Compliance to Multidisciplinary Lifestyle Intervention Decreases Blood Pressure in Patients with Resistant Hypertension: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Study: Overview
2.2. Participants
2.3. Clinical Assessment
2.4. Statistics
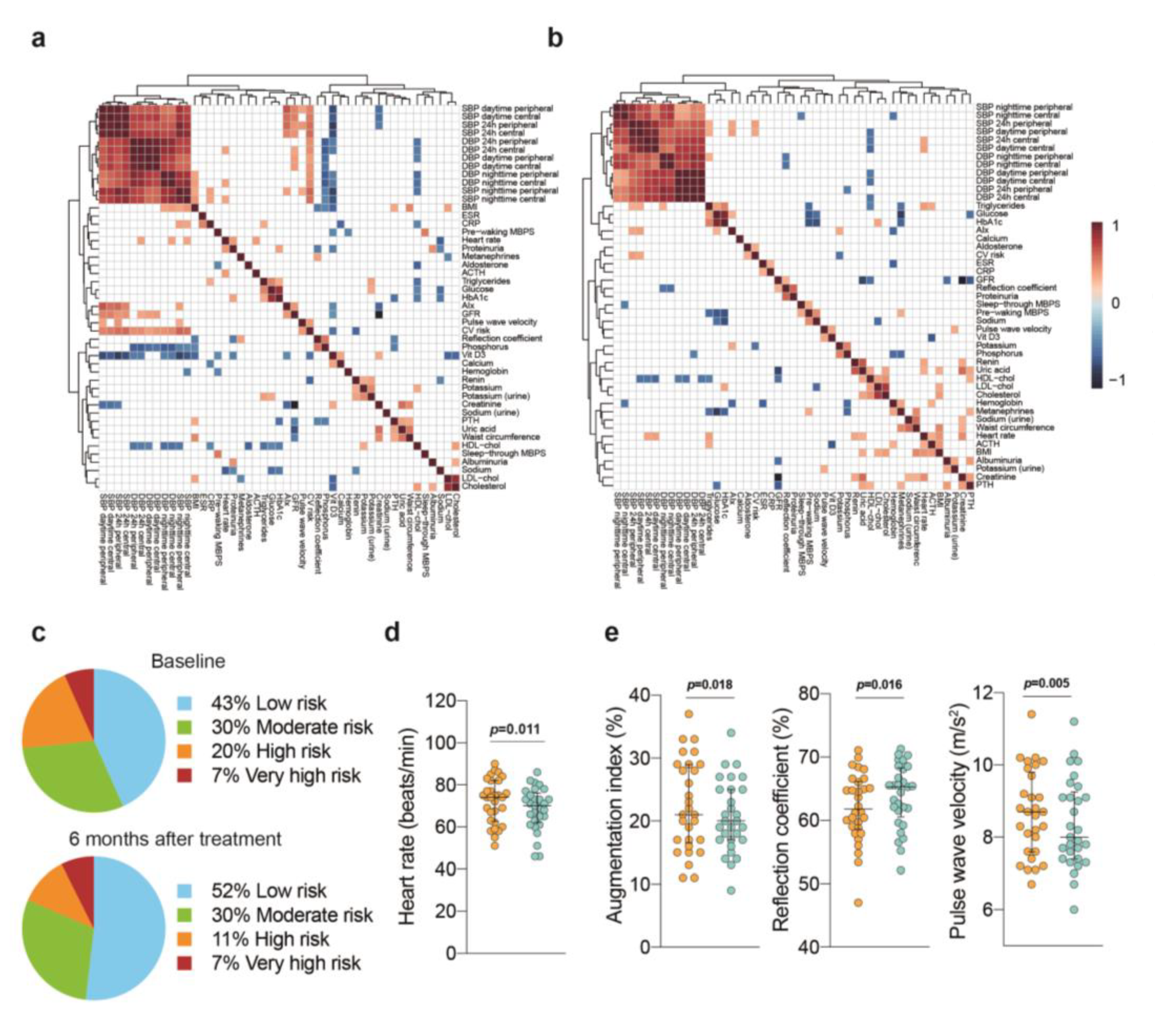
3. Results
3.1. Adherence to Intervention
3.2. Clinical Characteristics and Laboratory Testing
3.3. Blood Pressure Outcomes
3.4. Cardiovascular Risk Stratification
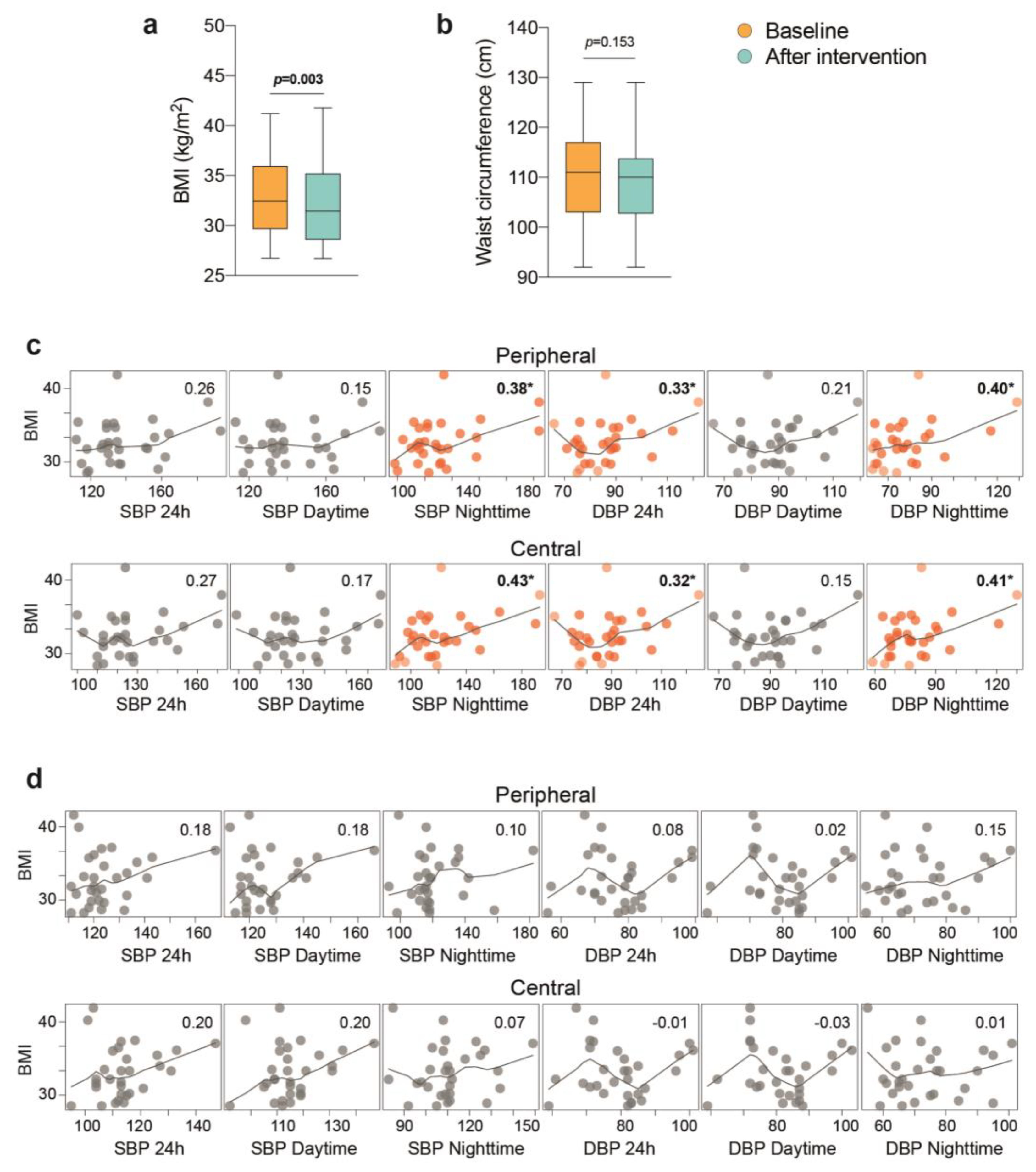
3.5. The Importance of Weight Loss
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Calhoun, D.A.; Mancia, G.; Carey, R.M. Resistant Hypertension Management: Comparison of the 2017 American and 2018 European High Blood Pressure Guidelines. Curr. Hypertens Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Simonetti, V.; D’Errico, M.M.; de Vito, C.; Flacco, M.E.; Forni, C.; la Torre, G.; Liguori, G.; Messina, G.; Mezzetti, A.; et al. (In)Accuracy of Blood Pressure Measurement in 14 Italian Hospitals. J. Hypertens 2012, 30, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Cicolini, G.; Pizzi, C.; Palma, E.; Bucci, M.; Schioppa, F.; Mezzetti, A.; Manzoli, L. Differences in Blood Pressure by Body Position (Supine, Fowler’s, and Sitting) in Hypertensive Subjects. Am. J. Hypertens 2011, 24, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Pandey, A.; Vongpatanasin, W. Resistant Hypertension-Defining the Scope of the Problem. Prog. Cardiovasc. Dis. 2020, 63, 46–50. [Google Scholar] [CrossRef]
- Bergler-Klein, J. What’s New in the ESC 2018 Guidelines for Arterial Hypertension: The Ten Most Important Messages. Wien Klin Wochenschr 2019, 131, 180–185. [Google Scholar] [CrossRef]
- de la Sierra, A.; Segura, J.; Banegas, J.R.; Gorostidi, M.; de la Cruz, J.J.; Armario, P.; Oliveras, A.; Ruilope, L.M. Clinical Features of 8295 Patients with Resistant Hypertension Classified on the Basis of Ambulatory Blood Pressure Monitoring. Hypertension 2011, 57, 898–902. [Google Scholar] [CrossRef]
- Achelrod, D.; Wenzel, U.; Frey, S. Systematic Review and Meta-Analysis of the Prevalence of Resistant Hypertension in Treated Hypertensive Populations. Am. J. Hypertens 2015, 28, 355–361. [Google Scholar] [CrossRef]
