Plasma Extracellular Vesicles as Liquid Biopsy to Unravel the Molecular Mechanisms of Cardiac Reverse Remodeling Following Resynchronization Therapy?
Abstract
1. Introduction
2. Myocardial Remodeling in Cardiac Dyssynchrony
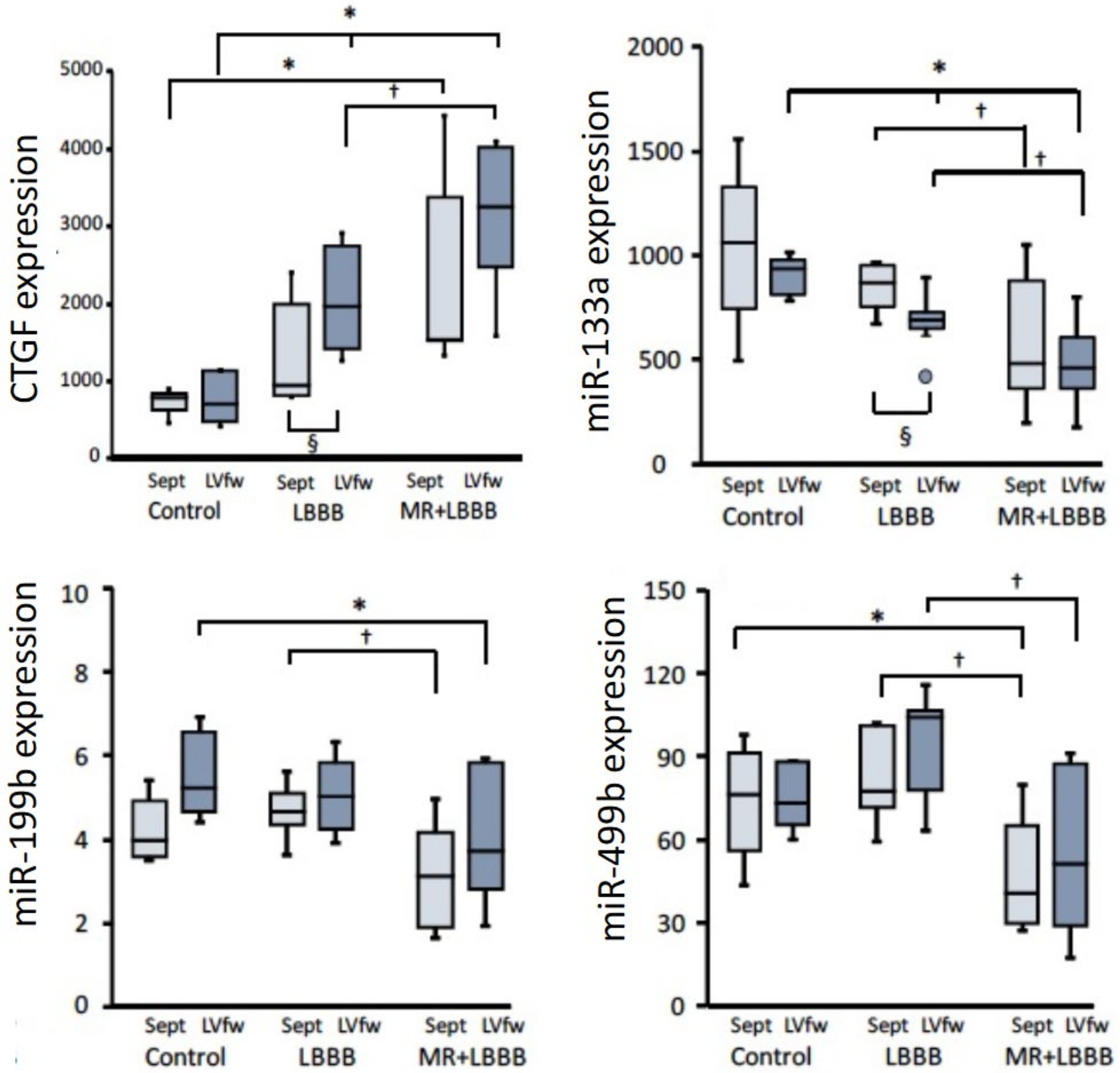
3. miRNAs in Remodeling in Dyssynchronous Hearts
4. The Role of Extracellular Vesicles and Their Cargos in Mechanisms and Diagnosis of Cardiac Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohn, J.N.; Ferrari, R.; Sharpe, N.; International Forum on Cardiac Remodeling. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Moss, A.J.; Hall, W.J.; Foster, E.; Goldberger, J.J.; Santucci, P.; Shinn, T.; Solomon, S.; Steinberg, J.S.; Wilber, D.; et al. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 124, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Saraon, T.; Katz, S.D. Reverse Remodeling in Systolic Heart Failure. Cardiol. Rev. 2015, 23, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, H.; Omens, J.H.; Ingels, N.B., Jr.; Covell, J.W. Transmural mechanics at left ventricular epicardial pacing site. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2401–H2407. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Hunter, W.C.; Wyman, B.T.; McVeigh, E.R. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J. Am. Coll. Cardiol. 1999, 33, 1735–1742. [Google Scholar] [CrossRef]
- Vernooy, K.; Verbeek, X.A.; Peschar, M.; Crijns, H.J.; Arts, T.; Cornelussen, R.N.; Prinzen, F.W. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur. Heart J. 2005, 26, 91–98. [Google Scholar] [CrossRef]
- Van Middendorp, L.B.; Kuiper, M.; Munts, C.; Wouters, P.; Maessen, J.G.; van Nieuwenhoven, F.A.; Prinzen, F.W. Local microRNA-133a downregulation is associated with hypertrophy in the dyssynchronous heart. ESC Heart Fail. 2017, 4, 241–251. [Google Scholar] [CrossRef]
- Barth, A.S.; Aiba, T.; Halperin, V.; DiSilvestre, D.; Chakir, K.; Colantuoni, C.; Tunin, R.S.; Dimaano, V.L.; Yu, W.; Abraham, T.P.; et al. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ. Cardiovasc. Genet. 2009, 2, 371–378. [Google Scholar] [CrossRef]
- Lin, J.M.; Lai, L.P.; Lin, C.S.; Chou, N.K.; Chiu, C.Y.; Lin, J.L. Left ventricular extracellular matrix remodeling in dogs with right ventricular apical pacing. J. Cardiovasc. Electrophysiol. 2010, 21, 1142–1149. [Google Scholar] [CrossRef]
- Spragg, D.D.; Akar, F.G.; Helm, R.H.; Tunin, R.S.; Tomaselli, G.F.; Kass, D.A. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc. Res. 2005, 67, 77–86. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Chen, H.; Qin, S.; Zhou, N.; Su, Y.; Ge, J. Effect of Cardiac Resynchronization Therapy on Myocardial Fibrosis and Relevant Cytokines in a Canine Model with Experimental Heart Failure. J. Cardiovasc. Electrophysiol. 2017, 28, 438–445. [Google Scholar] [CrossRef]
