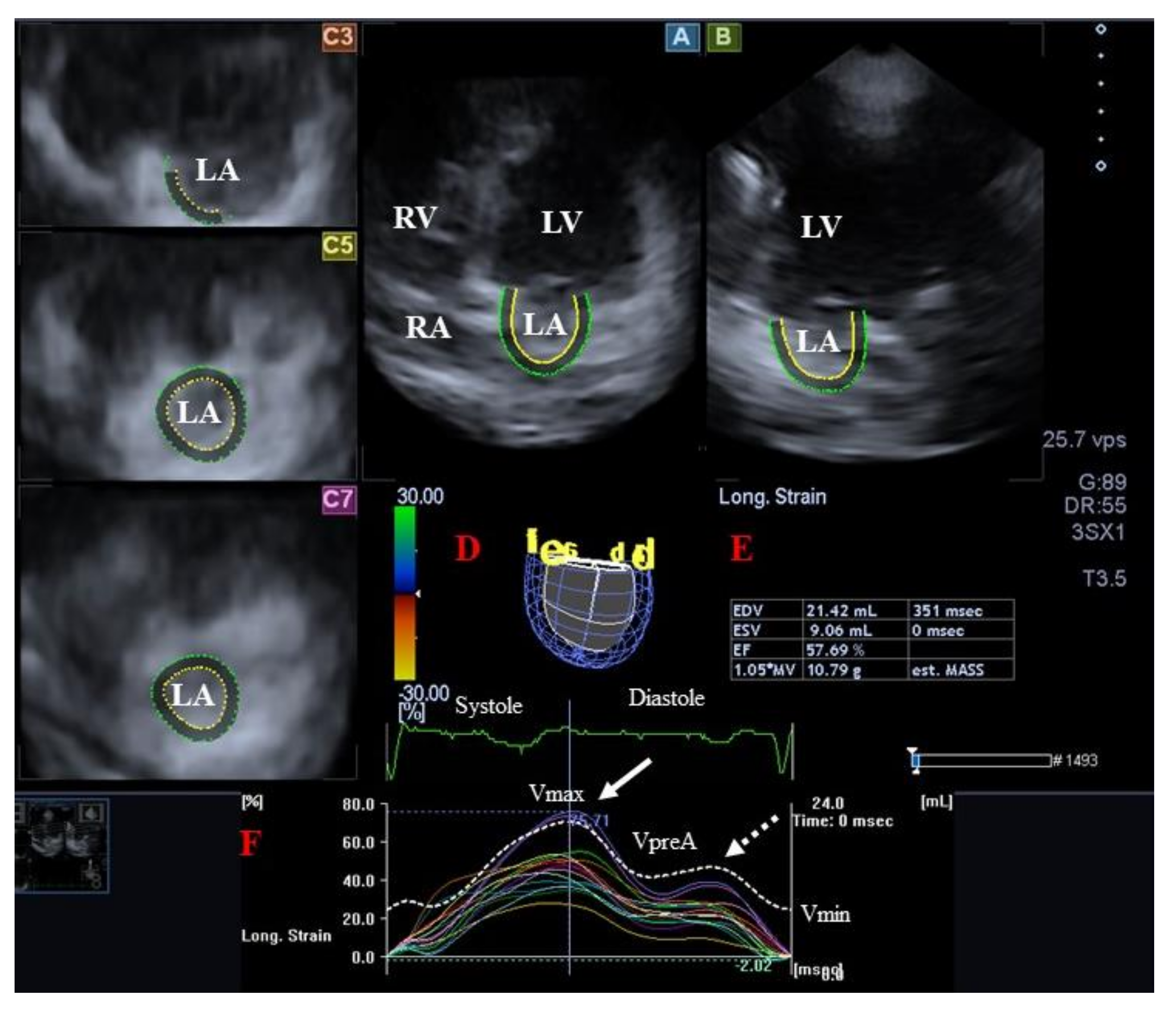
3.2.2. In Acromegaly
3.3. Mitral Valve
3.3.1. Under Healthy Circumstances
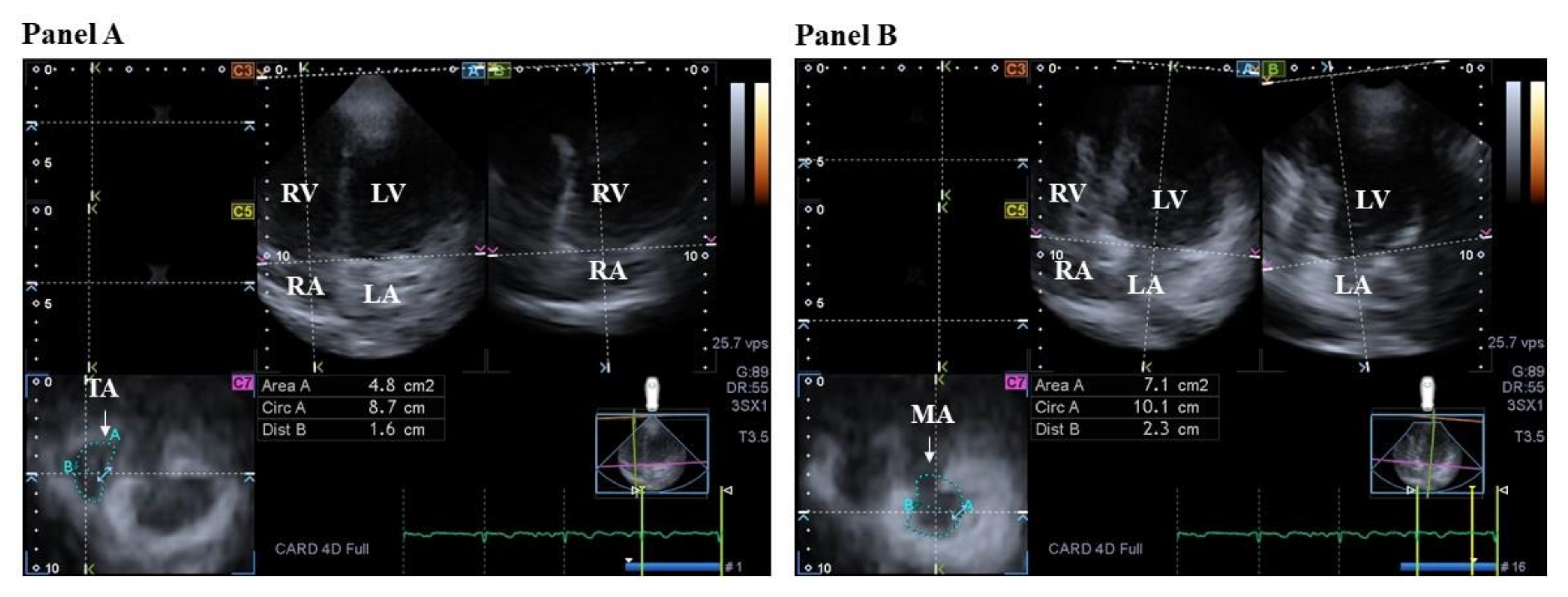
3.3.2. In Acromegaly
3.4. Aortic Valve
3.4.1. Under Healthy Circumstances
3.4.2. In Acromegaly
3.5. Aorta
3.5.1. Under Healthy Circumstances
3.5.2. In Acromegaly
4. The Right Heart and the Pulmonary Artery
4.1. Right Ventricle
4.1.1. Under Healthy Circumstances
4.1.2. In Acromegaly
4.2. Right Atrium
4.2.1. Under Healthy Circumstances
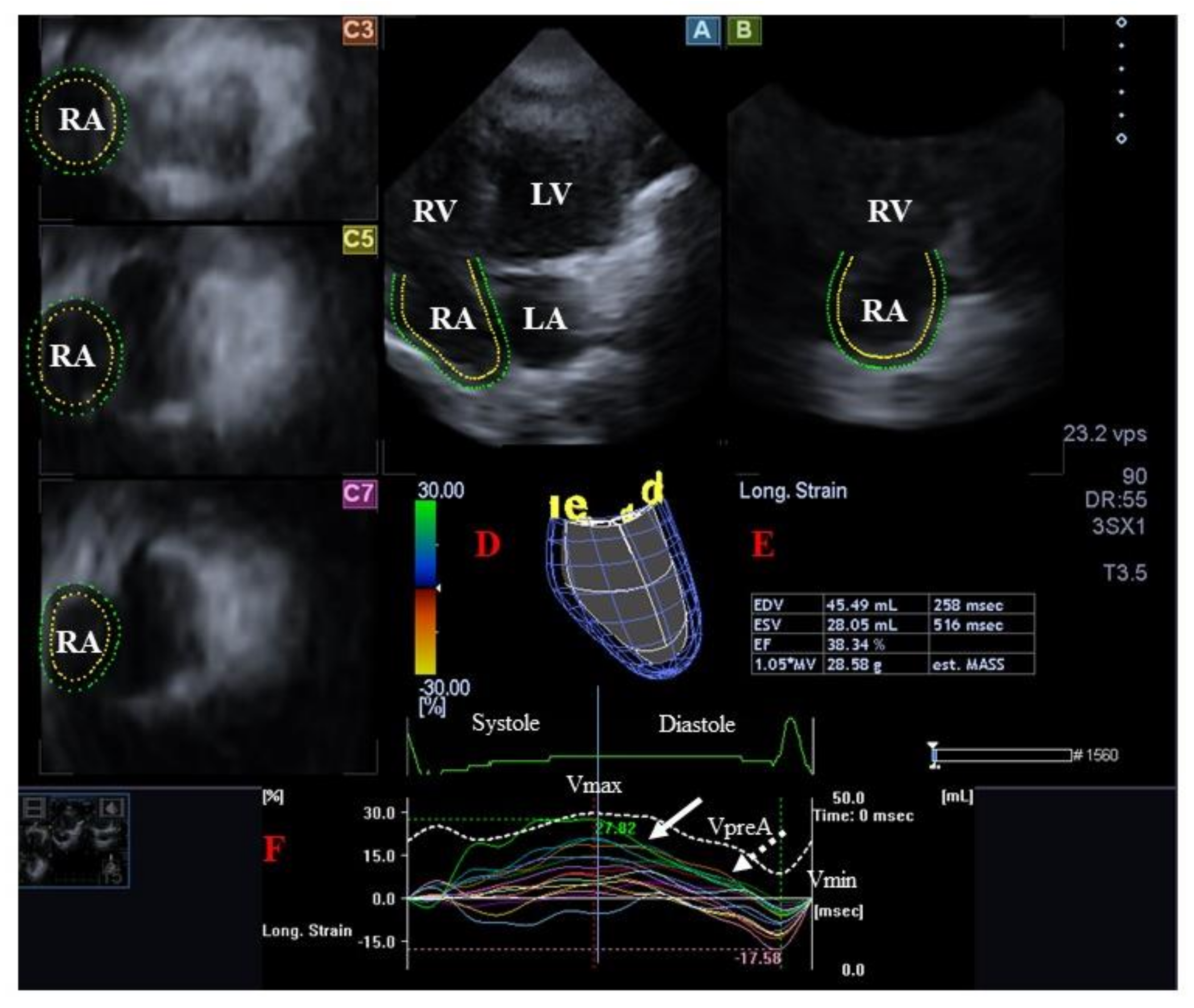
4.2.2. In Acromegaly
4.3. Tricuspid Valve
4.3.1. Under Healthy Circumstances
4.3.2. In Acromegaly
4.4. Pulmonary Valve
4.4.1. Under Healthy Circumstances
4.4.2. In Acromegaly
4.5. Pulmonary Artery
4.5.1. Under Healthy Circumstances
4.5.2. In Acromegaly
5. Pathophysiologic Background
6. Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giustina, A.; Barkan, A.; Beckers, A.; Biermasz, N.; Biller, B.M.K.; Boguszewski, C.; Bolanowski, M.; Bonert, V.; Bronstein, M.D.; Casanueva, F.F.; et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: An update. J. Clin. Endocrinol. Metab. 2020, 105, dgz096. [Google Scholar] [CrossRef]
- Ogedegbe, O.J.; Cheema, A.Y.; Khan, M.A.; Junaid, S.Z.S.; Erebo, J.K.; Ayirebi-Acquah, E.; Okpara, J.; Bofah, D.; Okon, J.G.; Munir, M.; et al. A Comprehensive Review of Four Clinical Practice Guidelines of Acromegaly. Cureus 2022, 14, e28722. [Google Scholar] [CrossRef]
- Bihan, H.; Espinosa, C.; Valdes-Socin, H.; Salenave, S.; Young, J.; Levasseur, S.; Assayag, P.; Beckers, A.; Chanson, P. Long-term outcome of patients with acromegaly and congestive heart failure. J. Clin. Endocrinol. Metab. 2004, 89, 5308–5313. [Google Scholar] [CrossRef]
- Clayton, R.N. Cardiovascular function in acromegaly. Endocr. Rev. 2003, 24, 272–277. [Google Scholar] [CrossRef]
- Ramos-Levi, A.M.; Marazuela, M. Cardiovascular comorbidities in acromegaly: An update on their diagnosis and management. Endocrine 2017, 55, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Popielarz-Grygalewicz, A.; Gasior, J.S.; Konwicka, A.; Grygalewicz, P.; Stelmachowska-Banaś, M.; Zgliczynski, W. Heart in acromegaly: The echocardiographic characteristics of patients diagnosed with acromegaly in various stages of the disease. Int. J. Endocrinol. 2018, 2018, 6935054. [Google Scholar] [CrossRef] [PubMed]
- Uziȩbło-Życzkowska, B.; Jurek, A.; Witek, P.; Zieliński, G.; Gielerak, G.; Krzesiński, P. Left Heart Dysfunction in Acromegaly Revealed by Novel Echocardiographic Methods. Front. Endocrinol. 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 97, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Lengyel, C.; Ambrus, N.; Valkusz, Z. Mitral annulus is dilated with preserved function in acromegaly regardless of its activity: Insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Rev. Port. Cardiol. 2021, 40, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kormányos, Á.; Domsik, P.; Kalapos, A.; Valkusz, Z.; Lengyel, C.; Forster, T.; Nemes, A. Three-dimensional speckle tracking echocardiography-derived left atrial deformation analysis in acromegaly (Results from the MAGYAR-Path Study). Echocardiography 2018, 35, 975–984. [Google Scholar] [CrossRef]
- Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Ambrus, N.; Valkusz, Z.; Lengyel, C.; Nemes, A. The right atrium in acromegaly-a three-dimensional speckle-tracking echocardiographic analysis from the MAGYAR-Path Study. Quant. Imaging Med. Surg. 2020, 10, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Kormányos, Á.; Domsik, P.; Kalapos, A.; Orosz, A.; Lengyel, C.; Valkusz, Z.; Trencsányi, A.; Forster, T.; Nemes, A. Left ventricular twist is impaired in acromegaly: Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. J. Clin. Ultrasound 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Gyenes, N.; Lengyel, C.; Valkusz, Z. Diabetes mellitus deteriorates left ventricular deformation in acromegaly-analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant. Imaging Med. Surg. 2021, 11, 410–414. [Google Scholar] [CrossRef]
- Gyenes, N.; Kormányos, Á.; Vágvölgyi, A.; Valkusz, Z.; Balogh, L.; Papp, G.; Lengyel, C.; Nemes, A. Left ventricular deformation: Similarities and dissimilarities in elite sport activity- and acromegaly-related abnormalities. Results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Sport and MAGYAR-Path Studies. Orv. Hetil. 2023, 164, 308–316. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Lengyel, C.; Valkusz, Z.; Forster, T. Left ventricular ‘rigid body rotation’ in a patient with acromegaly (from the MAGYAR-Path Study). Quant. Imaging Med. Surg. 2017, 7, 378–379. [Google Scholar] [CrossRef]
- Kormányos, Á.; Domsik, P.; Kalapos, A.; Gyenes, N.; Valkusz, Z.; Lengyel, C.; Forster, T.; Nemes, A. Active acromegaly is associated with enhanced left ventricular contractility: Results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Rev. Port. Cardiol. 2020, 39, 189–196. [Google Scholar] [CrossRef]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Buckberg, G.D.; Nanda, N.C.; Nguyen, C.; Kocica, M.L. What is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J. Cardiovasc. Dev. Dis. 2018, 5, 33. [Google Scholar] [CrossRef]
- Myers, J.A.; Lunn, K.F.; Bright, J.M. Echocardiographic findings in 11 cats with acromegaly. J. Vet. Intern. Med. 2014, 28, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Nobuoka, S.; Nagashima, J.; Hatano, S.; Yoshida, A.; Ando, H.; Miyake, F.; Murayama, M. Heart failure in 3 patients with acromegaly: Echocardiographic assessment. Intern. Med. 1998, 37, 599–603. [Google Scholar] [CrossRef]
- Csanady, M.; Gáspár, L.; Hogye, M.; Gruber, N. The heart in acromegaly: An echocardiographic study. Int. J. Cardiol. 1983, 2, 349–361. [Google Scholar] [CrossRef]
- Smallridge, R.C.; Rajfer, S.; Davia, J.; Schaaf, M. Acromegaly and the heart. An echocardiographic study. Am. J. Med. 1979, 66, 22–27. [Google Scholar] [CrossRef]
