Stress-Mediated Abnormalities in Regional Myocardial Wall Motion in Young Women with a History of Psychological Trauma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Echocardiography
2.2. Speckle Tracking Analysis
2.3. Serum Markers and Calculated Biomarkers
2.4. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aktaa, S.; Gencer, B.; Arbelo, E.; Davos, C.H.; Désormais, I.; Hollander, M.; Abreu, A.; Ambrosetti, M.; Bäck, M.; Carballo, D.; et al. European Society of Cardiology Quality Indicators for Cardiovascular Disease Prevention: Developed by the Working Group for Cardiovascular Disease Prevention Quality Indicators in collaboration with the European Association for Preventive Cardiology of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A. Behavioral cardiology: Current advances and future directions. J. Am. Coll. Cardiol. 2014, 64, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Abed, M.A.; Kloub, M.I.; Moser, D.K. Anxiety and adverse health outcomes among cardiac patients: A biobehavioral model. J. Cardiovasc. Nurs. 2014, 29, 354–363. [Google Scholar] [CrossRef]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e763–e783. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Shaffer, J.A.; Falzon, L.; Krupka, D.; Davidson, K.W.; Edmondson, D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am. J. Cardiol. 2012, 110, 1711–1716. [Google Scholar] [CrossRef]
- Chida, Y.; Steptoe, A. The association of anger and hostility with future coronary heart disease: A meta-analytic review of prospective evidence. J. Am. Coll. Cardiol. 2009, 53, 936–946. [Google Scholar] [CrossRef]
- Emdin, C.A.; Odutayo, A.; Wong, C.X.; Tran, J.; Hsiao, A.J.; Hunn, B.H.M. Meta-Analysis of Anxiety as a Risk Factor for Cardiovascular Disease. Am. J. Cardiol. 2016, 118, 511–519. [Google Scholar] [CrossRef]
- Nicholson, A.; Kuper, H.; Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef]
- Pänkäläinen, M.; Kerola, T.; Kampman, O.; Kauppi, M.; Hintikka, J. Pessimism and risk of death from coronary heart disease among middle-aged and older Finns: An eleven-year follow-up study. BMC Public Health 2016, 16, 1124. [Google Scholar] [CrossRef]
- Engemann, L.; Aweimer, A.; Ewers, A.; Afshari, F.; Maiß, C.; Kern, K.; Lücke, T.; Mügge, A.; Brüne, M. Altered Left Ventricular Myocardial Deformation in Young Women With Borderline Personality Disorder: An Echocardiographic Study. Psychosom. Med. 2022, 84, 581–587. [Google Scholar] [CrossRef]
- Bohus, M.; Kröger, C. Psychopathologie und Psychotherapie der Borderline-Persönlichkeitsstörung: Zum gegenwärtigen Stand der Forschung. Nervenarzt 2011, 82, 16–24. [Google Scholar] [CrossRef]
- Videler, A.C.; Hutsebaut, J.; Schulkens, J.E.M.; Sobczak, S.; van Alphen, S.P.J. A Life Span Perspective on Borderline Personality Disorder. Curr Psychiatry Rep. 2019, 21, 51. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Powers, A.D.; Oltmanns, T.F. Borderline personality pathology and chronic health problems in later adulthood: The mediating role of obesity. Personal. Disord. 2013, 4, 152–159. [Google Scholar] [CrossRef]
