Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Endpoints
2.3. Operative Technique
2.4. Data Analysis
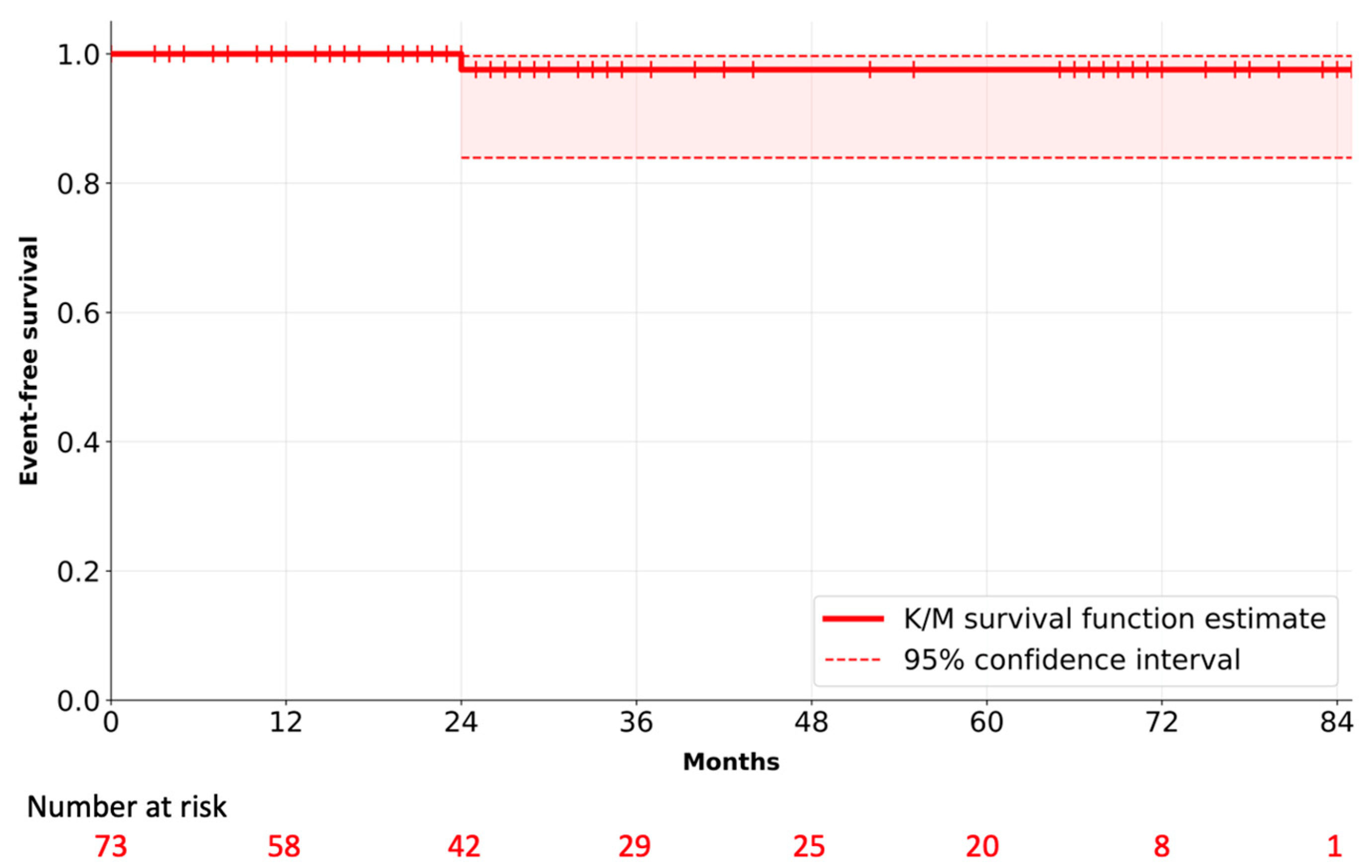
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2019: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2020, 68, 263–276. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, A.S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K.; et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef]
- Roberts, W.C.; Ko, J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef]
- Michelena, H.I.; Desjardins, V.A.; Avierinos, J.F.; Russo, A.; Nkomo, V.T.; Sundt, T.M.; Pellikka, P.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008, 117, 2776–2784. [Google Scholar] [CrossRef]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902, Erratum in Am. J. Cardiol. 1999, 84, 1143. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Briët, E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef]
- Chan, W.S.; Anand, S.; Ginsberg, J.S. Anticoagulation of pregnant women with mechanical heart valves: A systematic review of the literature. Arch. Intern. Med. 2000, 160, 191–196. [Google Scholar] [CrossRef]
- McClure, R.S.; Narayanasamy, N.; Wiegerinck, E.; Lipsitz, S.; Maloney, A.; Byrne, J.G.; Aranki, S.F.; Couper, G.S.; Cohn, L.H. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: Up to 17-year follow-up in 1000 patients. Ann. Thorac. Surg. 2010, 89, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Alperi, A.; Hernandez-Vaquero, D.; Pascual, I.; Diaz, R.; Silva, I.; Alvarez-Cabo, R.; Avanzas, P.; Moris, C. Aortic valve replacement in young patients: Should the biological prosthesis be recommended over the mechanical? Ann. Transl. Med. 2018, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Cebotari, S.; Tudorache, I.; Ciubotaru, A.; Boethig, D.; Sarikouch, S.; Goerler, A.; Lichtenberg, A.; Cheptanaru, E.; Barnaciuc, S.; Cazacu, A.; et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: Early report. Circulation 2011, 124, S115–S123. [Google Scholar] [CrossRef] [PubMed]
- Cvitkovic, T.; Bobylev, D.; Horke, A.; Avsar, M.; Beerbaum, P.; Martens, A.; Böthig, D.; Petenà, E.; Gutberlet, M.; Beyer, F.H.; et al. 4D-flow cardiac magnetic resonance imaging after aortic root replacement with long-valved decellularized aortic homografts: Comparison to valve-sparing aortic root replacement and healthy controls. Eur. J. Cardiothorac. Surg. 2022, 61, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Sarikouch, S.; Horke, A.; Tudorache, I.; Beerbaum, P.; Westhoff-Bleck, M.; Boethig, D.; Repin, O.; Maniuc, L.; Ciubotaru, A.; Haverich, A.; et al. Decellularized fresh homografts for pulmonary valve replacement: A decade of clinical experience. Eur. J. Cardiothorac. Surg. 2016, 50, 281–290. [Google Scholar] [CrossRef]
