Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics and Clinical Manifestations
3.2. Autoantibodies
3.3. Factors Associated to ILD Development
3.4. Treatment
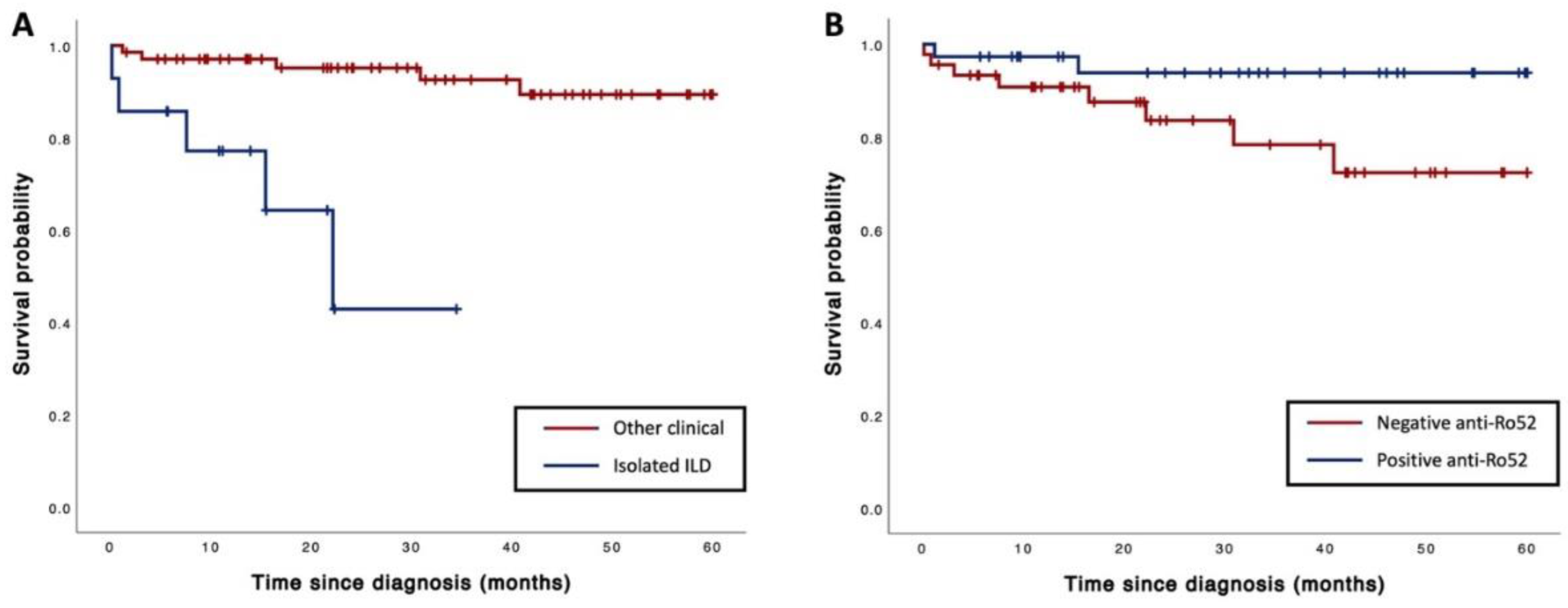
3.5. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connors, G.R.; Christopher-Stine, L.; Oddis, C.V.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest 2010, 138, 1464–1474. [Google Scholar] [CrossRef]
- Solomon, J.; Swigris, J.J.; Brown, K.K. Myositis-related interstitial lung disease and antisynthetase syndrome. J. Bras. Pneumol. 2011, 37, 100–109. [Google Scholar] [CrossRef]
- Nishikai, M.; Reichlin, M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1980, 23, 881–888. [Google Scholar] [CrossRef]
- Galindo-Feria, A.S.; Notarnicola, A.; Lundberg, I.E.; Horuluoglu, B. Aminoacyl-tRNA synthetases: On anti-synthetase syndrome and beyond. Front. Immunol. 2022, 13, 866087. [Google Scholar] [CrossRef]
- Opinc, A.H.; Makowska, J.S. Antisynthetase syndrome–much more than just a myopathy. Semin. Arthritis Rheum. 2021, 51, 72–83. [Google Scholar] [CrossRef]
- Hervier, B.; Devilliers, H.; Stanciu, R.; Meyer, A.; Uzunhan, Y.; Masseau, A.; Dubucquoi, S.; Hatron, P.-Y.; Musset, L.; Wallaert, B. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012, 12, 210–217. [Google Scholar] [CrossRef]
- Cavagna, L.; Trallero-Araguás, E.; Meloni, F.; Cavazzana, I.; Rojas-Serrano, J.; Feist, E.; Zanframundo, G.; Morandi, V.; Meyer, A.; Pereira da Silva, J.A. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J. Clin. Med. 2019, 8, 2013. [Google Scholar] [CrossRef]
- Aggarwal, R.; Cassidy, E.; Fertig, N.; Koontz, D.C.; Lucas, M.; Ascherman, D.P.; Oddis, C.V. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann. Rheum. Dis. 2014, 73, 227–232. [Google Scholar] [CrossRef]
- Ahn, S.S.; Park, Y.-B.; Lee, S.-W. Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis. J. Clin. Med. 2022, 11, 2052. [Google Scholar] [CrossRef]
- Witt, L.J.; Curran, J.J.; Strek, M.E. The diagnosis and treatment of antisynthetase syndrome. Clin. Pulm. Med. 2016, 23, 218. [Google Scholar] [CrossRef]
- Martins, P.; Dourado, E.; Melo, A.T.; Samões, B.; Sousa, M.; Freitas, R.; Lourenço, M.; Fernandes, B.M.; Costa, E.; Parente, H.; et al. Clinical characterisation of a multicentre nationwide cohort of patients with antisynthetase syndrome. ARP Rheumatol. 2022, 1, 190–196. [Google Scholar]
- Wang, R.; Zhao, Y.; Qi, F.; Wu, X.; Wang, Y.; Xu, Y.; Wu, Y.; Zhang, N.; Hou, H.; Sun, W. Analysis of the clinical features of antisynthetase syndrome: A retrospective cohort study in China. Clin. Rheumatol. 2023, 42, 703–709. [Google Scholar] [CrossRef]
