Reduction in Arterial Stiffness Index (SI) in Response to Combination Antioxidant Therapy
Abstract
:1. Introduction
2. Methods
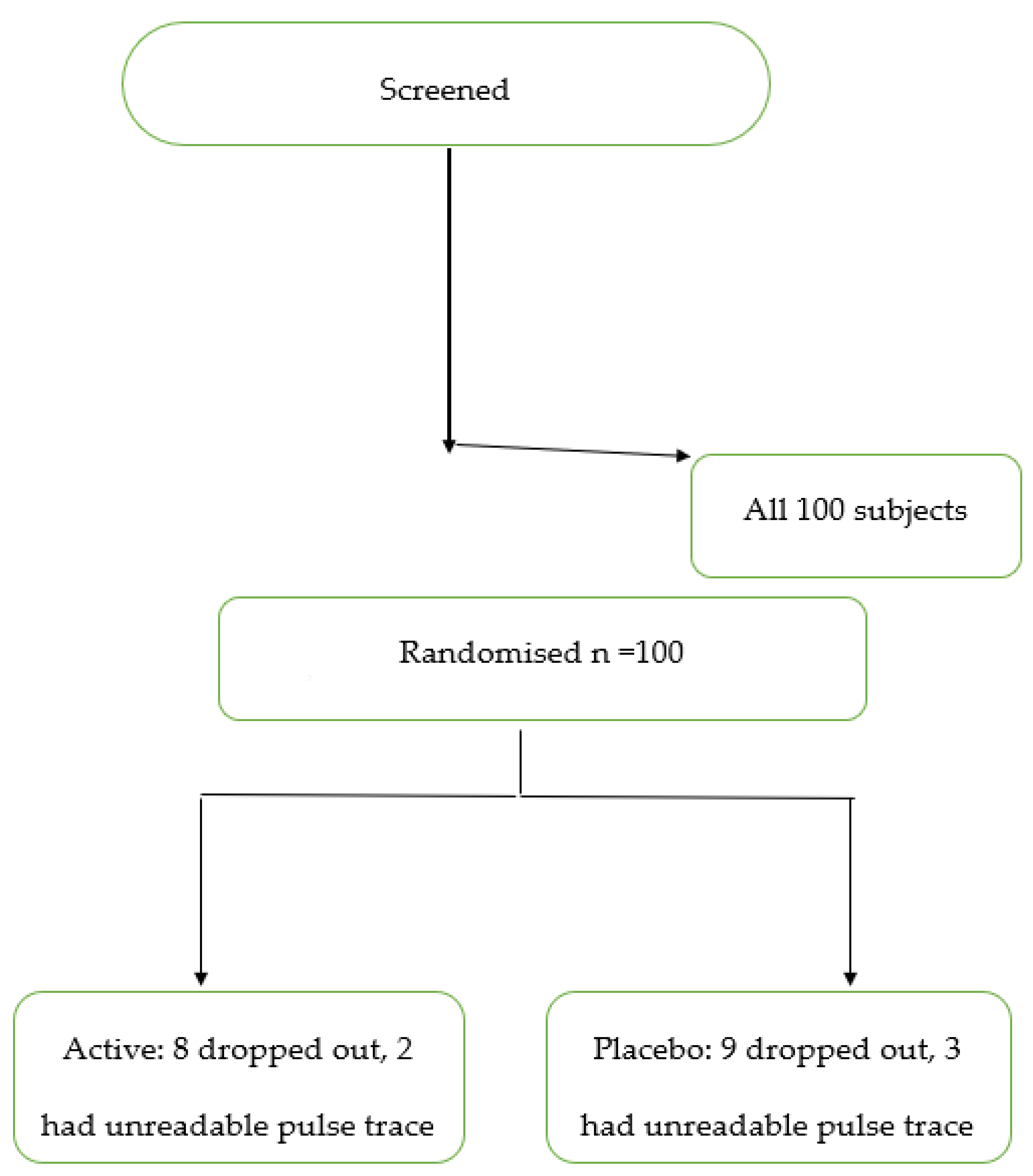
2.1. Study Design and Subjects
2.2. Study Procedures
2.3. Study Formulations
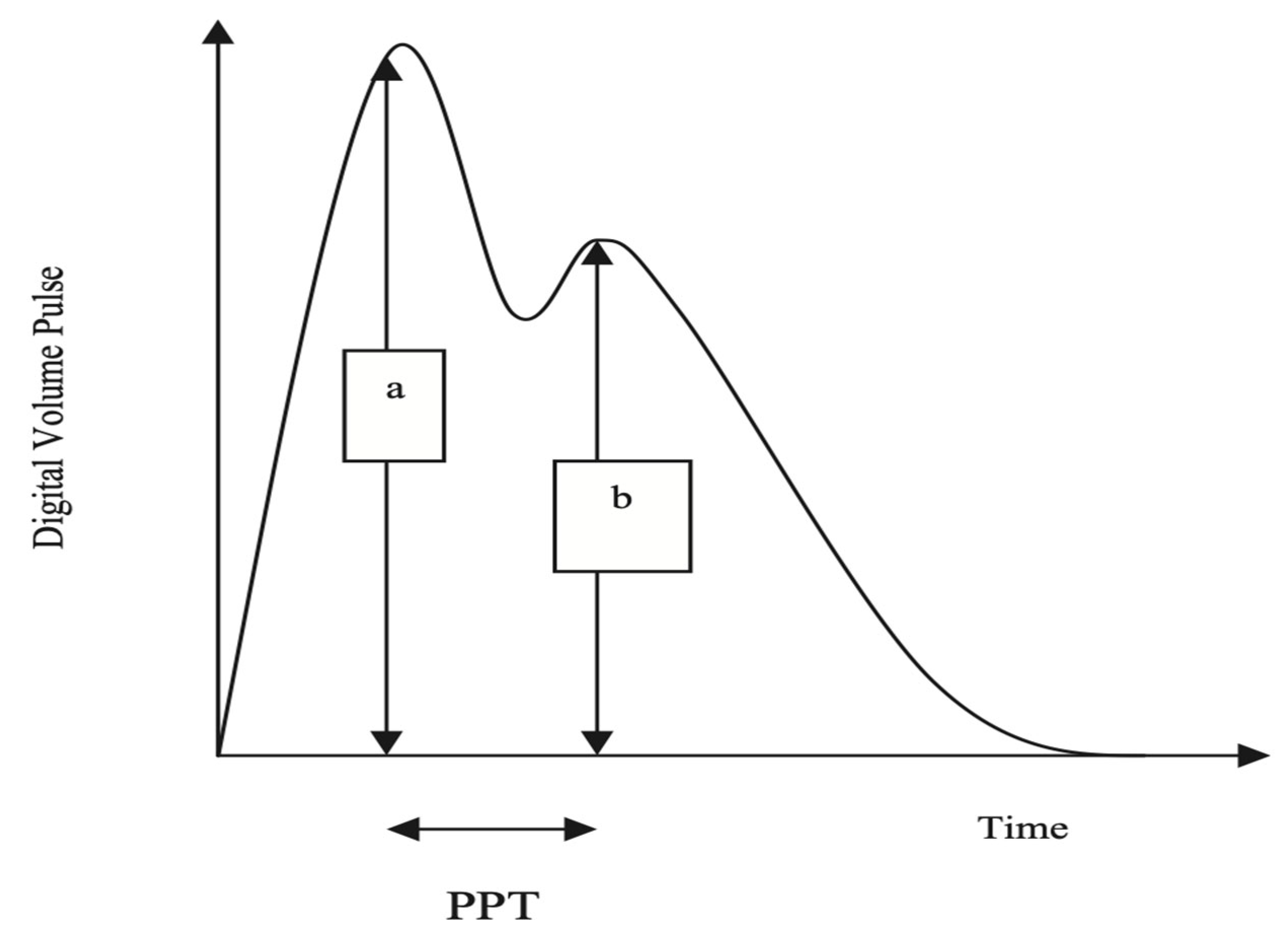
2.4. Measurement of SI
2.5. Statistical Analysis
2.6. Ethics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Med. Assoc. 2014, 63, 636–646. [Google Scholar]
- Patel, R.S.; Al Mheid, I.; Morris, A.A.; Ahmed, Y.; Kavtaradze, N.; Ali, S.; Dabhadkar, K.; Brigham, K.; Hooper, W.C.; Alexander, R.W.; et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis 2011, 218, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Mathers, J.C. Antioxidant vitamin supplementation reduces arterial stiffness in adults: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2014, 144, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Orella-Urugua, S.; Briones-Valdivics, C.; Chichirelli, S.; Saso, L.; Rodrig, R. Potential role of natural antioxidants in countering Reperfusion Injury in Acute Myocardional Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Bran, J.; Niewiara, L.; Podolec, J.; Siedlinski, M.; Josefczak, E.; Bernacik, A.; Badacz, R.; Przewlocki, T.; Pienazek, P.; Zmudka, K.; et al. Serum and vascular stiffness bio markers associated with the severity of degenerative aortic valve stenosis and cardiovascular outcomes. J. Cardiovasc. Dev. Dis. 2022, 9, 193. [Google Scholar]
- Sprince, H.; Parker, C.M.; Smith, G.G.; Gonzales, L.J. Protective action of Ascorbic acid and sulfer compounds against acetaldehyde toxicity: Implications in alcoholism and smoking. Agents Actions 1975, 5, 164–173. [Google Scholar] [CrossRef]