- Bobylev, D.; Sarikouch, S.; Tudorache, I.; Cvitkovic, T.; Söylen, B.; Boethig, D.; Theodoridis, K.; Bertram, H.; Beerbaum, P.; Haverich, A.; et al. Double semilunar valve replacement in complex congenital heart disease using decellularized homografts. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Böer, U.; Schridde, A.; Anssar, M.; Klingenberg, M.; Sarikouch, S.; Dellmann, A.; Harringer, W.; Haverich, A.; Wilhelmi, M. The immune response to crosslinked tissue is reduced in decellularized xenogeneic and absent in decellularized allogeneic heart valves. Int. J. Artif. Organs 2015, 38, 199–209. [Google Scholar] [CrossRef]
- Sarikouch, S.; Theodoridis, K.; Hilfiker, A.; Boethig, D.; Laufer, G.; Andreas, M.; Cebotari, S.; Tudorache, I.; Bobylev, D.; Neubert, L.; et al. Early Insight Into In Vivo Recellularization of Cell-Free Allogenic Heart Valves. Ann. Thorac. Surg. 2019, 108, 581–589. [Google Scholar] [CrossRef]
- Coti, I.; Wenda, S.; Andreeva, A.; Kocher, A.; Laufer, G.; Fischer, G.; Andreas, M. Donor-specific HLA antibodies after fresh decellularized vs cryopreserved native allograft implantation. HLA 2020, 96, 580–588. [Google Scholar] [CrossRef]
- Horke, A.; Tudorache, I.; Laufer, G.; Andreas, M.; Pomar, J.L.; Pereda, D.; Quintana, E.; Sitges, M.; Meyns, B.; Rega, F.; et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: The ARISE study and ARISE Registry data. Eur. J. Cardiothorac. Surg. 2020, 58, 1045–1053. [Google Scholar] [CrossRef]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L.; Takkenberg, J.J.; David, T.E.; Butchart, E.G.; Adams, D.H.; et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann. Thorac. Surg. 2008, 85, 1490–1495. [Google Scholar] [CrossRef]
- Urganci, E.; Aschacher, T.; Herbst, C.; Andreas, M.; Schlein, J.; Sandner, S.; Laufer, G.; Zimpfer, D. Implantation of a decellularized aortic homograft in a child. Multimed. Man. Cardiothorac. Surg. 2020, 2020. [Google Scholar] [CrossRef]