- Marguerie, C.; Bunn, C.; Beynon, H.; Bernstein, R.; Hughes, J.; So, A.; Walport, M. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. QJM Int. J. Med. 1990, 77, 1019–1038. [Google Scholar] [CrossRef]
- Marie, I.; Josse, S.; Hatron, P.; Dominique, S.; Hachulla, E.; Janvresse, A.; Cherin, P.; Mouthon, L.; Vittecoq, O.; Menard, J.F. Interstitial lung disease in anti-Jo-1 patients with antisynthetase syndrome. Arthritis Care Res. 2013, 65, 800–808. [Google Scholar] [CrossRef]
- Cavagna, L.; Nuno, L.; Scire, C.A.; Govoni, M.; Longo, F.J.L.; Franceschini, F.; Neri, R.; Castaneda, S.; Giraldo, W.A.S.; Caporali, R. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: Results from an international retrospective multicenter study. Medicine 2015, 94, e1144. [Google Scholar] [CrossRef]
- McHugh, N.J. Ro52, myositis, and interstitial lung disease. J. Rheumatol. 2023, 50, 161–163. [Google Scholar] [CrossRef]
- Ungprasert, P.; Leeaphorn, N.; Hosiriluck, N.; Chaiwatcharayut, W.; Ammannagari, N.; Raddatz, D.A. Clinical features of inflammatory myopathies and their association with malignancy: A systematic review in Asian population. Int. Sch. Res. Not. 2013, 2013, 509354. [Google Scholar] [CrossRef]
- Rojas-Serrano, J.; Herrera-Bringas, D.; Mejía, M.; Rivero, H.; Mateos-Toledo, H.; Figueroa, J.E. Prognostic factors in a cohort of antisynthetase syndrome (ASS): Serologic profile is associated with mortality in patients with interstitial lung disease (ILD). Clin. Rheumatol. 2015, 34, 1563–1569. [Google Scholar] [CrossRef]
- Xing, X.; Li, A.; Li, C. Anti-Ro52 antibody is an independent risk factor for interstitial lung disease in dermatomyositis. Respir. Med. 2020, 172, 106134. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Almeida, I.; Marinho, A.; Cerveira, C.; Vasconcelos, C. Anti-ro52 antibodies and interstitial lung disease in connective tissue diseases excluding scleroderma. Int. Sch. Res. Not. 2012, 2012, 415272. [Google Scholar] [CrossRef]
- Bozzalla-Cassione, E.; Zanframundo, G.; Biglia, A.; Bellis, E.; Bozzini, S.; Codullo, V.; Vertui, V.; Alpini, C.; Valentini, A.; Preda, L. Anti-Ro52 antibodies positivity in antisynthetase syndrome: A single centre cohort study. Clin. Exp. Rheumatol. 2022, 40, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Mora, A.L.; Kapetanaki, M.; Weathington, N.; Gladwin, M.; Eickelberg, O. Aging and lung disease. Clinical impact and cellular and molecular pathways. Ann. Am. Thorac. Soc. 2015, 12, S222–S227. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, S.; Yang, H.; Zhang, Y.; Peng, Q.; Lu, X.; Wang, G. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J. Rheumatol. 2017, 44, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Debray, M.-P.; Borie, R.; Revel, M.-P.; Naccache, J.-M.; Khalil, A.; Toper, C.; Israel-Biet, D.; Estellat, C.; Brillet, P.-Y. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings. Eur. J. Radiol. 2015, 84, 516–523. [Google Scholar] [CrossRef] [PubMed]