- Quinton, J.B.; Kemm RMHowes, L.G. A post marketing evaluation of Rapid Recovery in relieving symptoms of hangover. Clin. Res. Trials 2018, 4, 1–3. [Google Scholar]
- Mackus, M.; Van De Loo, A.J.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. J. Clin. Med. 2020, 9, 3421. [Google Scholar] [CrossRef]
- Millasseau, S.C.; Guigui, F.G.; Kelly, R.P.; Prasad, K.; Cockcroft, J.R.; Ritter, J.M.; Chowienczyk, P.J. Noninvasive assessment of the digital volume pulse: Comparison with the peripheral pressure pulse. Hypertension 2000, 36, 952–956. [Google Scholar] [CrossRef]
- Brillante, D.G.; O’Sullivan, A.J.; Howes, L.G. Arterial stiffness indices in healthy volunteers using non-invasive digital photoplethysmography. Blood Press. 2008, 17, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Ledesma, R.D.; Macbeth, G.; Cortada de Kohan, N.U. Computing effect size measures with ViSta-the visual statistics system. Tut. Quantit. Meth. Psychol. 2009, 5, 25–34. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using effect size—Or why the P value is not enough. J. Grad. Med. Ed. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Betuil, O.; Fuet, G.M.; Zeliha, S.; Engrin, S.E. The investigation of antioxidant and anti-inflammatory potentials of apitherapeutic agents on heart tissues in nitric oxide synthase inhibited rats via N-omega-nitro-L-arginine methyl ester. Clin. Exp. Hypertens. 2021, 43, 69–76. [Google Scholar]