- Tsioufis, C.; Kasiakogias, A.; Kordalis, A.; Dimitriadis, K.; Thomopoulos, C.; Tsiachris, D.; Vasileiou, P.; Doumas, M.; Makris, T.; Papademetriou, V.; et al. Dynamic Resistant Hypertension Patterns as Predictors of Cardiovascular Morbidity: A 4-Year Prospective Study. J. Hypertens 2014, 32, 415–422. [Google Scholar] [CrossRef]
- Bramlage, P.; Pittrow, D.; Wittchen, H.-U.; Kirch, W.; Boehler, S.; Lehnert, H.; Hoefler, M.; Unger, T.; Sharma, A.M. Hypertension in Overweight and Obese Primary Care Patients Is Highly Prevalent and Poorly Controlled. Am. J. Hypertens 2004, 17, 904–910. [Google Scholar] [CrossRef]
- Gruber, T.; Pan, C.; Contreras, R.E.; Wiedemann, T.; Morgan, D.A.; Skowronski, A.A.; Lefort, S.; de Bernardis Murat, C.; le Thuc, O.; Legutko, B.; et al. Obesity-Associated Hyperleptinemia Alters the Gliovascular Interface of the Hypothalamus to Promote Hypertension. Cell Metab. 2021, 33, 1155–1170.e10. [Google Scholar] [CrossRef]
- Persell, S.D. Prevalence of Resistant Hypertension in the United States, 2003-2008. Hypertension 2011, 57, 1076–1080. [Google Scholar] [CrossRef]
- Carey, R.M. Special Article-The Management of Resistant Hypertension: A 2020 Update. Prog. Cardiovasc. Dis. 2020, 63, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Appel, L.J.; Espeland, M.A.; Applegate, W.B.; Ettinger, W.H.J.; Kostis, J.B.; Kumanyika, S.; Lacy, C.R.; Johnson, K.C.; Folmar, S.; et al. Sodium Reduction and Weight Loss in the Treatment of Hypertension in Older Persons: A Randomized Controlled Trial of Nonpharmacologic Interventions in the Elderly (TONE). TONE Collaborative Research Group. JAMA 1998, 279, 839–846. [Google Scholar] [CrossRef]
- Clozel, M. Aprocitentan and the Endothelin System in Resistant Hypertension. Can. J. Physiol. Pharmacol. 2022, 100, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Siebenhofer, A.; Winterholer, S.; Jeitler, K.; Horvath, K.; Berghold, A.; Krenn, C.; Semlitsch, T. Long-Term Effects of Weight-Reducing Drugs in People with Hypertension. Cochrane Database Syst. Rev. 2021, 1, CD007654. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Ukena, C.; Schmieder, R.E.; Cremers, B.; Rump, L.C.; Vonend, O.; Weil, J.; Schmidt, M.; Hoppe, U.C.; Zeller, T.; et al. Ambulatory Blood Pressure Changes after Renal Sympathetic Denervation in Patients with Resistant Hypertension. Circulation 2013, 128, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Nadim, M.K.; Haller, H.; Lovett, E.G.; Schafer, J.E.; Bisognano, J.D. Baroreflex Activation Therapy Provides Durable Benefit in Patients with Resistant Hypertension: Results of Long-Term Follow-up in the Rheos Pivotal Trial. J. Am. Soc. Hypertens 2012, 6, 152–158. [Google Scholar] [CrossRef]
- White, W.B.; Galis, Z.S.; Henegar, J.; Kandzari, D.E.; Victor, R.; Sica, D.; Townsend, R.R.; Turner, J.R.; Virmani, R.; Mauri, L. Renal Denervation Therapy for Hypertension: Pathways for Moving Development Forward. J. Am. Soc. Hypertens 2015, 9, 341–350. [Google Scholar] [CrossRef]
- Cabré, N.; Luciano-Mateo, F.; Fernández-Arroyo, S.; Baiges-Gayà, G.; Hernández-Aguilera, A.; Fibla, M.; Fernández-Julià, R.; París, M.; Sabench, F.; del Castillo, D.; et al. Laparoscopic Sleeve Gastrectomy Reverses Non-Alcoholic Fatty Liver Disease Modulating Oxidative Stress and Inflammation. Metabolism 2019, 99, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cuffee, Y.; Ogedegbe, C.; Williams, N.J.; Ogedegbe, G.; Schoenthaler, A. Psychosocial Risk Factors for Hypertension: An Update of the Literature. Curr. Hypertens Rep. 2014, 16, 483. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; MacDonald, H.v.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens Rep. 2015, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) Diet on Blood Pressure: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Yusuf, S. Sodium Intake and Cardiovascular Health. Circ. Res. 2015, 116, 1046–1057. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Hinderliter, A.L.; Smith, P.J.; Mabe, S.; Watkins, L.L.; Craighead, L.; Ingle, K.; Tyson, C.; Lin, P.-H.; Kraus, W.E.; et al. Effects of Lifestyle Modification on Patients With Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation 2021, 144, 1212–1226. [Google Scholar] [CrossRef]