- Tudorache, I.; Horke, A.; Cebotari, S.; Sarikouch, S.; Boethig, D.; Breymann, T.; Beerbaum, P.; Bertram, H.; Westhoff-Bleck, M.; Theodoridis, K.; et al. Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur. J. Cardiothorac. Surg. 2016, 50, 89–97. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. Choice of prosthetic heart valve for adult patients. J. Am. Coll. Cardiol. 2003, 41, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.; Chan, V.; Bédard, P.; Kulik, A.; Ressler, L.; Lam, B.K.; Rubens, F.D.; Goldstein, W.; Hendry, P.J.; Masters, R.G.; et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation 2007, 116, I294–I300. [Google Scholar] [CrossRef]
- Ruel, M.; Kulik, A.; Lam, B.K.; Rubens, F.D.; Hendry, P.J.; Masters, R.G.; Bédard, P.; Mesana, T.G. Long-term outcomes of valve replacement with modern prostheses in young adults. Eur. J. Cardiothorac. Surg. 2005, 27, 425–433, discussion 433. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71, Erratum in Circulation 2021, 143, e228; Erratum in Circulation 2021, 143, e784. [Google Scholar] [CrossRef] [PubMed]
- Hammermeister, K.E.; Henderson, W.G.; Burchfiel, C.M.; Sethi, G.K.; Souchek, J.; Oprian, C.; Cantor, A.B.; Folland, E.; Khuri, S.; Rahimtoola, S. Comparison of outcome after valve replacement with a bioprosthesis versus a mechanical prosthesis: Initial 5 year results of a randomized trial. J. Am. Coll. Cardiol. 1987, 10, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Schnittman, S.R.; Adams, D.H.; Itagaki, S.; Toyoda, N.; Egorova, N.N.; Chikwe, J. Bioprosthetic aortic valve replacement: Revisiting prosthesis choice in patients younger than 50 years old. J. Thorac. Cardiovasc. Surg. 2018, 155, 539–547.e9. [Google Scholar] [CrossRef] [PubMed]
- Etnel, J.R.; Huygens, S.A.; Grashuis, P.; Pekbay, B.; Papageorgiou, G.; Roos Hesselink, J.W.; Bogers, A.J.; Takkenberg, J.J. Bioprosthetic Aortic Valve Replacement in Nonelderly Adults: A Systematic Review, Meta-Analysis, Microsimulation. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005481. [Google Scholar] [CrossRef]
- Werner, P.; Coti, I.; Kaider, A.; Gritsch, J.; Mach, M.; Kocher, A.; Laufer, G.; Andreas, M. Long-term durability after surgical aortic valve replacement with the Trifecta and the Intuity valve—A comparative analysis. Eur. J. Cardiothorac. Surg. 2022, 61, 416–424. [Google Scholar] [CrossRef]