- Correa, M.L.; Hayes, W.G. Arterial compliance and endothelial function. Curr. Diabetes Rep. 2007, 7, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, J.; Yuan, Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; McEniery, C.M.; Dhakam, Z.; Brown, M.J.; Cockcroft, J.R.; Wilkinson, I.B. Comparison of the Effects of Antihypertensive Agents on Central Blood Pressure and Arterial Stiffness in Isolated Systolic Hypertension. Hypertension 2009, 54, 409–413. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular stiffening and arterial compliance: Implications for systolic blood pressure. Am. J. Hypertens. 2004, 17, S39–S48. [Google Scholar] [CrossRef]
- Nichols, W.W.; Harripersaud, K.; Petersen, J.W. Nitrates and Arterial Function. Curr. Cardiovasc. Risk Rep. 2013, 7, 224–232. [Google Scholar] [CrossRef]
- D’elia, L.; La Fata, E.; Iannuzzi, A.; Rubba, P.O. Effect of statin therapy on pulse wave velocity: A meta-analysis of randomized controlled trials. Clin. Exp. Hypertens. 2018, 40, 601–608. [Google Scholar] [CrossRef]
- Kalenga, C.Z.; Hay, J.L.; Boreskie, K.F.; Duhamel, T.A.; MacRae, J.M.; Metcalfe, A.; Nerenberg, K.A.; Robert, M.; Ahmed, S.B. The association between route of post-menopausal estrogen administration and blood pressure and arterial stiffness in community-dwelling women. Front. Cardiovasc. Med. 2022, 9, 913609. [Google Scholar] [CrossRef]
- Man, B.; Cui, C.; Zhang, X.; Sugiyama, D.; Barinas-Mitchell, E.; Sekikawa, A. The effect of soy isoflavones on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 603–614. [Google Scholar] [CrossRef]
- Peterson, K.S.; Blanch, N.; Keoghan, J.B.; Clifton, P.M. Effect of weight loss on pulse wave velocity. Arterioscler. Throm. Vasc. Biol. 2015, 35, 243–252. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]