- Sabbahi, A.; Severin, R.; Laddu, D.; Sharman, J.E.; Arena, R.; Ozemek, C. Nonpharmacological Management of Resistant Hypertension. Curr. Cardiol. Rep. 2021, 23, 166. [Google Scholar] [CrossRef]
- Thomas, E.A.; Enduru, N.; Tin, A.; Boerwinkle, E.; Griswold, M.E.; Mosley, T.H.; Gottesman, R.F.; Fornage, M. Polygenic Risk, Midlife Life’s Simple 7, and Lifetime Risk of Stroke. J. Am. Heart Assoc. 2022, 11, e025703. [Google Scholar] [CrossRef]
- Julious, S.A. Pilot Studies in Clinical Research. Stat. Methods Med. Res. 2016, 25, 995–996. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension Practice Guidelines for Office and Out-of-Office Blood Pressure Measurement. J. Hypertens 2021, 39, 1293–1302. [Google Scholar] [CrossRef]
- Rosei, E.A.; Chiarini, G.; Rizzoni, D. How Important Is Blood Pressure Variability? Eur. Heart J. Suppl. 2020, 22, E1–E6. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Salles, G.F. Associations of the Nocturnal Blood Pressure Fall and Morning Surge with Cardiovascular Events and Mortality in Individuals with Resistant Hypertension. J. Hypertens 2021, 39, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine Learning for Neuroimaging with Scikit-Learn. Front. Neuroinform 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bertran, N.; Camps, J.; Fernandez-Ballart, J.; Arija, V.; Ferre, N.; Tous, M.; Simo, D.; Murphy, M.M.; Vilella, E.; Joven, J. Diet and Lifestyle Are Associated with Serum C-Reactive Protein Concentrations in a Population-Based Study. J. Lab. Clin. Med. 2005, 145, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.L.; Powers, J.D.; Magid, D.J.; Tavel, H.M.; Masoudi, F.A.; Margolis, K.L.; O’Connor, P.J.; Selby, J.v.; Ho, P.M. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation 2012, 125, 1635–1642. [Google Scholar] [CrossRef]
- Ozemek, C.; Tiwari, S.; Sabbahi, A.; Carbone, S.; Lavie, C.J. Impact of Therapeutic Lifestyle Changes in Resistant Hypertension. Prog. Cardiovasc. Dis. 2020, 63, 4–9. [Google Scholar] [CrossRef]
- Burke, V.; Beilin, L.J.; Cutt, H.E.; Mansour, J.; Wilson, A.; Mori, T.A. Effects of a Lifestyle Programme on Ambulatory Blood Pressure and Drug Dosage in Treated Hypertensive Patients: A Randomized Controlled Trial. J. Hypertens 2005, 23, 1241–1249. [Google Scholar] [CrossRef]