- Wexler, T.L.; Durst, R.; McCarty, D.; Picard, M.H.; Gunnell, L.; Omer, Z.; Fazeli, P.; Miller, K.K.; Klibanski, A. Growth hormone status predicts left ventricular mass in patients after cure of acromegaly. Growth Horm. IGF. Res. 2010, 20, 333–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bogazzi, F.; Lombardi, M.; Strata, E.; Aquaro, G.; Di Bello, V.; Cosci, C.; Sardella, C.; Talini, E.; Martino, E. High prevalence of cardiac hypertophy without detectable signs of fibrosis in patients with untreated active acromegaly: An in vivo study using magnetic resonance imaging. Clin. Endocrinol. 2008, 68, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ságová, I.; Dragula, M.; Mokáň, M.; Vaňuga, P. Filling the gap between the heart and the body in acromegaly: A case-control study. Endocrine 2023, 79, 365–375. [Google Scholar] [CrossRef]
- Leães, C.G.S.; Kramer, C.K.; Pereira-Lima, J.F.S.; Hatem, D.M.; Castro, I.; Oliveira Mda, C. Diastolic function study with conventional and pulsed tissue Doppler echocardiography imaging in acromegalic patients. Echocardiography 2009, 26, 651–656. [Google Scholar] [CrossRef]
- Cansu, G.B.; Yılmaz, N.; Yanıkoğlu, A.; Özdem, S.; Yıldırım, A.B.; Süleymanlar, G.; Altunbaş, H.A. Assessment of diastolic dysfunction, arterial stiffness, and carotid intima-media thickness in patients with acromegaly. Endocr. Pract. 2017, 23, 536–545. [Google Scholar] [CrossRef]
- Akdeniz, B.; Gedik, A.; Turan, O.; Ozpelit, E.; Ikiz, A.O.; Itil, O.; Badak, O.; Baris, N.; Cömlekçi, A. Evaluation of left ventricular diastolic function according to new criteria and determinants in acromegaly. Int. Heart J. 2012, 53, 299–305. [Google Scholar] [CrossRef][Green Version]
- Baykan, M.; Erem, C.; Gedikli, O.; Hacihasanoğlu, A.; Erdoğan, T.; Koçak, M.; Kaplan, S.; Kiriş, A.; Celik, S. Assessment of the Tei index by tissue Doppler imaging in patients with acromegaly: Serum growth hormone level is associated with the Tei index. Echocardiography 2008, 25, 374–380. [Google Scholar] [CrossRef]
- Toumanidis, S.T.H.; Evangelopoulos, M.E.; Ilias, I.; Pamboucas, C.; Trikka, C.; Alevizaki, M. Is left ventricular dysfunction reversed after treatment of active acromegaly? Pituitary 2011, 14, 75–79. [Google Scholar] [CrossRef]
- Guo, X.; Cao, Y.; Cao, J.; Li, X.; Liu, P.; Wang, Z.; Gao, L.; Bao, X.; Xing, B.; Wang, Y. Reversibility of Cardiac Involvement in Acromegaly Patients After Surgery: 12-Month Follow-up Using Cardiovascular Magnetic Resonance. Front. Endocrinol. 2020, 11, 598948. [Google Scholar] [CrossRef] [PubMed]
- Jaffrain-Rea, M.L.; Minniti, G.; Moroni, C.; Esposito, V.; Ferretti, E.; Santoro, A.; Infusino, T.; Tamburrano, G.; Cantore, G.; Cassone, R. Impact of successful transsphenoidal surgery on cardiovascular risk factors in acromegaly. Eur. J. Endocrinol. 2003, 148, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Bahl, A.; Bhagat, H.; Dutta, P.; Rai, A.; Devgun, J.S.; Kaur, R.; Kumar, K.; Mukherjee, K.K. Impact of transsphenoidal surgery on asymptomatic cardiomyopathy in patients with acromegaly. A single-blinded study. Neurol. India 2017, 65, 1312–1316. [Google Scholar] [PubMed]
- Annamalai, A.K.; Webb, A.; Kandasamy, N.; Elkhawad, M.; Moir, S.; Khan, F.; Maki-Petaja, K.; Gayton, E.L.; Strey, C.H.; O’Toole, S.; et al. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J. Clin. Endocrinol. Metab. 2013, 98, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Maison, P.; Tropeano, A.I.; Macquin-Mavier, I.; Giustina, A.; Chanson, P. Impact of somatostatin analogs on the heart in acromegaly: A metaanalysis. J. Clin. Endocrinol. Metab. 2007, 92, 1743–1747. [Google Scholar] [CrossRef]