- Korteland, N.M.; Etnel, J.R.G.; Arabkhani, B.; Mokhles, M.M.; Mohamad, A.; Roos-Hesselink, J.W.; Bogers, A.J.J.C.; Takkenberg, J.J.M. Mechanical aortic valve replacement in non-elderly adults: Meta-analysis and microsimulation. Eur. Heart J. 2017, 38, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.; Seebacher, G.; Reida, E.; Wiedemann, D.; Pees, C.; Rosenhek, R.; Heinze, G.; Moritz, A.; Kocher, A.; Laufer, G. A single-center experience with the ross procedure over 20 years. Ann. Thorac. Surg. 2014, 97, 182–188. [Google Scholar] [CrossRef]
- Etnel, J.R.G.; Grashuis, P.; Huygens, S.A.; Pekbay, B.; Papageorgiou, G.; Helbing, W.A.; Roos-Hesselink, J.W.; Bogers, A.J.J.C.; Mokhles, M.M.; Takkenberg, J.J.M. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004748. [Google Scholar] [CrossRef]
- Oeser, C.; Uyanik-Uenal, K.; Kocher, A.; Laufer, G.; Andreas, M. The Ross procedure in adult patients: A single-centre analysis of long-term results up to 28 years. Eur. J. Cardiothorac. Surg. 2022, 62, ezac379. [Google Scholar] [CrossRef]
- da Costa, F.D.; Costa, A.C.; Prestes, R.; Domanski, A.C.; Balbi, E.M.; Ferreira, A.D.; Lopes, S.V. The early and midterm function of decellularized aortic valve allografts. Ann. Thorac. Surg. 2010, 90, 1854–1860. [Google Scholar] [CrossRef]
- Zehr, K.J.; Yagubyan, M.; Connolly, H.M.; Nelson, S.M.; Schaff, H.V. Aortic root replacement with a novel decellularized cryopreserved aortic homograft: Postoperative immunoreactivity and early results. J. Thorac. Cardiovasc. Surg. 2005, 130, 1010–1015. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef]
- Tzemos, N.; Therrien, J.; Yip, J.; Thanassoulis, G.; Tremblay, S.; Jamorski, M.T.; Webb, G.D.; Siu, S.C. Outcomes in adults with bicuspid aortic valves. JAMA 2008, 300, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Khanna, A.D.; Mahoney, D.; Margaryan, E.; Topilsky, Y.; Suri, R.M.; Eidem, B.; Edwards, W.D.; Sundt, T.M., 3rd; Enriquez-Sarano, M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011, 306, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Forde, I.; Edwards, W.D.; Maleszewski, J.J.; Suri, R.M.; Schaff, H.V.; Enriquez-Sarano, M.; Michelena, H.I. Type A aortic dissection in patients with bicuspid aortic valves: Clinical and pathological comparison with tricuspid aortic valves. Heart 2013, 99, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey DEJr Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; Kouchoukos, N.T.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369, Erratum in Circulation 2010, 122, e410. [Google Scholar]