| Antioxidant n = 40 | Placebo n = 38 | p | |
|---|---|---|---|
| Gender: | |||
| Male | 40% | 34% | - |
| Female | 60% | 66% | |
| Age (years) | 62 ± 13 | 63 ± 10 | 0.83 |
| SBP (mmHg) | 138 ± 21 | 136 ± 14 | 0.62 |
| Change in SBP (mmHg) | −1 ± 17 | 2 ± 10 | 0.27 |
| DBP (mmHg) | 81 ± 11 | 83 ± 10 | 0.45 |
| Change in DBP (mmHg) | −2 ± 9 | 1 ± 9 | 0.20 |
| Heart rate (bpm) | 74 ± 11 | 73 ± 11 | 0.79 |
| Change in heart rate (bpm) | −1 ± 6 | −1 ± 9 | 0.88 |
| BMI (kg/m2) | 27 ± 4 | 30 ± 6 | 0.02 |
| SI (m/s) | 8.97 ± 3.20 | 8.78 ± 3.73 | 0.81 |
| Change in SI (m/s) | −1.95 ± 3.20 | −0.30 ± 1.14 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howes, L.G.; Unni, T.; Hamza, A.; Howes, J.B.; Jayasinghe, R. Reduction in Arterial Stiffness Index (SI) in Response to Combination Antioxidant Therapy. J. Clin. Med. 2023, 12, 6804. https://doi.org/10.3390/jcm12216804
Howes LG, Unni T, Hamza A, Howes JB, Jayasinghe R. Reduction in Arterial Stiffness Index (SI) in Response to Combination Antioxidant Therapy. Journal of Clinical Medicine. 2023; 12(21):6804. https://doi.org/10.3390/jcm12216804
Chicago/Turabian StyleHowes, Laurence Guy, Tanya Unni, Ameer Hamza, Jan B. Howes, and Rohan Jayasinghe. 2023. "Reduction in Arterial Stiffness Index (SI) in Response to Combination Antioxidant Therapy" Journal of Clinical Medicine 12, no. 21: 6804. https://doi.org/10.3390/jcm12216804
APA StyleHowes, L. G., Unni, T., Hamza, A., Howes, J. B., & Jayasinghe, R. (2023). Reduction in Arterial Stiffness Index (SI) in Response to Combination Antioxidant Therapy. Journal of Clinical Medicine, 12(21), 6804. https://doi.org/10.3390/jcm12216804





