Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Age >18 years
- (2)
- Velopharyngeal or lateral pharyngeal wall surgical treatment of OSAS patients,
- (3)
- Predictors of success related to phenotypes: anatomical, low arousal threshold, ventilatory instability, and poor muscle response
- (4)
- Pre-operative evaluations: DISE, body mass index (BMI), apnea-hypopnea index (AHI) with all levels of PSG, lateral wall space, positional OSA, Friedman classification, cephalometry, nasopharyngolaryngoscopy and nasopharyngeal tube
3. Results
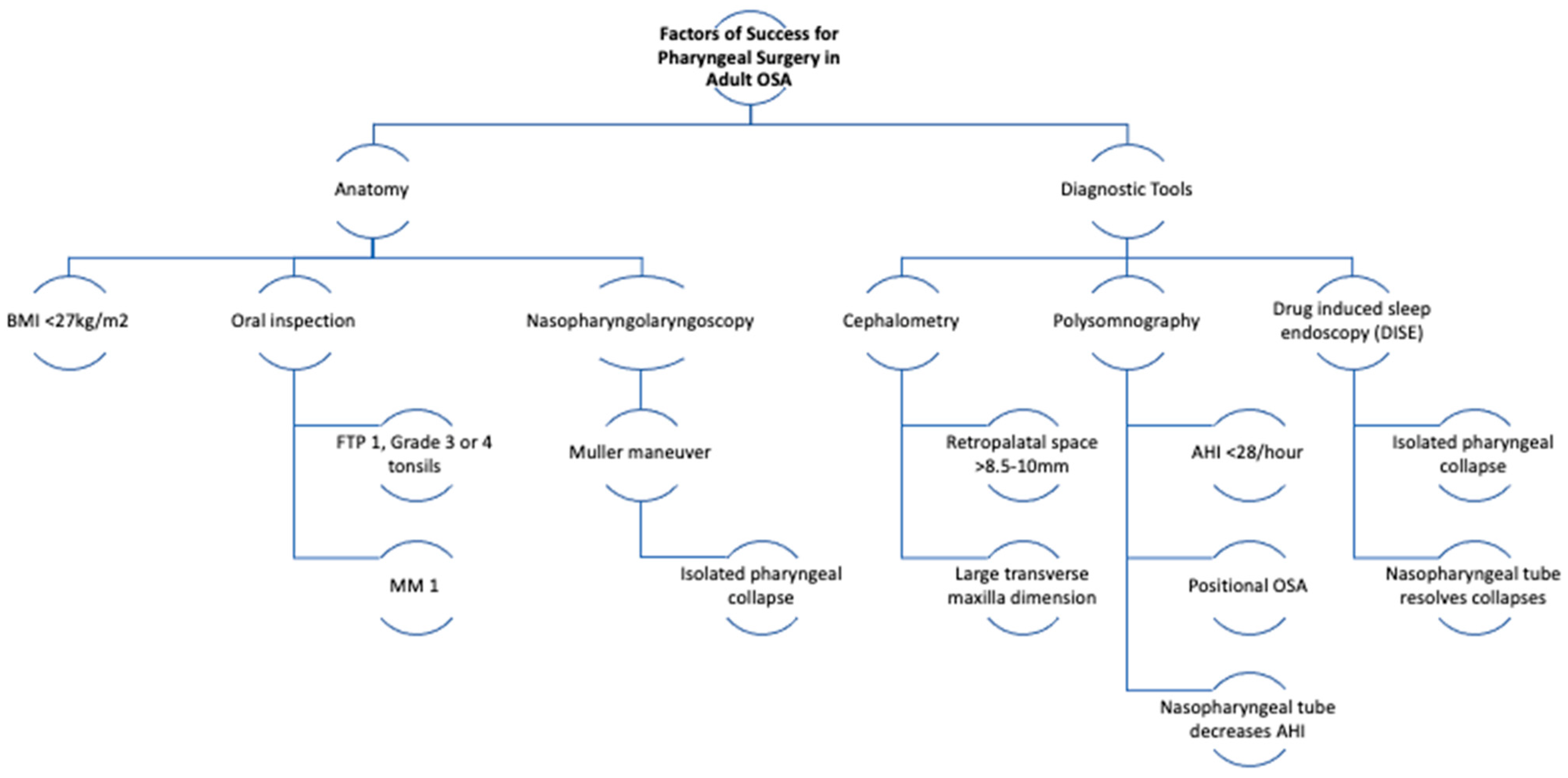
4. Discussion
4.1. BMI
4.2. Friedman
4.3. Cephalometry
4.4. AHI
4.5. Level and Type of Obstruction at Muller Maneuver
4.6. Positional Osa
4.7. Drug-Induced Sleep Endoscopy (DISE)
4.8. Nasopharyngeal Tube
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iannella, G.; Magliulo, G.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Pelucchi, S.; Ciorba, A.; Maniaci, A.; Cocuzza, S.; Gulotta, G.; et al. Effectiveness of drug-induced sleep endoscopy in improving outcomes of barbed pharyngoplasty for obstructive sleep apnea surgery: A prospective randomized trial. Sleep Breath. 2022, 26, 1621–1632. [Google Scholar] [CrossRef]
- Arnaud, C.; Bochaton, T.; Pépin, J.-L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Rundo, J.V. Obstructive sleep apnea basics. Cleve. Clin. J. Med. 2019, 86, 2–9. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Punjabi, N.M. The Epidemiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Batool-Anwar, S.; Goodwin, J.L.; Kushida, C.A.; Walsh, J.A.; Simon, R.D.; Nichols, D.A.; Quan, S.F. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). J. Sleep Res. 2016, 25, 731–738. [Google Scholar] [CrossRef]
- Borriboon, C.; Chaiard, J.; Tachaudomdach, C.; Turale, S. Continuous positive airway pressure adherence in people with obstructive sleep apnoea. J. Clin. Nurs. 2022, 31, 3477–3484. [Google Scholar] [CrossRef]
- Levrini, L.; Sacchi, F.; Milano, F.; Polimeni, A.; Cozza, P.; Bernkopf, E.; Segù, M.; Italian dentist work group about OSAS Collaborators; Zucconi, M.; Vicini, C.; et al. Italian recommendations on dental support in the treatment of adult obstructive sleep apnea syndrome (OSAS). Ann. Stomatol. 2015, 6, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Conway, W.; Zorick, F.; Roth, T. Surgical Correction of Anatomic Abnormalities in Obstructive Sleep Apnea Syndrome: Uvulopalatopharyngoplasty. Otolaryngol. Neck Surg. 1981, 89, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Carenfelt, C.; Haraldsson, P.O. Frequency of complications after uvulopalatopharyngoplasty. Lancet 1993, 341, 437. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, S.; Ye, J.; Wang, Y.; Bai, X.; Peng, K.A. Predicting outcome of velopharyngeal surgery in drug-induced sleep endoscopy by traction velum. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.A. Lessons from 50 Years of Uvulopalatopharyngoplasty. J. Sleep Disord. Ther. 2016, 5, 3–5. [Google Scholar] [CrossRef]