- Cervio, E.; Barile, L.; Moccetti, T.; Vassalli, G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015, 2015, 482171. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies—From exosomes to microvesicles. Cardiovasc. Res. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Ziegler, O.; Yeri, A.; Liu, X.; Murthy, V.; Rabideau, D.; Xiao, C.Y.; Hanspers, K.; Belcher, A.; Tackett, M.; et al. MicroRNAs Associated with Reverse Left Ventricular Remodeling in Humans Identify Pathways of Heart Failure Progression. Circ. Heart Fail. 2018, 11, e004278. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Barile, L. Fndc5/irisin-enriched extracellular vesicles: A new hormonal relay in the regular race against vascular ageing. Eur. Heart J. 2022, 43, 4596–4598. [Google Scholar] [CrossRef]
- Costard-Jackle, A.; Goetsch, B.; Antz, M.; Franz, M.R. Slow and long-lasting modulation of myocardial repolarization produced by ectopic activation in isolated rabbit hearts. Evidence for cardiac “memory”. Circulation 1989, 80, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, D.; Wilson, L.D.; Zhong, J.; Flask, C.; Saffitz, J.E.; Deschenes, I.; Yu, X.; Rosenbaum, D.S. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation 2007, 115, 3145–3155. [Google Scholar] [CrossRef]
- Akar, F.G.; Nass, R.D.; Hahn, S.; Cingolani, E.; Shah, M.; Hesketh, G.G.; DiSilvestre, D.; Tunin, R.S.; Kass, D.A.; Tomaselli, G.F. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1223–H1230. [Google Scholar] [CrossRef]
- Vernooy, K.; Cornelussen, R.N.; Verbeek, X.A.; Vanagt, W.Y.; van Hunnik, A.; Kuiper, M.; Arts, T.; Crijns, H.J.; Prinzen, F.W. Cardiac resynchronization therapy cures dyssynchronopathy in canine left bundle-branch block hearts. Eur. Heart J. 2007, 28, 2148–2155. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Miller, M.A.; Neuzil, P.; Sogaard, P.; Butter, C.; Seifert, M.; Delnoy, P.P.; van Erven, L.; Schalji, M.; Boersma, L.V.A.; et al. Cardiac Resynchronization Therapy with Wireless Left Ventricular Endocardial Pacing: The SELECT-LV Study. J. Am. Coll. Cardiol. 2017, 69, 2119–2129. [Google Scholar] [CrossRef]
- Van Oosterhout, M.F.; Prinzen, F.W.; Arts, T.; Schreuder, J.J.; Vanagt, W.Y.; Cleutjens, J.P.; Reneman, R.S. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 1998, 98, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Sridhar, A.; Viatchenko-Karpinski, S.; Shaw, C.; Bonagura, J.D.; Abraham, W.T.; Joshi, M.S.; Bauer, J.A.; Hamlin, R.L.; Györke, S.; et al. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007, 81, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, M.; Mullens, W.; Delrue, L.; Goethals, M.; de Bruyne, B.; Wijns, W.; Geelen, P.; Verstreken, S.; Wellens, F.; Bartunek, J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J. Am. Coll. Cardiol. 2008, 51, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.; Geleijnse, M.L.; Theuns, D.A.; Nemes, A.; Vletter, W.B.; van Dalen, B.M.; Motawea, A.K.; Jordaens, L.J.; Cate, F.J.T. Reverse of left ventricular volumetric and structural remodeling in heart failure patients treated with cardiac resynchronization therapy. Am. J. Cardiol. 2008, 101, 651–657. [Google Scholar] [CrossRef]