- El-Gabalawy, R.; Katz, L.Y.; Sareen, J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom. Med. 2010, 72, 641–647. [Google Scholar] [CrossRef]
- Moran, P.; Stewart, R.; Brugha, T.; Bebbington, P.; Bhugra, D.; Jenkins, R.; Coid, J.W. Personality Disorder and Cardiovascular Disease: Results From a National Household Survey. J. Clin. Psychiatry 2007, 68, 69–74. [Google Scholar] [CrossRef]
- Barber, T.A.; Ringwald, W.R.; Wright, A.G.C.; Manuck, S.B. Borderline personality disorder traits associate with midlife cardiometabolic risk. Personal. Disord. 2020, 11, 151–156. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; De Boeck, B.W.; Melman, P.G.; Sieswerda, G.T.; Doevendans, P.A.; Cramer, M.J.M. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc. Ultrasound 2007, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; d’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Dalen, H.; Thorstensen, A.; Aase, S.A.; Ingul, C.B.; Torp, H.; Vatten, L.J.; Stoylen, A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: The HUNT study in Norway. Eur. J. Echocardiogr. 2010, 11, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Landgrafe-Mende, G.; Wiora, E.; Chatzitomaris, A.; Klein, H.H.; Midgley, J.E.; Hoermann, R. Calculated Parameters of Thyroid Homeostasis: Emerging Tools for Differential Diagnosis and Clinical Research. Front. Endocrinol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, R.; Midgley, J.E.M.; Larisch, R.; Dietrich, J.W. Lessons from Randomised Clinical Trials for Triiodothyronine Treatment of Hypothyroidism: Have They Achieved Their Objectives? J. Thyroid. Res. 2018, 2018, 3239197. [Google Scholar] [CrossRef] [PubMed]
- Snelder, S.M.; de Groot-de Laat, L.E.; Biter, L.U.; Castro Cabezas, M.; Pouw, N.; Birnie, E.; Boxma-de Klerk, B.M.; Klaassen, R.A.; Zijlstra, F.; van Dalen, B.M. Subclinical cardiac dysfunction in obesity patients is linked to autonomic dysfunction: Findings from the CARDIOBESE study. ESC Heart Fail. 2020, 7, 3726–3737. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B.; Akpınar, O.; Cerit, L.; Kemal, H.S.; Usalp, S.; Yüksek, Ü.; Açıkgöz, E.; Coşkun, U.; Duygu, H. Effects of chronic cigarette smoking on myocardial deformation parameters by two-dimensional speckle tracking echocardiography. Echocardiography 2019, 36, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Morariu, V.I.; Arnautu, D.A.; Morariu, S.I.; Popa, A.M.; Parvanescu, T.; Andor, M.; Abhinav, S.; David, V.L.; Ionescu, A.; Tomescu, M.C. 2D speckle tracking: A diagnostic and prognostic tool of paramount importance. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3903–3910. [Google Scholar] [CrossRef]