- O’Brien, M.F.; Harrocks, S.; Stafford, E.G.; Gardner, M.A.; Pohlner, P.G.; Tesar, P.J.; Stephens, F. The homograft aortic valve: A 29-year, 99.3% follow up of 1,022 valve replacements. J. Heart Valve Dis. 2001, 10, 334–344, discussion 335. [Google Scholar]
- Bobylev, D.; Horke, A.; Boethig, D.; Hazekamp, M.; Meyns, B.; Rega, F.; Dave, H.; Schmiady, M.; Ciubotaru, A.; Cheptanaru, E.; et al. 5-Year results from the prospective European multi-centre study on decellularized homografts for pulmonary valve replacement ESPOIR Trial and ESPOIR Registry data. Eur. J. Cardiothorac. Surg. 2022, 62, ezac219. [Google Scholar] [CrossRef]
- Helder, M.R.; Kouchoukos, N.T.; Zehr, K.; Dearani, J.A.; Maleszewski, J.J.; Leduc, C.; Heins, C.N.; Schaff, H.V. Late durability of decellularized allografts for aortic valve replacement: A word of caution. J. Thorac. Cardiovasc. Surg. 2016, 152, 1197–1199. [Google Scholar] [CrossRef]
- Andreas, M.; Freystaetter, K.; Bernardi, M.H.; Zuckermann, A. Accelerated acute severe antibody-mediated graft failure related to a Ross procedure 17 years earlier. Eur. J. Cardiothorac. Surg. 2018, 54, 402–403. [Google Scholar] [CrossRef]
- Oripov, F.; Ramm, R.; Falk, C.; Goecke, T.; Ebken, J.; Jashari, R.; Böthig, D.; Horke, A.; Avsar, M.; Bobylev, D.; et al. Serial assessment of early antibody binding to decellularized valved allografts. Front. Cardiovasc. Med. 2022, 9, 895943. [Google Scholar] [CrossRef]
- Ebken, J.; Mester, N.; Smart, I.; Ramm, R.; Goecke, T.; Jashari, R.; Böthig, D.; Horke, A.; Cebotari, S.; Tudorache, I.; et al. Residual immune response towards decellularized homografts may be highly individual. Eur. J. Cardiothorac. Surg. 2021, 59, 773–782. [Google Scholar] [CrossRef]
- Bobylev, D.; Horke, A.; Avsar, M.; Cvitkovic, T.; Boethig, D.; Hazekamp, M.; Meyns, B.; Rega, F.; Dave, H.; Schmiady, M.; et al. Matched comparison of decellularized homografts and bovine jugular vein conduits for pulmonary valve replacement in congenital heart disease. Cell Tissue Bank 2023. [Google Scholar] [CrossRef] [PubMed]
- Huygens, S.A.; Rutten-van Mölken, M.P.M.H.; Noruzi, A.; Etnel, J.R.G.; Corro Ramos, I.; Bouten, C.V.C.; Kluin, J.; Takkenberg, J.J.M. What Is the Potential of Tissue-Engineered Pulmonary Valves in Children? Ann. Thorac. Surg. 2019, 107, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Huygens, S.A.; Ramos, I.C.; Bouten, C.V.C.; Kluin, J.; Chiu, S.T.; Grunkemeier, G.L.; Takkenberg, J.J.M.; Rutten-van Mölken, M.P.M.H. Early cost-utility analysis of tissue-engineered heart valves compared to bioprostheses in the aortic position in elderly patients. Eur. J. Health Econ. 2020, 21, 557–572. [Google Scholar] [CrossRef] [PubMed]