- Merola, B.; Cittadini, A.; Colao, A.; Ferone, D.; Fazio, S.; Sabatini, D.; Biondi, B.; Saccá, L.; Lombardi, G. Chronic treatment with the somatostatin analog octreotide improves cardiac abnormalities in acromegaly. J. Clin. Endocrinol. Metab. 1993, 77, 790–793. [Google Scholar]
- Colao, A.; Auriemma, R.S.; Galdiero, M.; Lombardi, G.; Pivonello, R. Effects of initial therapy for five years with somatostatin analogs for acromegaly on growth hormone and insulin-like growth factor-I levels, tumor shrinkage, and cardiovascular disease: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 3746–3756. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Grasso, L.F.; Galdiero, M.; Galderisi, M.; Pivonello, C.; Siemoli, C.; De Martino, M.C.; Ferrigno, R.; Negri, M.; de Angelis, C.; et al. Effects of long-term combined treatment with somatostatin analogues and pegvisomant on cardiac structure and performance in acromegaly. Endocrine 2017, 55, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Pivonello, R.; Galderisi, M.; Cappabianca, P.; Auriemma, R.S.; Galdiero, M.; Cavallo, L.M.; Esposito, F.; Lombardi, G. Impact of treating acromegaly first with surgery or somatostatin analogs on cardiomyopathy. J. Clin. Endocrinol. Metab. 2008, 93, 2639–2646. [Google Scholar] [CrossRef]
- Pivonello, R.; Galderisi, M.; Auriemma, R.S.; De Martino, M.C.; Galdiero, M.; Ciccarelli, A.; D’Errico, A.; Kourides, I.; Burman, P.; Lombardi, G.; et al. Treatment with growth hormone receptor antagonist in acromegaly: Effect on cardiac structure and performance. J. Clin. Endocrinol. Metab. 2007, 92, 476–482. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yu, W.C.; Kuo, C.S.; Chen, H.S. Growth hormone control and cardiovascular function in patients with acromegaly. J. Chin. Med. Assoc. 2021, 84, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Shafie, D.; Aminorroaya, A.; Sarrafzadegan, N.; Farajzadegan, Z.; Nouri, R.; Najimi, A.; Dimopolou, C.; Stalla, G. Effects of somatostatin analog treatment on cardiovascular parameters in patients with acromegaly: A systematic review. J. Res. Med. Sci. 2019, 24, 29. [Google Scholar]
- Volschan, I.C.M.; Kasuki, L.; Silva, C.M.S.; Alcantara, M.L.; Saraiva, R.M.; Xavier, S.S.; Gadelha, M.R. Two-dimensional speckle tracking echocardiography demonstrates no effect of active acromegaly on left ventricular strain. Pituitary 2017, 20, 349–357. [Google Scholar] [CrossRef]
- Popielarz-Grygalewicz, A.; Stelmachowska-Banaś, M.; Gąsior, J.S.; Grygalewicz, P.; Czubalska, M.; Zgliczyński, W.; Dąbrowski, M.; Kochman, W. Subclinical left ventricular systolic dysfunction in patients with naive acromegaly—Assessment with two-dimensional speckle-tracking echocardiography: Retrospective study. Endokrynol. Pol. 2020, 71, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, V.; Bogazzi, F.; Di Cori, A.; Palagi, C.; Delle Donne, M.G.; Gavioli, S.; Talini, E.; Cosci, C.; Sardella, C.; Tonti, G.; et al. Myocardial systolic strain abnormalities in patients with acromegaly: A prospective color Doppler imaging study. J. Endocrinol. Invest. 2006, 29, 544–550. [Google Scholar] [CrossRef]