- Braga, A.; Grechi, T.H.; Eckeli, A.; Vieira, B.B.; Itikawa, C.E.; Küpper, D.S.; Matsumoto, M.A.N.; Trawitzki, L.V.V.; Felício, C.M.; Fernandes, R.M.F.; et al. Predictors of uvulopalatopharyngoplasty success in the treatment of obstructive sleep apnea syndrome. Sleep Med. 2013, 14, 1266–1271. [Google Scholar] [CrossRef]
- Missale, F.; Fragale, M.; Incandela, F.; Roustan, V.; Arceri, C.; Barbieri, A.; Canevari, F.R.; Peretti, G.; Barbieri, M. Outcome predictors for non-resective pharyngoplasty alone or as a part of multilevel surgery, in obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2020, 24, 1397–1406. [Google Scholar] [CrossRef]
- Xiong, Y.P.; Yi, H.L.; Yin, S.K.; Meng, L.L.; Tang, X.L.; Guan, J.; Luo, H.P.; Zhang, W.T.; Chen, B. Predictors of surgical outcomes of uvulopalatopharyngoplasty for obstructive sleep apnea hypopnea syndrome. Otolaryngol. Head Neck Surg. 2011, 145, 1049–1054. [Google Scholar] [CrossRef]
- Liu, S.R.; Yi, H.L.; Yin, S.K.; Guan, J.; Chen, B.; Meng, L.L.; Su, K.M. Associated predictors of therapeutic response to uvulopharyngopalatoplasty for severe obstructive sleep apnea hypopnea syndrome. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 1411–1417. [Google Scholar] [CrossRef]
- Millman, R.P.; Carlisle, C.C.; Rosenberg, C.; Kahn, D.; McRae, R.; Kramer, N.R. Simple Predictors of Uvulopalatopharyngoplasty Outcome in the Treatment of Obstructive Sleep Apnea. Chest 2000, 118, 1025–1030. [Google Scholar] [CrossRef][Green Version]
- Yin, G.; He, M.; Cao, X.; Xu, J.; Zhang, Y.; Kang, D.; Ye, J. Five-Year Objective and Subjective Outcomes of Velopharyngeal Surgery for Patients with Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2020, 162, 148–154. [Google Scholar] [CrossRef]
- Li, H.Y.; Chen, N.H.; Lee, L.A.; Shu, Y.H.; Fang, T.J.; Wang, P.C. Use of morphological indicators to predict outcomes of palatopharyngeal surgery in patients with obstructive sleep apnea. Orl 2004, 66, 119–123. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.; Xian, J.; Qu, Y.; Cao, X.; Ye, J. The Combination of Anatomy and Genioglossus Activity in Predicting the Outcomes of Velopharyngeal Surgery. Otolaryngol. Head Neck Surg. 2017, 156, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, Y.; Qu, Y.; Zhang, J.; Cao, X.; Ye, J. The Role of Genioglossus Activity in Predicting Uvulopalatopharyngoplasty Outcomes. Otolaryngol. Head Neck Surg. 2020, 162, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Shie, D.-Y.; Tsou, Y.-A.; Tai, C.-J.; Tsai, M.-H. Impact of obesity on uvulopalatopharyngoplasty success in patients with severe obstructive sleep apnea: A retrospective single-center study in Taiwan. Acta Otolaryngol. 2013, 133, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Doghramji, K.; Jabourian, Z.H.; Pilla, M.; Farole, A.; Lindholm, R.N. Predictors of outcome for uvulopalatopharyngoplasty. Laryngoscope 1995, 105, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Ibrahim, H.; Bass, L. Clinical staging for sleep-disordered breathing. Otolaryngol. Head Neck Surg. 2002, 127, 13–21. [Google Scholar] [CrossRef]