- O’Brien, E.; Kario, K.; Staessen, J.A.; de la Sierra, A.; Ohkubo, T. Patterns of Ambulatory Blood Pressure: Clinical Relevance and Application. J. Clin. Hypertens 2018, 20, 1112–1115. [Google Scholar] [CrossRef]
- Perret-Guillaume, C.; Joly, L.; Benetos, A. Heart Rate as a Risk Factor for Cardiovascular Disease. Prog. Cardiovasc. Dis. 2009, 52, 6–10. [Google Scholar] [CrossRef] [PubMed]
- van Popele, N.M.; Mattace-Raso, F.U.S.; Vliegenthart, R.; Grobbee, D.E.; Asmar, R.; van der Kuip, D.A.M.; Hofman, A.; de Feijter, P.J.; Oudkerk, M.; Witteman, J.C.M. Aortic Stiffness Is Associated with Atherosclerosis of the Coronary Arteries in Older Adults: The Rotterdam Study. J. Hypertens 2006, 24, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Grillo, A.; Guida, V.; Parati, G. Morning Blood Pressure Surge: Pathophysiology, Clinical Relevance and Therapeutic Aspects. Integr. Blood Press Control 2018, 11, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Chernofsky, A.; Spartano, N.L.; Tanguay, M.; Blodgett, J.B.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Velagaleti, R.S.; Murabito, J.M.; et al. Physical Activity and Fitness in the Community: The Framingham Heart Study. Eur. Heart J. 2021, 42, 4565–4575. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, C.A.; Ikeoka, D.; Santucci, E.v.; Santos, R.N.; Damiani, L.P.; Bueno, P.T.; Oliveira, J.D.; Torreglosa, C.R.; Bersch-Ferreira, A.C.; Miranda, T.A.; et al. Effects of Bariatric Surgery Versus Medical Therapy on the 24-Hour Ambulatory Blood Pressure and the Prevalence of Resistant Hypertension. Hypertension 2019, 73, 571–577. [Google Scholar] [CrossRef]
- Harsha, D.W.; Bray, G.A. Weight Loss and Blood Pressure Control (Pro). Hypertension 2008, 51, 1420–1425. [Google Scholar] [CrossRef]
- Hall, M.E.; Cohen, J.B.; Ard, J.D.; Egan, B.M.; Hall, J.E.; Lavie, C.J.; Ma, J.; Ndumele, C.E.; Schauer, P.R.; Shimbo, D. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2021, 78, E38–E50. [Google Scholar] [CrossRef]
- Green, L.W.; Glasgow, R.E. Evaluating the Relevance, Generalization, and Applicability of Research: Issues in External Validation and Translation Methodology. Eval. Health Prof. 2006, 29, 126–153. [Google Scholar] [CrossRef]