- La Corte, R.; Lo Monaco, A.; Locaputo, A.; Dolzani, F.; Trotta, F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006, 39, 249–253. [Google Scholar] [CrossRef]
- Marie, I.; Hatron, P.Y.; Dominique, S.; Cherin, P.; Mouthon, L.; Menard, J.-F.; Levesque, H.; Jouen, F. Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody. Semin. Arthritis Rheum. 2012, 41, 890–899. [Google Scholar] [CrossRef]
- Tranah, E.; MacBrayne, A.; Bhadauria, N.; Mukerjee, D. A case of antisynthetase syndrome presenting solely with life-threatening interstitial lung disease. Clin. Med. 2023, 23, 85–87. [Google Scholar] [CrossRef]
- Kuwana, M.; Okazaki, Y. A multianalyte assay for the detection of dermatomyositis-related autoantibodies based on immunoprecipitation combined with immunoblotting. Mod. Rheumatol. 2023, 33, 543–548. [Google Scholar] [CrossRef]
| Total (N = 82) | ILD (N = 64) | Non-ILD (N = 18) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 25 (30) | 23 (36) | 2 (11) | 0.04 |
| Age at diagnosis, years § | 60.2 ± 14.5 | 63.3 ± 13.0 | 48.9 ± 13.9 | <0.001 |
| Smoking, n (%) | 21 (26) | 18 (28) | 3 (17) | 0.54 |
| Overlapping diseases | ||||
| Sjogren’s syndrome, n (%) | 10 (12) | 7 (11) | 3 (17) | 0.68 |
| SLE, n (%) | 8 (10) | 4 (6) | 4 (22) | 0.07 |
| Systemic sclerosis, n (%) | 8 (10) | 8 (13) | 0 (0) | 0.19 |
| Rheumatoid arthritis, n (%) | 7 (9) | 5 (8) | 2 (11) | 0.65 |
| Clinical features (throughout the follow-up period) | ||||
| Arthritis, n (%) | 47 (57) | 32 (50) | 15 (83) | 0.01 |
| Raynaud’s phenomenon, n (%) | 26 (32) | 19 (30) | 7 (39) | 0.46 |
| Mechanic’s hands, n (%) | 24 (29) | 23 (36) | 1 (6) | 0.01 |
| Fever, n (%) | 21 (26) | 17 (27) | 4 (22) | 1.00 |
| Rash, n (%) | 31 (38) | 24 (38) | 7 (39) | 0.92 |
| Oral ulcer, n (%) | 4 (5) | 3 (5) | 1 (6) | 1.00 |
| Hair loss, n (%) | 20 (24) | 14 (22) | 6 (33) | 0.36 |
| Sicca symptoms, n (%) | 25 (31) | 19 (30) | 6 (33) | 0.77 |
| Weakness, n (%) | 22 (27) | 17 (27) | 5 (28) | 1.00 |
| Myositis, n (%) | 14 (17) | 11 (17) | 3 (17) | 1.00 |
| Autoantibodies (at diagnosis) | ||||
| Anti-Jo-1, n (%) | 22 (27) | 19 (30) | 3 (17) | 0.37 |
| Anti-PL-7, n (%) | 24 (29) | 16 (25) | 8 (44) | 0.11 |
| Anti-PL-12, n (%) | 13 (16) | 11 (17) | 2 (11) | 0.72 |
| Anti-EJ, n (%) | 14 (17) | 13 (20) | 1 (6) | 0.29 |
| Anti-OJ, n (%) | 9 (11) | 5 (8) | 4 (22) | 0.10 |