- Friedman, M.; Vidyasagar, R.; Bliznikas, D.; Joseph, N. Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? Laryngoscope 2005, 115, 2109–2113. [Google Scholar] [CrossRef]
- Hegstrom, T.; Emmons, L.L.; Hoddes, E.; Kennedy, T.; Christopher, K.; Collins, T.; Spofford, B. Obstructive sleep apnea syndrome: Preoperative radiologic evaluation. Am. J. Roentgenol. 1988, 150, 67–69. [Google Scholar] [CrossRef]
- Lee, C.H.; Mo, J.-H.; Seo, B.S.; Kim, D.-Y.; Yoon, I.-Y.; Kim, J.-W. Mouth opening during sleep may be a critical predictor of surgical outcome after uvulopalatopharyngoplasty for obstructive sleep apnea. J. Clin. Sleep Med. 2010, 6, 157–162. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Xian, J.; Wang, J.; Dong, J. Upper airway anatomical changes after velopharyngeal surgery in obstructive sleep apnea patients with small tonsils. Otolaryngol. Head Neck Surg. 2013, 149, 335–341. [Google Scholar] [CrossRef]
- Rollheim, J.; Miljeteig, H.; Osnes, T. Body mass index less than 28 kg/m 2 is a predictor of subjective improvement after laser-assisted uvulopalatoplasty for snoring. Laryngoscope 1999, 109, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Matarredona-Quiles, S.; Carrasco-Llatas, M.; Apodaca, P.M.-R.D.; Ortega-Beltrá, N.; Dalmau-Galofre, J. Is there a relationship between tonsil volume and the success of pharyngeal surgery among adult patients with obstructive sleep apnea? Braz. J. Otorhinolaryngol. 2022, 88, S156–S161. [Google Scholar] [CrossRef] [PubMed]
- Elsobki, A.; Moussa, H.H.; Eldeeb, M.E.; Fayed, A.; Elzayat, S. Efficacy, predictors of success and failure of an updated lateral pharyngoplasty approach as an independent procedure in treating obstructive sleep apnea with CPAP failures. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Beigh, Z.; Khursheed, R.S.; Jallu, A.S.; Pampoori, R.A. Clinical Predictors for Successful Uvulopalatopharyngoplasty in the Management of Obstructive Sleep Apnea. Int. J. Otolaryngol. 2013, 2013, 290265. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Yin, G.; Cao, X.; Ye, J. The potential effects of obesity on predicting outcomes of velopharyngeal surgery for obstructive sleep apnea. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1951–1956. [Google Scholar] [CrossRef]
- Friedman, M.; Salapatas, A.M.; Bonzelaar, L.B. Updated Friedman Staging System for Obstructive Sleep Apnea. In Sleep-Related Breathing Disorders; Karger Publishers: Basel, Switzerland, 2017; pp. 41–48. [Google Scholar]
- Choi, J.H.; Cho, S.H.; Kim, S.-N.; Suh, J.D.; Cho, J.H. Predicting Outcomes after Uvulopalatopharyngoplasty for Adult Obstructive Sleep Apnea: A Meta-analysis. Otolaryngol. Head. Neck Surg. 2016, 155, 904–913. [Google Scholar] [CrossRef]
- Thuler, E.; Rabelo, F.A.W.; Yui, M.; Tominaga, Q.; dos Santos, V.; Arap, S.S. Correlation between the transverse dimension of the maxilla, upper airway obstructive site, and OSA severity. J. Clin. Sleep Med. 2021, 17, 1465–1473. [Google Scholar] [CrossRef]