- Araujo-Gutierrez, R.; Chitturi, K.R.; Xu, J.; Wang, Y.; Kinder, E.; Senapati, A.; Chebrolu, L.B.; Kassi, M.; Trachtenberg, B.H. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardiooncology 2021, 7, 4. [Google Scholar] [CrossRef]
- Orde, S.R.; Pulido, J.N.; Masaki, M.; Gillespie, S.; Spoon, J.N.; Kane, G.C.; Oh, J.K. Outcome prediction in sepsis: Speckle tracking echocardiography based assessment of myocardial function. Crit. Care 2014, 18, R149. [Google Scholar] [CrossRef]
- Barssoum, K.; Altibi, A.M.; Rai, D.; Kumar, A.; Kharsa, A.; Chowdhury, M.; Thakkar, S.; Shahid, S.; Abdelazeem, M.; Abuzaid, A.S.; et al. Speckle tracking echocardiography can predict subclinical myocardial involvement in patients with sarcoidosis: A meta-analysis. Echocardiography 2020, 37, 2061–2070. [Google Scholar] [CrossRef]
- Rosman, L.; Dunsiger, S.; Salmoirago-Blotcher, E. Cumulative Impact of Stressful Life Events on the Development of Takotsubo Cardiomyopathy. Ann. Behav. Med. 2017, 51, 925–930. [Google Scholar] [CrossRef]
- Aweimer, A.; El-Battrawy, I.; Akin, I.; Borggrefe, M.; Mügge, A.; Patsalis, P.C.; Urban, A.; Kummer, M.; Vasileva, S.; Stachon, A.; et al. Abnormal thyroid function is common in takotsubo syndrome and depends on two distinct mechanisms: Results of a multicentre observational study. J. Intern. Med. 2021, 289, 675–687. [Google Scholar] [CrossRef]
- Templin, C.; Hänggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Lüscher, T.F.; Ghadri, J.R.; Jäncke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P.; Kim, D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003, 27, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazk, R.R.; El-Sehrawy, A.A.; Ghoniem, M.G.M.; Amer, M.Z. Speckle tracking echocardiographic assessment of left ventricular longitudinal strain in female patients with subclinical hyperthyroidism. Cardiovasc. Endocrinol. Metab. 2020, 10, 182–185. [Google Scholar] [CrossRef]
- Sinai, C.; Hirvikoski, T.; Nordström, A.L.; Nordström, P.; Nilsonne, Å.; Wilczek, A.; Åsberg, M.; Jokinen, J. Thyroid hormones and adult interpersonal violence among women with borderline personality disorder. Psychiatry Res. 2015, 227, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Karin, O.; Swisa, A.; Glaser, B.; Dor, Y.; Alon, U. Dynamical compensation in physiological circuits. Mol. Syst. Biol. 2016, 12, 886. [Google Scholar] [CrossRef]
- Korem Kohanim, Y.; Milo, T.; Raz, M.; Karin, O.; Bar, A.; Mayo, A.; Mendelson Cohen, N.; Toledano, Y.; Alon, U. Dynamics of thyroid diseases and thyroid-axis gland masses. Mol. Syst. Biol. 2022, 18, e10919. [Google Scholar] [CrossRef]
- Chaker, L.; van den Berg, M.E.; Niemeijer, M.N.; Franco, O.H.; Dehghan, A.; Hofman, A.; Rijnbeek, P.R.; Deckers, J.W.; Eijgelsheim, M.; Stricker, B.H.; et al. Thyroid Function and Sudden Cardiac Death: A Prospective Population-Based Cohort Study. Circulation 2016, 134, 713–722. [Google Scholar] [CrossRef]
- Müller, P.; Leow, M.K.; Dietrich, J.W. Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence. Front. Cardiovasc. Med. 2022, 9, 942971. [Google Scholar] [CrossRef]