- Koca, H.; Koc, M.; Sumbul, H.E.; Icen, Y.K.; Gulumsek, E.; Koca, F.; Ozturk, H.A.; Baykan, A.O.; Kaypakli, O. Subclinical Left Atrial and Ventricular Dysfunction in Acromegaly Patients: A Speckle Tracking Echocardiography Study. Arq. Bras. Cardiol. 2022, 118, 634–645. [Google Scholar]
- Jurcut, R.; Găloiu, S.; Florian, A.; Vlădaia, A.; Ioniţă, O.R.; Amzulescu, M.S.; Baciu, I.; Popescu, B.A.; Coculescu, M.; Ginghina, C. Quantifying subtle changes in cardiovascular mechanics in acromegaly: A Doppler myocardial imaging study. J. Endocrinol. Invest. 2014, 37, 1081–1090. [Google Scholar] [CrossRef]
- Gadelha, P.; Santos, E.C.L.; Castillo, J.; Vilar, L. Subclinical ventricular dysfunction in long-term acromegaly assessed by speckle-tracking echocardiography. Front. Endocrinol. 2022, 13, 812964. [Google Scholar] [CrossRef] [PubMed]
- Popielarz-Grygalewicz, A.; Stelmachowska-Banaś, M.; Raczkiewicz, D.; Czajka-Oraniec, I.; Zieliński, G.; Kochman, W.; Dąbrowski, M.; Zgliczyński, W. Effects of acromegaly treatment on left ventricular systolic function assessed by speckle tracking echocardiography in relation to sex differences: Results from a prospective single center study. Front. Endocrinol. 2023, 14, 1154615. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ho, S.Y.; Gibson, D.G.; Anderson, R.H. Architecture of atrial musculature in humans. Br. Heart J. 1995, 73, 559–565. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Badano, L.P.; Nour, A.; Muraru, D. Left atrium as a dynamic three-dimensional entity: Implications for echocardiographic assessment. Rev. Esp. Cardiol. 2013, 66, 1–4. [Google Scholar] [CrossRef]
- Todaro, M.C.; Choudhuri, I.; Belohlavek, M.; Jahangir, A.; Carerj, S.; Oreto, L.; Chandheria, B.K. New echocardiographic techniques for evaluation of left atrial mechanics. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 973–984. [Google Scholar] [CrossRef]
- Yayla, Ç.; Canpolat, U.; Şahinarslan, A.; Özkan, Ç.; Altinova, A.E.; Yayla, K.G.; Akboğa, M.K.; Eyiol, A.; Boyaci, B. The Assessment of Atrial Electromechanical Delay in Patients With Acromegaly. Can. J. Cardiol. 2015, 31, 1012–1018. [Google Scholar] [CrossRef]
- Ilter, A.; Kiris, A.; Kaplan, S.; Kutlu, M.; Şahin, M.; Erem, C.; Civan, N.; Kangül, F. Atrial conduction times and left atrium mechanical functions in patients with active acromegaly. Endocrine 2015, 48, 653–660. [Google Scholar] [CrossRef]
- Dal-Bianco, J.P.; Levine, R.A. Anatomy of the mitral valve apparatus—Role of 2D and 3D echocardiography. Cardiol. Clin. 2013, 31, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J.; Bazaz, R. The anatomic substrate of mitral annular contraction. Int. J. Cardiol. 2020, 306, 158–161. [Google Scholar] [CrossRef]
- Mihaila, S.; Muraru, D.; Miglioranza, M.H.; Piasentini, E.; Peluso, D.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Normal mitral annulus dynamics and its relationships with left ventricular and left atrial function. Int. J. Cardiovasc. Imaging 2015, 31, 279–290. [Google Scholar] [CrossRef]