| Multiple positive MSA, n (%) | 17 (21) | 14 (22) | 3 (17) | 0.75 |
| Anti-Ro52, n (%) | 37 (45) | 34 (53) | 3 (17) | 0.01 |
| Antinuclear antibodies, n (%) † | 53 (76) | 42 (76) | 11 (73) | 1.00 |
| -Speckle pattern, n (%) | 30 (57) | 23 (55) | 7 (64) | 0.74 |
| -Homogeneous pattern, n (%) | 16 (30) | 12 (29) | 4 (36) | 0.72 |
| -Nucleolar pattern, n (%) | 13 (25) | 8 (19) | 5 (46) | 0.11 |
| -Multiple nuclear dot pattern, n (%) | 7 (23) | 7 (30) | 0 (0) | 0.15 |
| -Centromere pattern, n (%) | 2 (4) | 2 (5) | 0 (0) | 1.00 |
| -Cytoplasmic staining pattern, n (%) | 17 (24) | 14 (25) | 3 (20) | 1.00 |
| Laboratory data (at diagnosis) | ||||
| ESR, mm/hour ¶ | 35 (20–62) | 35.5 (20–58) | 33 (18–68) | 0.87 |
| Creatine kinase, IU/L ¶ | 90 (60–153) | 91 (61–201) | 87 (50–103) | 0.21 |
| C-reactive protein, mg/dL ¶ | 5.9 (1.1–18.9) | 6.2 (1.2–9.1) | 3.4 (0.6–16.1) | 0.40 |
| Hemoglobin levels, g/dL § | 12.1 ± 1.8 | 12.3 ± 1.7 | 11.6 ± 2.0 | 0.16 |
| White blood cell count, 109/L ¶ | 7.9 (5.7–10.6) | 8.5 (6.3–11.5) | 6.3 (5.5–7.9) | 0.01 |
| Platelet count, 109/L ¶ | 288 (216–358) | 290 (218–360) | 282 (212–354) | 0.50 |
| Underlying diseases | ||||
| Hypertension, n (%) | 35 (43) | 32 (50) | 3 (17) | 0.01 |
| Diabetes mellitus, n (%) | 20 (24) | 14 (22) | 6 (33) | 0.32 |
| COPD, n (%) | 5 (6) | 5 (8) | 0 (0) | 0.22 |
| Cerebrovascular disease, n (%) | 3 (4) | 3 (5) | 0 (0) | 0.35 |
| Coronary artery disease, n (%) | 9 (11) | 7 (11) | 2 (11) | 0.98 |
| Cirrhosis, n (%) | 3 (4) | 3 (5) | 0 (0) | 0.35 |
| Malignancy, n (%) | 10 (12) | 8 (13) | 2 (11) | 0.87 |
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | |
|---|---|---|---|---|---|
| Anti-Jo-1 | |||||
| Anti-PL-7 | 0 | ||||
| Anti-PL-12 | 0 | 0 | |||
| Anti-EJ | 0 | 1 | 0 | ||
| Anti-OJ | 0 | 0 | 0 | 1 | |
| Anti-SRP | 1 | 0 | 0 | 0 | 0 |
| Anti-Mi2 | 2 | 3 | 3 | 0 | 1 |
| Anti-MDA5 | 0 | 0 | 0 | 1 | 0 |
| Anti-TIF1γ | 0 | 1 | 1 | 0 | 0 |
| Adjusted Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Age at diagnosis | 1.10 (1.03–1.18) | 0.01 |
| Arthritis | 0.09 (0.01–0.75) | 0.03 |
| Positive anti-Ro52 | 17.92 (2.13–138.68) | 0.01 |
| History of hypertension | 7.27 (2.13–138.68) | 0.06 |
| Mechanic’s hands | 13.05 (0.72–235.38) | 0.08 |
| Total (N = 82) | ILD (N = 64) | Non-ILD (N = 18) | p-Value | |
|---|---|---|---|---|
| Oral prednisolone | 69 (84) | 54 (84) | 15 (83) | 1.00 |
| Hydroxychloroquine | 60 (73) | 42 (67) | 18 (100) | <0.01 |
| Azathioprine | 44 (54) | 33 (52) | 11 (61) | 0.60 |
| Oral cyclophosphamide | 36 (44) | 33 (52) | 3 (17) | <0.01 |
| Mycophenolate mofetil | 27 (33) | 25 (39) | 2 (11) | 0.03 |
| Methotrexate | 16 (20) | 9 (14) | 7 (39) | 0.04 |