- Dietrich, J.W.; Hoermann, R.; Midgley, J.E.M.; Bergen, F.; Müller, P. The Two Faces of Janus: Why Thyrotropin as a Cardiovascular Risk Factor May Be an Ambiguous Target. Front. Endocrinol. 2020, 11, 542710. [Google Scholar] [CrossRef] [PubMed]
- Candan, O.; Gecmen, C.; Bayam, E.; Guner, A.; Celik, M.; Doğan, C. Mechanical dispersion and global longitudinal strain by speckle tracking echocardiography: Predictors of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy. Echocardiography 2017, 34, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Chugh, S.S. The 12-lead electrocardiogram and risk of sudden death: Current utility and future prospects. Europace 2015, 17 (Suppl. S2), ii7–ii13. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef]
- Nagata, Y.; Kado, Y.; Onoue, T.; Otani, K.; Nakazono, A.; Otsuji, Y.; Takeuchi, M. Impact of image quality on reliability of the measurements of left ventricular systolic function and global longitudinal strain in 2D echocardiography. Echo Res. Pract. 2018, 5, 27–39. [Google Scholar] [CrossRef]
| BPD (n = 50) | Control Group (n = 50) | p Value | |
|---|---|---|---|
| Age | 23.85 (±5.2) | 24.10 (±3.9) | n.s. |
| Pack years | 3.9 (±6.0) | 0.2 (±1.1) | <0.001 * |
| SBP (mmHg) | 126.9 (±12.8) | 115.5 (±9.5) | <0.001 * |
| DBP (mmHg) | 82.9 (±9.5) | 75.8 (±7.3) | <0.001 * |
| WtH ratio | 0.77 (±0.1) | 0.74 (±0.04) | <0.001 * |
| BMI (kg/m2) | 28.37 (±6.6) | 22.40 (±3.0) | <0.001 * |
| Height (cm) | 167.4(±6.3) | 166.7(±5.6) | 0.53 |
| Cholesterol/HDL ratio | 3.29 (±1.2) | 2.77 (±0.55) | 0.004 * |
| HDL (mg/dL) | 55.94 (±15.6) | 71.68 (±16.4) | <0.001 * |
| HbA1c (%) | 5.2 (±0.29) | 4.9 (±0.22) | <0.001 * |
| CRP (mg/dL) | 0.47 (±0.79) | 0.17 (±0.33) | 0.010 * |
| TSH (mIU/L) | 1.45 (±0.68) | 1.82 (±0.78) | 0.012 * |
| fT3 (pmol/L) | 5.41 (±0.69) | 5.11 (±0.45) | 0.012 * |
| fT4 (ng/L) | 8.46 (±1.30) | 7.54 (±1.16) | <0.001 * |
| SPINA-GT (pmol/s) | 2.82 (±0.17) | 2.17 (±0.14) | <0.001 * |
| SPINA-GD (nmol/s) | 46.89 (±1.14) | 49.39 (±1.17) | n.s. |
| JTI | 1.73 (±0.07) | 1.79 (±0.07 | n.s. |
| BNP (pg/mL) | 17.41 (±9.59) | 29.02 (±18.97) | <0.001 * |
| hsTroponine (pg/mL) | 2.42 (±0.46) | 2.80 (±2.71) | 0.334 |
| PR (ms) | 139 (±22.7) | 141.7 (±25.1) | 0.62 |
| QRS (ms) | 83.6 (±13.3) | 87 (±11.8) | 0.18 |
| QTc (ms) | 403.7 (±31.2) | 402.3 (±25.7) | 0.80 |
| Tpe (ms) | 62.3 (±11.4) | 61.4 (±13.3) | 0.74 |
| BPD (n = 42) | Control Group (n = 49) | p-Value | |
|---|---|---|---|
| PW (mm) | 8.0 (±1.3) | 7.7 (±1.2) | 0.240 |
| IVS (mm) | 8.5 (±1.1) | 7.5 (±1.5) | <0.001 * |
| Heart rate (bpm) | 72 (±12) | 69 (±13) | 0.259 |
| LVEDD (mm) | 45.9 (±3.8) | 45.9 (±4.7) | 0.990 |
| LVESD (mm) | 29.6 (±3.6) | 28.0 (±5.1) | 0.076 |
| LVEDV (mL) | 90.6 (±24.6) | 91.6 (±17.4) | 0.806 |
| LVESV (mL) | 37.7 (±10.2) | 38.2 (±8.3) | 0.788 |
| SV (mL) | 52.5 (±14.4) | 53.3 (±11.2) | 0.739 |