- Green, L.W. Public Health Asks of Systems Science: To Advance Our Evidence-Based Practice, Can You Help Us Get More Practice-Based Evidence? Am. J. Public Health 2006, 96, 406–409. [Google Scholar] [CrossRef]

| Patients with RHT (n = 30) | |
|---|---|
| Age (years) | 59.6 ± 8.8 |
| Sex, men, n (%) | 22 (66) |
| BMI (kg/m2) | 33.1 ± 4.4 |
| Waist circumference (m) | 1.1 ± 0.1 |
| 24 h SBP (mmHg) | 138.3 ± 20.1 |
| 24 h DBP (mmHg) | 85.7 ± 12.1 |
| T2DM, n (%) | 12 (36) |
| Dyslipidemia, n (%) | 23 (76) |
| OSAS, n (%) | 18 (60) |
| Metabolic syndrome, n (%) | 19 (63) |
| Atrial fibrillation, n (%) | 1 (3) |
| Right branch bundle block, n (%) | 4 (13) |
| Left branch bundle block, n (%) | 2 (6) |
| Left ventricular hypertrophy, n (%) | 27 (90) |
| Heart rate (beats/min) | 72.2 ± 10.4 |
| PWV > 10 m/seg, n (%) | 7 (23) |
| Ankle-brachial index < 0.9, n (%) | 1 (3) |
| Left atrial enlargement, n (%) | 25 (83) |
| Carotid artery plaque, n (%) | 23 (76) |
| Baseline | After Intervention | p-Value | |
|---|---|---|---|
| Peripheral blood pressure | |||
| 24 h SBP (mmHg) | 138.3 ± 20.1 | 124.3 ± 11.8 | 1.6 × 10−5 |
| 24 h DBP (mmHg) | 85.7 ± 12.1 | 77.2 ± 10.4 | 2 × 10−5 |
| Daytime SBP (mmHg) | 141.2 ± 18.8 | 126.1 ± 11 | 2.5 × 10−6 |
| Daytime DBP (mmHg) | 88.6 ± 11.6 | 79.7 ± 10.4 | 7.3 × 10−6 |
| Nighttime SBP (mmHg) | 131 ± 25.3 | 121.1 ± 17.7 | 0.013 |
| Nighttime DBP (mmHg) | 77.9 ± 15.3 | 72 ± 11.9 | 0.014 |
| Heart rate (beats/min) | 72.2 ± 10.4 | 69.3 ± 10.9 | 0.038 |
| Central hemodynamics | |||
| Central SBP 24 h (mmHg) | 126.9 ± 17.7 | 114.1 ± 10.6 | 6.7 × 10−6 |
| Central DBP 24 h (mmHg) | 85.5 ± 12.3 | 79.5 ± 10.4 | 1.8 × 10−5 |
| Central daytime SBP (mmHg) | 128.4 ± 16.5 | 115.4 ± 10.8 | 2.5 × 10−6 |
| Central nighttime DBP (mmHg) | 90.2 ± 12.1 | 81.4 ± 10.3 | 1.3 × 10−6 |
| Central daytime SBP (mmHg) | 122.3 ± 25.8 | 110.9 ± 14.3 | 0.007 |
| Central nighttime DBP (mmHg) | 80.1 ± 15.7 | 73.4 ± 12.1 | 0.013 |
| Cardiac output (L/min) | 6.3 ± 7 | 6.3 ± 8.6 | 0.013 |
| Variability of arterial pressure | |||