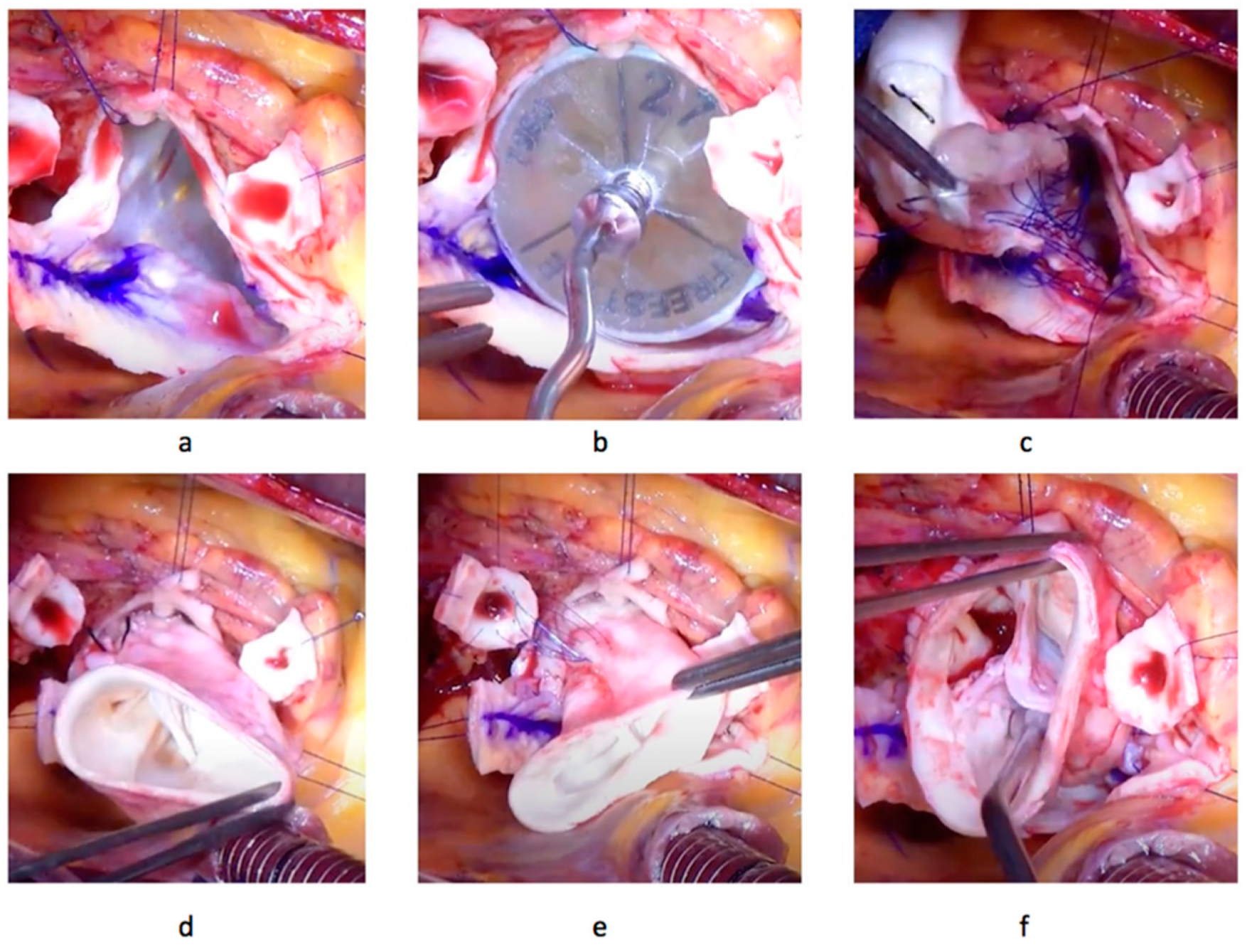
| Baseline Characteristics | DAH |
|---|---|
| Patients, n | 73 |
| Age at surgery, y, mean (SD) | 47.0 (11.3) |
| Sex, male, % | 68.4 |
| Weight, kg, mean (SD) | 81.1 (20.1) |
| Height, cm, mean (SD) | 173.6 (10.4) |
| Body mass index, kg/m2, mean (SD) | 26.8 (5.7) |
| EuroScore II, %, median (IQR) | 1.8 [0.8, 2.9] |
| Hypertension, n (%) | 24 (32.9) |
| Chronical lung disease, n (%) | 2 (2.7) |
| Renal insufficiency, n (%) | 1 (1.4) |
| Hemodialysis, n (%) | 1 (1.4) |
| Diabetes, n (%) | 3 (4.1) |
| Dyslipidemia, n (%) | 19 (26.0) |
| Coronary artery disease, n (%) | 5 (6.8) |
| Cerebrovascular disease, n (%) | 2 (2.7) |
| Preoperative creatinine, mg/dl, mean (SD) | 1.1 (0.8) |
| Indication for surgery: | |
| aortic stenosis, n (%) | 27 (36.9) |
| aortic regurgitation, n (%) | 24 (32.9) |
| mixed aortic disease, n (%) | 21 (28.8) |
| other, n (%) | 1 (1.4) |
| Bicuspid aortic valve, n (%) | 47 (64.4) |
| Previous aortic valve replacement, n (%) | 2 (2.7) |
| Previous aortic valve reconstruction, n (%) | 5 (8.2) |
| Previous Ross procedure, n (%) | 1 (1.4) |
| Previous balloon valvuloplasty, n (%) | 1 (1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, A.; Coti, I.; Werner, P.; Scherzer, S.; Kocher, A.; Laufer, G.; Andreas, M. Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience. J. Clin. Med. 2023, 12, 6713. https://doi.org/10.3390/jcm12216713
Andreeva A, Coti I, Werner P, Scherzer S, Kocher A, Laufer G, Andreas M. Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience. Journal of Clinical Medicine. 2023; 12(21):6713. https://doi.org/10.3390/jcm12216713
Chicago/Turabian StyleAndreeva, Alexandra, Iuliana Coti, Paul Werner, Sabine Scherzer, Alfred Kocher, Günther Laufer, and Martin Andreas. 2023. "Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience" Journal of Clinical Medicine 12, no. 21: 6713. https://doi.org/10.3390/jcm12216713
APA StyleAndreeva, A., Coti, I., Werner, P., Scherzer, S., Kocher, A., Laufer, G., & Andreas, M. (2023). Aortic Valve Replacement in Adult Patients with Decellularized Homografts: A Single-Center Experience. Journal of Clinical Medicine, 12(21), 6713. https://doi.org/10.3390/jcm12216713