- Zhang, Q.; Fung, J.W.; Auricchio, A.; Chan, J.Y.; Kum, L.C.; Wu, L.W.; Yu, C.-M. Differential change in left ventricular mass and regional wall thickness after cardiac resynchronization therapy for heart failure. Eur. Heart J. 2006, 27, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- D’Ascia, C.; Cittadini, A.; Monti, M.G.; Riccio, G.; Saccà, L. Effects of biventricular pacing on interstitial remodelling, tumor necrosis factor-alpha expression, and apoptotic death in failing human myocardium. Eur. Heart J. 2006, 27, 201–206. [Google Scholar] [CrossRef]
- Orrego, C.M.; Nasir, N.; Oliveira, G.H.; Flores-Arredondo, J.H.; Cordero-Reyes, A.M.; Loebe, M.; Youker, K.A.; Torre-Amione, G. Cellular evidence of reverse cardiac remodeling induced by cardiac resynchronization therapy. Congest. Heart Fail. 2011, 17, 140–146. [Google Scholar] [CrossRef]
- Lieberman, R.; Padeletti, L.; Schreuder, J.; Jackson, K.; Michelucci, A.; Colella, A.; Eastman, W.; Valsecchi, S.; Hettrick, D.A. Ventricular pacing lead location alters systemic hemodynamics and left ventricular function in patients with and without reduced ejection fraction. J. Am. Coll. Cardiol. 2006, 48, 1634–1641. [Google Scholar] [CrossRef]
- Nahlawi, M.; Waligora, M.; Spies, S.M.; Bonow, R.O.; Kadish, A.H.; Goldberger, J.J. Left ventricular function during and after right ventricular pacing. J. Am. Coll. Cardiol. 2004, 44, 1883–1888. [Google Scholar] [CrossRef]
- Strik, M.; Rademakers, L.M.; van Deursen, C.J.; van Hunnik, A.; Kuiper, M.; Klersy, C.; Auricchio, A.; Prinzen, F.W. Endocardial left ventricular pacing improves cardiac resynchronization therapy in chronic asynchronous infarction and heart failure models. Circ. Arrhythm. Electrophysiol. 2012, 5, 191–200. [Google Scholar] [CrossRef]
- Kirk, J.A.; Kass, D.A. Cellular and Molecular Aspects of Dyssynchrony and Resynchronization. Heart Fail. Clin. 2017, 13, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.C.; Verzaal, N.J.; van Nieuwenhoven, F.A.; Vernooy, K.; Prinzen, F.W. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace 2018, 20, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Delnoy, P.P.; Ottervanger, J.P.; Luttikhuis, H.O.; Nicastia, D.M.; Elvan, A.; Misier, A.R.; Beukema, W. Sustained benefit of cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2007, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Duckett, S.G.; Ginks, M.; Shetty, A.K.; Bostock, J.; Gill, J.S.; Hamid, S.; Kapetanakis, S.; Cunliffe, E.; Razavi, R.; Carr-White, G.; et al. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2011, 58, 1128–1136. [Google Scholar] [CrossRef]