| Systolic mean (mmHg) | 132.5 ± 16 | 126.3 ± 14.1 | 0.064 |
| Diastolic mean (mmHg) | 82.5 ± 11.2 | 78.5 ± 10.9 | 0.049 |
| Systolic SD (mmHg) | 16.8 ± 4.9 | 15.1 ± 3.9 | 0.184 |
| Diastolic SD (mmHg) | 12.6 ± 3.1 | 11.7 ± 2.6 | 0.178 |
| Systolic CV (mmHg) | 12.6± 3.2 | 11.9 ± 2.5 | 0.229 |
| Diastolic CV (mmHg) | 15.4 ± 3.9 | 15.1 ± 3.3 | 0.685 |
| Systolic ARV (mmHg) | 14.5 ± 5.3 | 13.6 ± 5 | 0.375 |
| Diastolic ARV (mmHg) | 11.3 ± 3.6 | 10.3 ± 3.4 | 0.243 |
| Weighted SD (mmHg) | 22.3 ± 2 | 22.4 ± 1.5 | 0.948 |
| Surge morning | |||
| Sleep-through MBPS (mmHg) | 26 ± 14.5 | 18.4 ± 14.9 | 0.061 |
| Pre-waking MBPS (mmHg) | 23.2 ± 20.5 | 12.4 ± 11.5 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinel, E.; Azancot, M.A.; Gomez, A.; Beneria, A.; Caraben, A.; Andurell, L.; Delgado, P.; Castañé, H.; Joven, J.; Seron, D. Compliance to Multidisciplinary Lifestyle Intervention Decreases Blood Pressure in Patients with Resistant Hypertension: A Cross-Sectional Pilot Study. J. Clin. Med. 2023, 12, 679. https://doi.org/10.3390/jcm12020679
Espinel E, Azancot MA, Gomez A, Beneria A, Caraben A, Andurell L, Delgado P, Castañé H, Joven J, Seron D. Compliance to Multidisciplinary Lifestyle Intervention Decreases Blood Pressure in Patients with Resistant Hypertension: A Cross-Sectional Pilot Study. Journal of Clinical Medicine. 2023; 12(2):679. https://doi.org/10.3390/jcm12020679
Chicago/Turabian StyleEspinel, Eugenia, María Antonia Azancot, Alba Gomez, Anna Beneria, Anna Caraben, Laura Andurell, Pilar Delgado, Helena Castañé, Jorge Joven, and Daniel Seron. 2023. "Compliance to Multidisciplinary Lifestyle Intervention Decreases Blood Pressure in Patients with Resistant Hypertension: A Cross-Sectional Pilot Study" Journal of Clinical Medicine 12, no. 2: 679. https://doi.org/10.3390/jcm12020679
APA StyleEspinel, E., Azancot, M. A., Gomez, A., Beneria, A., Caraben, A., Andurell, L., Delgado, P., Castañé, H., Joven, J., & Seron, D. (2023). Compliance to Multidisciplinary Lifestyle Intervention Decreases Blood Pressure in Patients with Resistant Hypertension: A Cross-Sectional Pilot Study. Journal of Clinical Medicine, 12(2), 679. https://doi.org/10.3390/jcm12020679