- Colao, A.; Spinelli, L.; Marzullo, P.; Pivonello, R.; Petretta, M.; Di Somma, C.; Vitale, G.; Bonaduce, D.; Lombardi, G. High prevalence of cardiac valve disease in acromegaly: An observational, analytical, case-control study. J. Clin. Endocrinol. Metab. 2003, 88, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; van Thiel, S.W.; Lindner, J.R.; Roelfsema, F.; van der Wall, E.E.; Morreau, H.; Smit, J.W.; Romijn, J.A.; Bax, J.J. Increased prevalence of regurgitant valvular heart disease in acromegaly. J. Clin. Endocrinol. Metab. 2004, 89, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention 2022, 17, e1126–e1196. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y. Arterial-cardiac interaction: The concept and implications. J. Cardiovasc. Ultrasound 2011, 19, 62–66. [Google Scholar] [CrossRef]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gao, L.; Zhang, S.; Li, Y.; Fang, L.; Xing, B. Case-control study of expansion of heart cavity and great vessel diameters in acromegaly. Zhonghua Yi Xue Za Zhi 2015, 95, 3343–3346. [Google Scholar]
- van der Klaauw, A.A.; Bax, J.J.; Smit, J.W.A.; Holman, E.R.; Delgado, V.; Bleeker, G.B.; Biermasz, N.R.; Roelfsema, F.; Romijn, J.A.; Pereira, A.M. Increased aortic root diameters in patients with acromegaly. Eur. J. Endocrinol. 2008, 159, 97–103. [Google Scholar] [CrossRef]
- Lehmann, E.D. Noninvasive measurements of aortic stiffness: Methodological considerations. Pathol. Biol. 1999, 47, 716–730. [Google Scholar]
- Nemes, A.; Gavallér, H.; Csajbók, E.; Julesz, J.; Forster, T.; Csanády, M. Aortic stiffness is increased in acromegaly—A transthoracic echocardiographic study. Int. J. Cardiol. 2008, 124, 121–123. [Google Scholar] [CrossRef]
- Topaloglu, O.; Sayki Arslan, M.; Turak, O.; Ginis, Z.; Sahin, M.; Cebeci, M.; Ucan, B.; Cakir, E.; Karbek, B.; Ozbek, M.; et al. Three noninvasive methods in the evaluation of subclinical cardiovascular disease in patients with acromegaly: Epicardial fat thickness, aortic stiffness and serum cell adhesion molecules. Clin. Endocrinol. 2014, 80, 726–734. [Google Scholar] [CrossRef]
- Smith, J.C.; Lane, H.; Davies, N.; Evans, L.M.; Cockcroft, J.; Scanlon, M.F.; Davies, J.S. The effects of depot long-acting somatostatin analog on central aortic pressure and arterial stiffness in acromegaly. J. Clin. Endocrinol. Metab. 2003, 88, 2556–2561. [Google Scholar] [CrossRef][Green Version]
- Paisley, A.N.; Banerjee, M.; Rezai, M.; Schofield, R.E.; Balakrishnannair, S.; Herbert, A.; Lawrance, J.A.L.; Trainer, P.J.; Cruickshank, J.K. Changes in arterial stiffness but not carotid intimal thickness in acromegaly. J. Clin. Endocrinol. Metab. 2011, 96, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.R.; Evans, P.; Davies, B.; Baker, J.S. Arterial pulse wave velocity, inflammatory markers, pathological GH and IGF states, cardiovascular and cerebrovascular disease. Vasc. Health Risk Manag. 2008, 4, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Unübol, M.; Güney, E.; Türe, M.; Eryılmaz, U. Mean platelet volume and arterial stiffness in patients with acromegaly. Anadolu Kardiyol. Derg. 2014, 14, 456–463. [Google Scholar] [CrossRef][Green Version]