- Kezirian, E.J.; Hohenhorst, W.; de Vries, N. Drug-induced sleep endoscopy: The VOTE classification. Eur. Arch. Otorhinolaryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef]
- Li, H.-Y.; Cheng, W.-N.; Chuang, L.-P.; Fang, T.-J.; Hsin, L.-J.; Kang, C.-J.; Lee, L.-A. Positional dependency and surgical success of relocation pharyngoplasty among patients with severe obstructive sleep apnea. Otolaryngol. Head. Neck Surg. 2013, 149, 506–512. [Google Scholar] [CrossRef]
- Croft, C.B.; Pringle, M. Sleep nasendoscopy: A technique of assessment in snoring and obstructive sleep apnoea. Clin. Otolaryngol. 1991, 16, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Vroegop, A.V.; Vanderveken, O.M.; Verbraecken, J.A. Drug-Induced Sleep Endoscopy: Evaluation of a Selection Tool for Treatment Modalities for Obstructive Sleep Apnea. Respiration 2020, 99, 451–457. [Google Scholar] [CrossRef]
- Certal, V.F.; Pratas, R.; Guimarães, L.; Lugo, R.; Tsou, Y.; Camacho, M.; Capasso, R. Awake examination versus DISE for surgical decision making in patients with OSA: A systematic review. Laryngoscope 2016, 126, 768–774. [Google Scholar] [CrossRef]
- Huntley, C.; Chou, D.; Doghramji, K.; Boon, M. Preoperative Drug Induced Sleep Endoscopy Improves the Surgical Approach to Treatment of Obstructive Sleep Apnea. Ann. Otol. Rhinol. Laryngol. 2017, 126, 478–482. [Google Scholar] [CrossRef]
- Zhang, P.; Ye, J.; Pan, C.; Sun, N.; Kang, D. The Role of Obstruction Length and Height in Predicting Outcome of Velopharyngeal Surgery. Otolaryngol.—Head Neck Surg. 2015, 153, 144–149. [Google Scholar] [CrossRef]
- Green, K.K.; Kent, D.T.; D’agostino, M.A.; Hoff, P.T.; Lin, H.-S.; Soose, R.J.; Gillespie, M.B.; Yaremchuk, K.L.; Carrasco-Llatas, M.; Woodson, B.T.; et al. Drug-induced sleep endoscopy and surgical outcomes: A multicenter cohort study HHS Public Access. Laryngoscope 2019, 129, 761–770. [Google Scholar] [CrossRef]
- Koutsourelakis, I.; Safiruddin, F.; Ravesloot, M.; Zakynthinos, S.; de Vries, N. Surgery for obstructive sleep apnea: Sleep endoscopy determinants of outcome. Laryngoscope 2012, 122, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.P.; Baptista, P.M.; Olszewska, E.; Braverman, I.; Carrasco-Llatas, M.; Kishore, S.; Chandra, S.; Yang, H.C.; Wang, C.M.Z.; Chan, Y.H.; et al. Does drug-induced sleep endoscopy affect surgical outcome? A multicenter study of 326 obstructive sleep apnea patients. Laryngoscope 2020, 130, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.; Sinawe, H.; Folbe, A.J.; Yoo, G.; Badr, S.; Rowley, J.A.; Lin, H.-S. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope 2012, 122, 473–479. [Google Scholar] [CrossRef]
- Lin, H.-S.; Rowley, J.A.; Folbe, A.J.; Yoo, G.H.; Badr, M.S.; Chen, W. Transoral robotic surgery for treatment of obstructive sleep apnea: Factors predicting surgical response. Laryngoscope 2015, 125, 1013–1020. [Google Scholar] [CrossRef]