| Cyclosporine | 13 (16) | 11 (17) | 2 (11) | 0.72 |
| Intravenous cyclophosphamide | 12 (15) | 10 (16) | 2 (11) | 1.00 |
| Intravenous methylprednisolone | 10 (12) | 8 (13) | 2 (11) | 1.00 |
| Rituximab | 10 (12) | 10 (16) | 0 (0) | 0.11 |
| Tacrolimus | 3 (4) | 3 (5) | 0 (0) | 1.00 |
| Death (N = 10) | Survival (N = 72) | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 4 (40) | 21 (29) | 1.6 (0.5–5.8) | 0.45 |
| Age at diagnosis, years § | 66.9 ± 10.5 | 59.2 ± 14.7 | 1.05 (0.90–1.10) | 0.06 |
| Smoking, n (%) | 6 (60) | 15 (21) | 4.4 (1.3–15.8) | 0.02 |
| Overlapping diseases | ||||
| Rheumatoid arthritis, n (%) | 0 (0) | 7 (10) | 0.04 (0–707) | 0.33 |
| Sjogren’s syndrome, n (%) | 1 (10) | 9 (13) | 0.6 (0.1–4.8) | 0.64 |
| SLE, n (%) | 1 (10) | 7 (10) | 0.9 (0.1–7.5) | 0.95 |
| Systemic sclerosis, n (%) | 1 (10) | 7 (10) | 1.2 (0.2–9.8) | 0.84 |
| Clinical features | ||||
| ILD, n (%) | 7 (70) | 57 (79) | 0.7 (0.2–2.8) | 0.66 |
| Rapid progressive, n (%) | 2 (20) | 4 (6) | 3.5 (0.7–16.4) | 0.11 |
| Isolated ILD, n (%) | 5 (50) | 9 (13) | 10.9 (2.8–43.2) | <0.01 |
| Arthritis, n (%) | 4 (40) | 43 (60) | 0.4 (0.1–1.3) | 0.13 |
| Raynaud’s phenomenon, n (%) | 2 (20) | 24 (33) | 0.5 (0.1–2.3) | 0.36 |
| Mechanic’s hands, n (%) | 0 (0) | 24 (33) | 0.03 (0–6.3) | 0.19 |
| Fever, n (%) | 2 (20) | 19 (26) | 0.5 (0.1–2.6) | 0.45 |
| Rash, n (%) | 3 (30) | 28 (39) | 0.5 (0.1–2.1) | 0.36 |
| Oral ulcer, n (%) | 0 (0) | 4 (6) | 0.1 (0–19,116) | 0.64 |
| Hair loss, n (%) | 1 (10) | 19 (26) | 0.3 (0.1–2.3) | 0.25 |
| Sicca symptoms, n (%) | 2 (20) | 23 (32) | 0.8 (0.2–3.7) | 0.76 |
| Weakness, n (%) | 3 (30) | 19 (26) | 1.1 (0.3–4.1) | 0.92 |
| Autoantibodies | ||||
| Anti-Jo-1, n (%) | 0 (0) | 22 (31) | 0.03 (0–14.7) | 0.27 |
| Anti-PL-7, n (%) | 5 (50) | 19 (26) | 2.4 (0.7–8.4) | 0.16 |
| Anti-PL-12, n (%) | 3 (30) | 10 (14) | 2.4 (0.7–9.3) | 0.21 |
| Anti-EJ, n (%) | 1 (10) | 13 (18) | 0.4 (0.1–3.3) | 0.40 |
| Anti-OJ, n (%) | 1 (10) | 8 (11) | 0.8 (0.1–6.5) | 0.85 |
| Multiple positive MSA, n (%) | 4 (40) | 13 (18) | 2.8 (0.8–9.9) | 0.11 |
| Anti-Ro52, n (%) | 2 (20) | 35 (49) | 0.3 (0.1–1.2) | 0.08 |
| Antinuclear antibodies, n (%) † | 7 (78) | 46 (75) | 1.3 (0.3–6.2) | 0.76 |
| -Speckle pattern, n (%) | 3 (43) | 27 (59) | 0.5 (0.1–2.4) | 0.42 |
| -Homogeneous pattern, n (%) | 3 (43) | 13 (28) | 1.6 (0.4–7.4) | 0.53 |
| -Nucleolar pattern, n (%) | 3 (43) | 10 (22) | 2.2 (0.5–10.2) | 0.31 |
| -Multiple nuclear dot pattern, n (%) | 0 (0) | 7 (27) | 0.03 (0–490) | 0.48 |
| -Centromere pattern, n (%) | 1 (14) | 1 (2) | 12.0 (1.1–132) | 0.04 |
| -Cytoplasmic staining pattern, n (%) | 2 (22) | 15 (25) | 0.6 (0.1–2.9) | 0.49 |