| LVEF (%) | 58.1 (±4.1) | 58.3 (±4.5) | 0.860 |
| TAPSE (mm) | 22.8 (±4.3) | 24.7 (±3.7) | 0.019 * |
| LV mass (g) | 117.3 (±42) | 109.3 (±41) | 0.02 |
| LV mass-index (g/m2) | 61.9 (±21) | 65.5 (±22) | 0.81 |
| RWT | 0.35 (±0.06) | 0.56 (±1.6) | 0.32 |
| GLS (%) | −19.04 (±1.9) | −20.7 (±1.7) | <0.001 * |
| LV Mechanical dispersion (ms) | 35.2 (±8.5) | 39.5 (±13.9) | 0.30 |
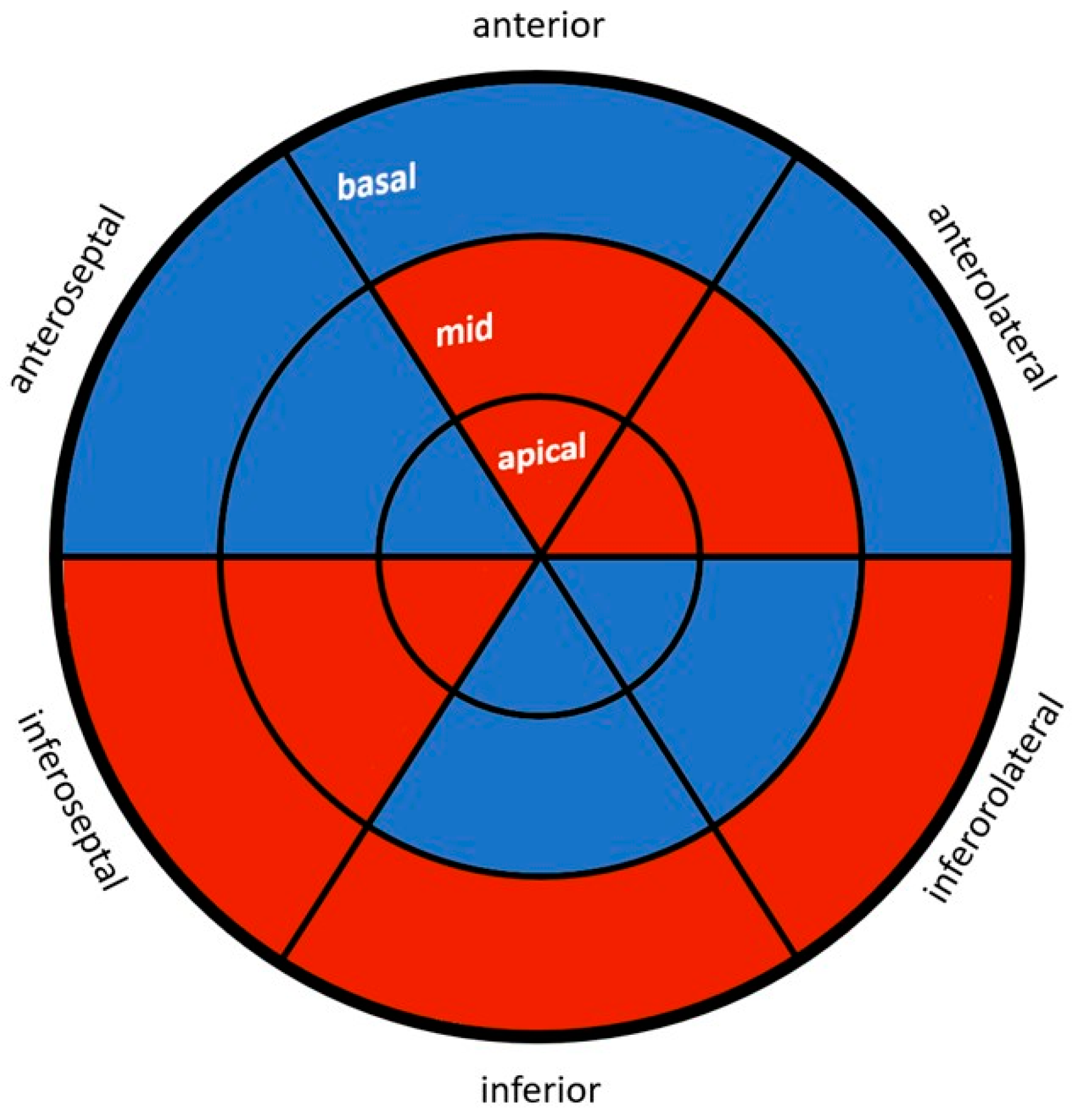
| Anteroseptal | |||
| Basal (%) | −16.5 (±2.8) | −18.1 (±3.2) | 0.01 * |
| Mid (%) | −19.5 (±2.6) | −21.8 (±3.0) | <0.001 * |
| Apical (%) | −23.2 (±4.2) | −27.0 (±4.8) | <0.001 * |
| Inferolateral | |||
| Basal (%) | −18.8 (±3.8) | −19.6 (±4.5) | 0.369 |
| Mid (%) | −18.0 (±3.2) | −20.7 (±3.3) | <0.001 * |
| Apical (%) | −18.9 (±4.3) | −21.5 (±4.9) | 0.01 * |
| Inferoseptal | |||
| Basal (%) | −19.6 (±3.7) | −20.9 (±4.1) | 0.126 |
| Mid (%) | −18.8 (±8.2) | −20.4 (±3.1) | 0.191 |
| Apical (%) | −23.3 (±4.3) | −24.6 (±4.6) | 0.171 |
| Anterolateral | |||
| Basal (%) | −17.7 (±5.0) | −19.8 (±4.7) | 0.048 * |
| Mid (%) | −19.3 (±4.1) | −20.3 (±4.8) | 0.333 |
| Apical (%) | −18.7 (±5.0) | −18.9 (±5.7) | 0.842 |
| Inferior | |||
| Basal (%) | −17.9 (±4.4) | −19.9 (±4.1) | 0.028 |
| Mid (%) | −18.4 (±2.9) | −20.6 (±3.3) | 0.001 * |
| Apical (%) | −20.4 (±3.4) | −23.3 (±4.9) | 0.001 * |
| Anterior | |||
| Basal (%) | −16.0 (±3.1) | −17.9 (±3.5) | 0.007 * |
| Mid (%) | −21.1 (±4.1) | −21.9 (±3.3) | 0.315 |
| Apical (%) | −21.4 (±4.3) | −23.0 (±5.4) | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aweimer, A.; Engemann, L.; Amar, S.; Ewers, A.; Afshari, F.; Maiß, C.; Kern, K.; Lücke, T.; Mügge, A.; El-Battrawy, I.; et al. Stress-Mediated Abnormalities in Regional Myocardial Wall Motion in Young Women with a History of Psychological Trauma. J. Clin. Med. 2023, 12, 6702. https://doi.org/10.3390/jcm12216702
Aweimer A, Engemann L, Amar S, Ewers A, Afshari F, Maiß C, Kern K, Lücke T, Mügge A, El-Battrawy I, et al. Stress-Mediated Abnormalities in Regional Myocardial Wall Motion in Young Women with a History of Psychological Trauma. Journal of Clinical Medicine. 2023; 12(21):6702. https://doi.org/10.3390/jcm12216702
Chicago/Turabian StyleAweimer, Assem, Luisa Engemann, Sameh Amar, Aydan Ewers, Faegheh Afshari, Clara Maiß, Katharina Kern, Thomas Lücke, Andreas Mügge, Ibrahim El-Battrawy, and et al. 2023. "Stress-Mediated Abnormalities in Regional Myocardial Wall Motion in Young Women with a History of Psychological Trauma" Journal of Clinical Medicine 12, no. 21: 6702. https://doi.org/10.3390/jcm12216702
APA StyleAweimer, A., Engemann, L., Amar, S., Ewers, A., Afshari, F., Maiß, C., Kern, K., Lücke, T., Mügge, A., El-Battrawy, I., Dietrich, J. W., & Brüne, M. (2023). Stress-Mediated Abnormalities in Regional Myocardial Wall Motion in Young Women with a History of Psychological Trauma. Journal of Clinical Medicine, 12(21), 6702. https://doi.org/10.3390/jcm12216702