- Carrasco-Llatas, M.; Marcano-Acuña, M.; Zerpa-Zerpa, V.; Dalmau-Galofre, J. Surgical results of different palate techniques to treat oropharyngeal collapse. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Hessel, N.S.; de Vries, N. Results of uvulopalatopharyngoplasty after diagnostic workup with polysomnography and sleep endoscopy: A report of 136 snoring patients. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Qin, J.; Xing, D.; Li, S.; Wu, D. Diagnosis of Retrolingual Obstruction during Drug-Induced Sleep Endoscopy versus Polysomnography with Nasopharyngeal Tube in Patients with Obstructive Sleep Apnea. Ann. Otol. Rhinol. Laryngol. 2021, 130, 1285–1291. [Google Scholar] [CrossRef]
- Victores, A.J.; Olson, K.; Takashima, M. Interventional Drug-Induced Sleep Endoscopy: A Novel Technique to Guide Surgical Planning for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, D.; Bao, J.; Qin, J. Nasopharyngeal Tube: A Simple and Effective Tool to Screen Patients Indicated for Glossopharyngeal Surgery. J. Clin. Sleep Med. 2014, 10, 385–389. [Google Scholar] [CrossRef][Green Version]
- Dellweg, A.; Kampmann, M.; Tschopp, K. Evaluation of a nasopharyngeal stent in patients with obstructive sleep-related breathing disorders. J. Int. Med. Res. 2022, 50, 733. [Google Scholar] [CrossRef]

| Title | Year of Publication | Predictor for Success | Number of Patients | Success Rate |
|---|---|---|---|---|
| Predictors of uvulopalatopharyngoplasty success in the treatment of obstructive sleep apnea syndrome [16] | 2013 | BMI, AHI, Cephalometry | 54 | - |
| Outcome predictors for non-resective pharyngoplasty alone or as a part of multilevel surgery, in obstructive sleep apnea-hypopnea syndrome [17]. | 2020 | AHI, Cephalometry | 70 | - |
| Predictors of surgical outcomes of uvulopalatopharyngoplasty for obstructive sleep apnea hypopnea syndrome [18]. | 2011 | AHI | 39 | 56.4% |
| Associated predictors of therapeutic response to uvulopharyngopalatoplasty for severe obstructive sleep apnea hypopnea syndrome [19]. | 2013 | AHI, Cephalometry | 51 | 45.1% |
| Simple predictors of uvulopalatopharyngoplasty outcome in the treatment of obstructive sleep apnea [20]. | 2000 | BMI, AHI, Cephalometry | 46 | - |
| Five-Year Objective and Subjective Outcomes of Velopharyngeal Surgery for Patients with Obstructive Sleep Apnea [21]. | 2019 | Tonsil Size and ODI | 63 | 66.6% |
| Use of morphological indicators to predict outcomes of palatopharyngeal surgery in patients with obstructive sleep apnea [22]. | 2004 | BMI, Tonsil Size, Mallampati, AHI | 105 | - |
| The Combination of Anatomy and Genioglossus Activity in Predicting the Outcomes of Velopharyngeal Surgery [23]. | 2017 | Cephalometry, Genioglossus Electromyography | 40 | 62.5% |
| The Role of Genioglossus Activity in Predicting Uvulopalatopharyngoplasty Outcomes [24]. | 2019 | Tonsil Size, Genioglossus Electromyography | 44 | 92.9% |
| Impact of obesity on uvulopalatopharyngoplasty success in patients with severe obstructive sleep apnea: a retrospective single-center study in Taiwan [25]. | 2012 | BMI, AHI, Friedman, Epworth | 117 | 24.6% |