| Laboratory data (at diagnosis) | ||||
| ESR, mm/hour ¶ | 53 (27–67) | 34 (20–62) | 1.01 (0.99–1.03) | 0.41 |
| C-reactive protein, mg/dL ¶ | 17.5 (9.9–68.2) | 4.3 (1.0–17.6) | 1.01 (0.99–1.03) | 0.11 |
| Creatine kinase, IU/L ¶ | 50 (20–69) | 93 (68–164) | 0.99 (0.98–1.01) | 0.26 |
| Hemoglobin levels, g/dL § | 10.5 ± 1.5 | 12.3 ± 1.8 | 0.6 (0.4–0.9) | < 0.01 |
| White blood cell count, 109/L ¶ | 7.5 (5.8–11.0) | 8.0 (5.8–11.2) | 0.9 (0.8–1.1) | 0.37 |
| Lymphocyte count, 109/L | 0.9 (0.4–1.3) | 1.8 (1.3–2.5) | 0.2 (0.1–0.6) | <0.01 |
| Platelet count, 109/L ¶ | 234 (163–384) | 290 (217–357) | 0.99 (0.98–1.00) | 0.21 |
| HRCT pattern | ||||
| Emphysema, n (%) | 3 (30) | 3 (4) | 7.4 (1.9–29.1) | <0.01 |
| Evidence of air trapping, n (%) | 2 (20) | 22 (31) | 0.8 (0.2–4.1) | 0.82 |
| NSIP, n (%) | 6 (60) | 38 (53) | 1.3 (0.4–4.5) | 0.72 |
| Honeycombing, n (%) | 1 (10) | 6 (8) | 1.3 (0.2–10.3) | 0.80 |
| UIP or probable UIP, n (%) | 0 (0) | 6 (8) | 0.04 (0–767) | 0.53 |
| OP, n (%) | 3 (30) | 19 (26) | 1.1 (0.3–4.4) | 0.84 |
| Underlying disease | ||||
| Hypertension, n (%) | 8 (80) | 27 (38) | 6.8 (1.4–32.2) | 0.02 |
| Diabetes mellitus, n (%) | 6 (60) | 14 (19) | 6.0 (1.7–21.5) | <0.01 |
| COPD, n (%) | 2 (20) | 3 (4) | 4.1 (0.9–19.4) | 0.07 |
| Cerebrovascular disease, n (%) | 0 (0) | 3 (4) | 0.05 (0–>10,000) | 0.77 |
| Coronary artery disease, n (%) | 1 (10) | 8 (11) | 0.99 (0.13–7.87) | 1.00 |
| Cirrhosis, n (%) | 2 (20) | 1 (1) | 9.6 (2.0–45.4) | <0.01 |
| Malignancy, n (%) | 6 (60) | 4 (6) | 21.5 (5.1–89.7) | <0.01 |
| Adjusted Hazard Ratio (95% CI) | p-Value | |
|---|---|---|
| Increasing lymphocyte counts per 100 × 109/L | 0.74 (0.61–0.91) | <0.01 |
| Isolated ILD | 9.59 (1.52–60.61) | 0.02 |
| Presence of anti-Ro52 | 0.14 (0.02–0.93) | 0.04 |
| History of cirrhosis | 8.55 (0.95–77.08) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodsri, T.; Petnak, T.; Ngamjanyaporn, P. Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 6849. https://doi.org/10.3390/jcm12216849
Sodsri T, Petnak T, Ngamjanyaporn P. Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study. Journal of Clinical Medicine. 2023; 12(21):6849. https://doi.org/10.3390/jcm12216849
Chicago/Turabian StyleSodsri, Tulaton, Tananchai Petnak, and Pintip Ngamjanyaporn. 2023. "Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study" Journal of Clinical Medicine 12, no. 21: 6849. https://doi.org/10.3390/jcm12216849
APA StyleSodsri, T., Petnak, T., & Ngamjanyaporn, P. (2023). Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study. Journal of Clinical Medicine, 12(21), 6849. https://doi.org/10.3390/jcm12216849