- Kass, D.A.; Chen, C.H.; Curry, C.; Talbot, M.; Berger, R.; Fetics, B.; Nevo, E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999, 99, 1567–1573. [Google Scholar] [CrossRef]
- Kumari, R.; Ranjan, P.; Suleiman, Z.G.; Goswami, S.K.; Li, J.; Prasad, R.; Verma, S.K. mRNA modifications in cardiovascular biology and disease: With a focus on m6A modification. Cardiovasc. Res. 2022, 118, 1680–1692. [Google Scholar] [CrossRef]
- Thum, T.; Galuppo, P.; Kneitz, S.; Fiedler, J.; van Laake, L.; Mummery, C.; Ertl, G.; Bauersachs, J. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef]
- Zhu, L.; Li, N.; Sun, L.; Zheng, D.; Shao, G. Non-coding RNAs: The key detectors and regulators in cardiovascular disease. Genomics 2021, 113 Pt 2, 1233–1246. [Google Scholar] [CrossRef]
- Foglieni, C.; Lombardi, M.; Lazzeroni, D.; Zerboni, R.; Lazzarini, E.; Bertoli, G.; Pisano, A.; Girolami, F.; Andolfo, A.; Magagnotti, C.; et al. Myosins and MyomiR Network in Patients with Obstructive Hypertrophic Cardiomyopathy. Biomedicines 2022, 10, 2180. [Google Scholar] [CrossRef]
- Heymans, S.; Corsten, M.F.; Verhesen, W.; Carai, P.; van Leeuwen, R.E.; Custers, K.; Peters, T.; Hazebroek, M.; Stöger, L.; Wijnands, E.; et al. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation 2013, 128, 1420–1432. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007, 316, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Oslon, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Guedes, E.C.; França, G.S.; Lino, C.A.; Koyama, F.C.; Moreira, L.D.N.; Alexandre, J.G.; Barreto-Chaves, M.L.M.; Galante, P.A.F.; Diniz, G.P. MicroRNA Expression Signature Is Altered in the Cardiac Remodeling Induced by High Fat Diets. J. Cell. Physiol. 2016, 231, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Care, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Hong, C.; Chen, I.Y.; Lypowy, J.; Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 2007, 100, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol. Cell. Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef]
- Creemers, E.E.; van Rooij, E. Function and Therapeutic Potential of Noncoding RNAs in Cardiac Fibrosis. Circ. Res. 2016, 118, 108–118. [Google Scholar] [CrossRef]
- Strik, M.; van Middendorp, L.B.; Vernooy, K. Animal models of dyssynchrony. J. Cardiovasc. Transl. Res. 2012, 5, 135–145. [Google Scholar] [CrossRef]
- Gabisonia, K.; Khan, M.; Recchia, F.A. Extracellular vesicle-mediated bidirectional communication between heart and other organs. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H769–H784. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Liu, Y.; Li, J.; Yang, X.; Hu, R.; Liu, J.; Zhang, Y.; Zuo, K.; Li, K.; et al. Myofibroblast-Derived Exosomes Contribute to Development of a Susceptible Substrate for Atrial Fibrillation. Cardiology 2020, 145, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Jiao, H. LncRNA NRON promotes M2 macrophage polarization and alleviates atrial fibrosis through suppressing exosomal miR-23a derived from atrial myocytes. J. Formos. Med. Assoc. 