- Yaron, M.; Izkhakov, E.; Sack, J.; Azzam, I.; Osher, E.; Tordjman, K.; Stern, N.; Greenman, Y. Arterial properties in acromegaly: Relation to disease activity and associated cardiovascular risk factors. Pituitary 2016, 19, 322–331. [Google Scholar] [CrossRef]
- Parolin, M.; Dassie, F.; Martini, C.; Mioni, R.; Russo, L.; Fallo, F.; Rossato, M.; Vettor, R.; Maffei, P.; Pagano, C. Preclinical markers of atherosclerosis in acromegaly: A systematic review and meta-analysis. Pituitary 2018, 21, 653–662. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kawate, H.; Matsuzaki, C.; Sakamoto, R.; Abe, I.; Shibue, K.; Kohno, M.; Adachi, M.; Ohnaka, K.; Nomura, M.; et al. Reduced arterial stiffness in patients with acromegaly: Non-invasive assessment by the cardio-ankle vascular index (CAVI). Endocr. J. 2013, 60, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dassie, F.; Grillo, A.; Carretta, R.; Fabris, B.; Macaluso, L.; Bardelli, M.; Martini, C.; Paoletta, A.; Vettor, R.; Sicolo, N.; et al. Ambulatory arterial stiffness indexes in acromegaly. Eur. J. Endocrinol. 2012, 166, 199–205. [Google Scholar] [CrossRef]
- Can, M.; Kocabas, M.; Karakose, M.; Yerlikaya, F.H.; Alsancak, Y.; Turkmen, K.; Kulaksizoglu, M.; Karakurt, F. Arterial Stiffness, Carotid Intima-Media Thickness, Endocan, and A Disintegrin and Metalloproteinase With Thrombospondin Type I Motif 9 Levels and Their Relationship With Disease Activity in Patients With Acromegaly With and Without Cardiovascular Risk Factors. Endocr. Pract. 2022, 28, 298–303. [Google Scholar] [PubMed]
- Filchenko, I.; Korostovtseva, L.; Bochkarev, M.; Boyarinova, M.; Alieva, A.; Rotar, O.; Sviryaev, Y.; Tsoi, U.; Grineva, E. Pulse wave velocity is decreased in acromegaly compared to non-acromegaly study participants with similar cardiovascular risk profile. Growth Horm. IGF Res. 2021, 57–58, 101395. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Gavallér, H.; Csajbók, É.; Lengyel, C.; Forster, T.; Csanády, M. Does diabetes mellitus facilitate aortic stiffening in acromegaly? Diabetes Res. Clin. Pract. 2007, 78, e7–e8. [Google Scholar] [CrossRef]
- Csajbók, E.; Kalapos, A.; Gavallér, H.; Wittmann, T.; Csanády, M.; Forster, T.; Nemes, A. Prognostic significance of aortic stiffness index in acromegaly—Results from a 4-year follow-up. Int. J. Cardiol. 2011, 147, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Bassareo, P.P.; Marras, A.R.; Mercuro, G. Adolescent acromegaly and decreased arterial distensibility despite successful treatment. Clin. Endocrinol. 2012, 77, 79–85. [Google Scholar] [CrossRef]
- Akgul, E.; Tokgozoglu, S.L.; Erbas, T.; Kabakci, G.; Aytemir, K.; Haznedaroglu, I.; Oto, A.; Kes, S.K. Evaluation of the impact of treatment on endothelial function and cardiac performance in acromegaly. Echocardiography 2010, 27, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, C.; Altinova, A.E.; Cerit, E.T.; Yayla, C.; Sahinarslan, A.; Sahin, D.; Dincel, A.S.; Toruner, F.B.; Akturk, M.; Arslan, M. Markers of early atherosclerosis, oxidative stress and inflammation in patients with acromegaly. Pituitary 2015, 18, 621–629. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92 (Suppl. S1), i2–i13. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, Part I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Colao, A. Improvement of cardiac parameters in patients with acromegaly treated with medical therapies. Pituitary 2012, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Cittadini, A.; Sabatini, D.; Merola, B.; Colao, A.M.; Biondi, B.; Lombardi, G.; Saccá, L. Evidence for biventricular involvement in acromegaly: A Doppler echocardiographic study. Eur. Heart J. 1993, 14, 26–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pirhan, O.; Ertuğrul, A.S.; Yıldız, C.; Karabulut, D.; Pehlivan, B.; Piskinpasa, H.; Dogansen, S.C.; Mert, M. Assessment of right ventricular functions in acromegaly: Comparison of active disease with remission. Kardiologiia 2022, 62, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Natchev, E.; Kundurdjiev, A.; Zlatareva, N.; Vandeva, S.; Kirilov, G.; Kundurzhiev, T.; Zacharieva, S. Echocardiographic myocardial changes in acromegaly: A cross-sectional analysis in a tertiary center in Bulgaria. Acta Endocrinol. 2019, 5, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M. The right atrium, a forgotten cardiac chamber: An updated review of multimodality imaging. J. Clin. Ultrasound 2015, 43, 335–345. [Google Scholar] [CrossRef]
- Dahou, A.; Levin, D.; Reisman, M.; Hahn, R.T. Anatomy and physiology of the tricuspid valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef]
- Kivelitz, D.E.; Dohmen, P.M.; Lembcke, A.; Kroencke, T.J.; Klingebiel, R.; Hamm, B.; Konertz, W.; Taupitz, M. Visualization of the pulmonary valve using cine MR imaging. Acta Radiol. 2003, 44, 172–176. [Google Scholar] [CrossRef]
- Goldberg, M.D.; Vadera, N.; Yandrapalli, S.; Frishman, W.H. Acromegalic Cardiomyopathy: An Overview of Risk Factors, Clinical Manifestations, and Therapeutic Options. Cardiol. Rev. 2018, 26, 307–311. [Google Scholar] [CrossRef]
- Thompson, B.J.; Shang, C.A.; Waters, M.J. Identification of genes induced by growth hormone in rat liver using cDNA arrays. Endocrinology 2000, 141, 4321–4324. [Google Scholar] [CrossRef]
- Kamenický, P.; Maione, L.; Chanson, P. Cardiovascular complications of acromegaly. Ann. Endocrinol. 2021, 82, 206–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C.; Valkusz, Z. Myocardial, Valvular, and Vascular Structural and Functional Properties in Acromegaly. J. Clin. Med. 2023, 12, 6857. https://doi.org/10.3390/jcm12216857
Nemes A, Kormányos Á, Ambrus N, Lengyel C, Valkusz Z. Myocardial, Valvular, and Vascular Structural and Functional Properties in Acromegaly. Journal of Clinical Medicine. 2023; 12(21):6857. https://doi.org/10.3390/jcm12216857
Chicago/Turabian StyleNemes, Attila, Árpád Kormányos, Nóra Ambrus, Csaba Lengyel, and Zsuzsanna Valkusz. 2023. "Myocardial, Valvular, and Vascular Structural and Functional Properties in Acromegaly" Journal of Clinical Medicine 12, no. 21: 6857. https://doi.org/10.3390/jcm12216857
APA StyleNemes, A., Kormányos, Á., Ambrus, N., Lengyel, C., & Valkusz, Z. (2023). Myocardial, Valvular, and Vascular Structural and Functional Properties in Acromegaly. Journal of Clinical Medicine, 12(21), 6857. https://doi.org/10.3390/jcm12216857