| Predictors of outcome for uvulopalatopharyngoplasty [26] | 1995 | Fiberoptic nasopharyngolaryngoscopy with the Müller maneuver (FNMM), Cephalometry | 53 | 32.1% |
| Clinical staging for sleep-disordered breathing [27]. | 2016 | BMI, Tonsil Size, Palate Position at Cephalometry | 134 | 80.6% |
| Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? [28] | 2009 | AHI, Friedman | 134 | 31.3% |
| Obstructive sleep apnea syndrome: preoperative radiologic evaluation. [29] | 1988 | Palate Position with Cephalometry | 12 | - |
| Mouth opening during sleep may be a critical predictor of surgical outcome after uvulopalatopharyngoplasty for obstructive sleep apnea [30]. | 2010 | Oral Cavity Anatomy | 69 | - |
| Upper airway anatomical changes after velopharyngeal surgery in obstructive sleep apnea patients with small tonsils [31]. | 2009 | Tonsil Size, Cephalometry | 44 | 72.4% |
| Body mass index less than 28 kg/m2 is a predictor of subjective improvement after laser-assisted uvulopalatoplasty for snoring [32] | 2009 | BMI | 119 | - |
| Is there a relationship between tonsil volume and the success of pharyngeal surgery among adult patients with obstructive sleep apnea? [33] | 2022 | Tonsil Size | 44 | 65.6% |
| Efficacy, predictors of success and failure of an updated lateral pharyngoplasty approach as an independent procedure in treating obstructive sleep apnea with CPAP failures [34] | 2021 | Type of collapse in DISE | 46 | 69.9% |
| Clinical predictors for successful uvulopalatopharyngoplasty in the management of obstructive sleep apnea [35] | 2013 | BMI, Friedman, Muller Maneuver, Neck Circumference | 50 | 95.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, H.d.S.S.; Vaz de Castro, J.; Favier, V.; Carsuzaa, F.; Kim, M.H.R.; Mira, F.A.; Meccariello, G.; Vicini, C.; De Vito, A.; Lechien, J.R.; et al. Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review. J. Clin. Med. 2023, 12, 6773. https://doi.org/10.3390/jcm12216773
Nunes HdSS, Vaz de Castro J, Favier V, Carsuzaa F, Kim MHR, Mira FA, Meccariello G, Vicini C, De Vito A, Lechien JR, et al. Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review. Journal of Clinical Medicine. 2023; 12(21):6773. https://doi.org/10.3390/jcm12216773
Chicago/Turabian StyleNunes, Heloisa dos Santos Sobreira, Joana Vaz de Castro, Valentin Favier, Florent Carsuzaa, Marina He Ryi Kim, Felipe Ahumada Mira, Giuseppe Meccariello, Claudio Vicini, Andrea De Vito, Jerome R. Lechien, and et al. 2023. "Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review" Journal of Clinical Medicine 12, no. 21: 6773. https://doi.org/10.3390/jcm12216773
APA StyleNunes, H. d. S. S., Vaz de Castro, J., Favier, V., Carsuzaa, F., Kim, M. H. R., Mira, F. A., Meccariello, G., Vicini, C., De Vito, A., Lechien, J. R., Chiesa Estomba, C., Maniaci, A., Iannella, G., & Cammaroto, G. (2023). Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review. Journal of Clinical Medicine, 12(21), 6773. https://doi.org/10.3390/jcm12216773