2021, 120, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Moreira, D.A.R.; Gun, C.; Wang, H.L.; Hirata, M.H.; de Freitas Germano, J.; Leite, G.G.S.; Farsky, P. Analysis of Circulating miR-1, miR-23a, and miR-26a in Atrial Fibrillation Patients Undergoing Coronary Bypass Artery Grafting Surgery. Ann. Hum. Genet. 2017, 81, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, I.; Volinsky, N.; Grosman-Rimon, L.; Haviv, I.; Rozen, G.; Andria, N.; Asulin, N.; Margalit, N.; Marai, I.; Amir, O. Cardiac-peripheral transvenous gradients of microRNA expression in systolic heart failure patients. ESC Heart Fail. 2020, 7, 835–843. [Google Scholar] [CrossRef]
- Moscoso, I.; Cebro-Marquez, M.; Martinez-Gomez, A.; Abou-Jokh, C.; Martinez-Monzonis, M.A.; Martinez-Sande, J.L.; González-Melchor, L.; García-Seara, J.; Fernández-López, X.A.; Moraña-Fernández, S.; et al. Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy. Cells 2022, 11, 271. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.A.; et al. Circulating MicroRNA-30d Is Associated with Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 2015, 131, 2202–2216. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.; et al. Response to Letter Regarding Article, “Circulating MicroRNA-30d Is Associated with Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study”. Circulation 2016, 133, e389–e390. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Letter by Sardu et al Regarding Article, “Circulating MicroRNA-30d Is Associated with Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study”. Circulation 2016, 133, e388. [Google Scholar] [CrossRef] [PubMed]
- Bridge, G.; Monteiro, R.; Henderson, S.; Emuss, V.; Lagos, D.; Georgopoulou, D.; Patient, R.; Boshoff, C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood 2012, 120, 5063–5072. [Google Scholar] [CrossRef] [PubMed]
- Forini, F.; Kusmic, C.; Nicolini, G.; Mariani, L.; Zucchi, R.; Matteucci, M.; Iervasi, G.; Pitto, L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014, 155, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Van Stipdonk, A.M.W.; Ter Horst, I.; Kloosterman, M.; Engels, E.B.; Rienstra, M.; Crijns, H.J.G.M.; Vos, M.A.; van Gelder, I.C.; Prinzen, F.W.; Meine, M.; et al. QRS Area Is a Strong Determinant of Outcome in Cardiac Resynchronization Therapy. Circ. Arrhythm. Electrophysiol. 2018, 11, e006497. [Google Scholar] [CrossRef] [PubMed]
- Maffessanti, F.; Jadczyk, T.; Wilczek, J.; Conte, G.; Caputo, M.L.; Gołba, K.S.; Biernat, J.; Cybulska, M.; Caluori, G.; Regoli, F.; et al. Electromechanical factors associated with favourable outcome in cardiac resynchronization therapy. Europace 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Fyenbo, D.B.; Sommer, A.; Nørgaard, B.L.; Kronborg, M.B.; Kristensen, J.; Gerdes, C.; Jensen, H.K.; Jensen, J.M.; Nielsen, J.C. Long-term outcomes in a randomized controlled trial of multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy. Europace 2022, 24, 828–834. [Google Scholar] [CrossRef]
- Chen, X.; Ye, Y.; Wang, Z.; Jin, Q.; Qiu, Z.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: A prospective, multi-centre, observational study. Europace 2022, 24, 807–816. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Sieniewicz, B.; Gould, J.; Elliott, M.K.; Mehta, V.S.; Betts, T.R.; James, S.; Turley, A.J.; Butter, C.; Seifert, M.; et al. Leadless left ventricular endocardial pacing for CRT upgrades in previously failed and high-risk patients in comparison with coronary sinus CRT upgrades. Europace 2021, 23, 1577–1585. [Google Scholar] [CrossRef]
- Glikson, M.; Beinart, R.; Golovchiner, G.; Bar Sheshet, A.; Swissa, M.; Bolous, M.; Rosso, R.; Medina, A.; Haim, M.; Friedman, P.; et al. Radial strain imaging-guided lead placement for improving response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy: The Raise CRT trial. Europace 2022, 24, 835–844. [Google Scholar] [CrossRef]
- Moriña-Vázquez, P.; Moraleda-Salas, M.T.; Manovel-Sánchez, A.J.; Fernández-Gómez, J.M.; Arce-Léon, Á.; Venegas-Gamero, J.; Barba-Pichardo, R. Early improvement of left ventricular ejection fraction by cardiac resynchronization through His bundle pacing in patients with heart failure. Europace 2020, 22, 125–132. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Nieuwenhoven, F.A.; Schroen, B.; Barile, L.; van Middendorp, L.; Prinzen, F.W.; Auricchio, A. Plasma Extracellular Vesicles as Liquid Biopsy to Unravel the Molecular Mechanisms of Cardiac Reverse Remodeling Following Resynchronization Therapy? J. Clin. Med. 2023, 12, 665. https://doi.org/10.3390/jcm12020665
van Nieuwenhoven FA, Schroen B, Barile L, van Middendorp L, Prinzen FW, Auricchio A. Plasma Extracellular Vesicles as Liquid Biopsy to Unravel the Molecular Mechanisms of Cardiac Reverse Remodeling Following Resynchronization Therapy? Journal of Clinical Medicine. 2023; 12(2):665. https://doi.org/10.3390/jcm12020665
Chicago/Turabian Stylevan Nieuwenhoven, Frans A., Blanche Schroen, Lucio Barile, Lars van Middendorp, Frits W. Prinzen, and Angelo Auricchio. 2023. "Plasma Extracellular Vesicles as Liquid Biopsy to Unravel the Molecular Mechanisms of Cardiac Reverse Remodeling Following Resynchronization Therapy?" Journal of Clinical Medicine 12, no. 2: 665. https://doi.org/10.3390/jcm12020665
APA Stylevan Nieuwenhoven, F. A., Schroen, B., Barile, L., van Middendorp, L., Prinzen, F. W., & Auricchio, A. (2023). Plasma Extracellular Vesicles as Liquid Biopsy to Unravel the Molecular Mechanisms of Cardiac Reverse Remodeling Following Resynchronization Therapy? Journal of Clinical Medicine, 12(2), 665. https://doi.org/10.3390/jcm12020665






