Impact of Antifibrotic Treatment on Postoperative Complications in Patients with Interstitial Lung Diseases Undergoing Lung Transplantation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
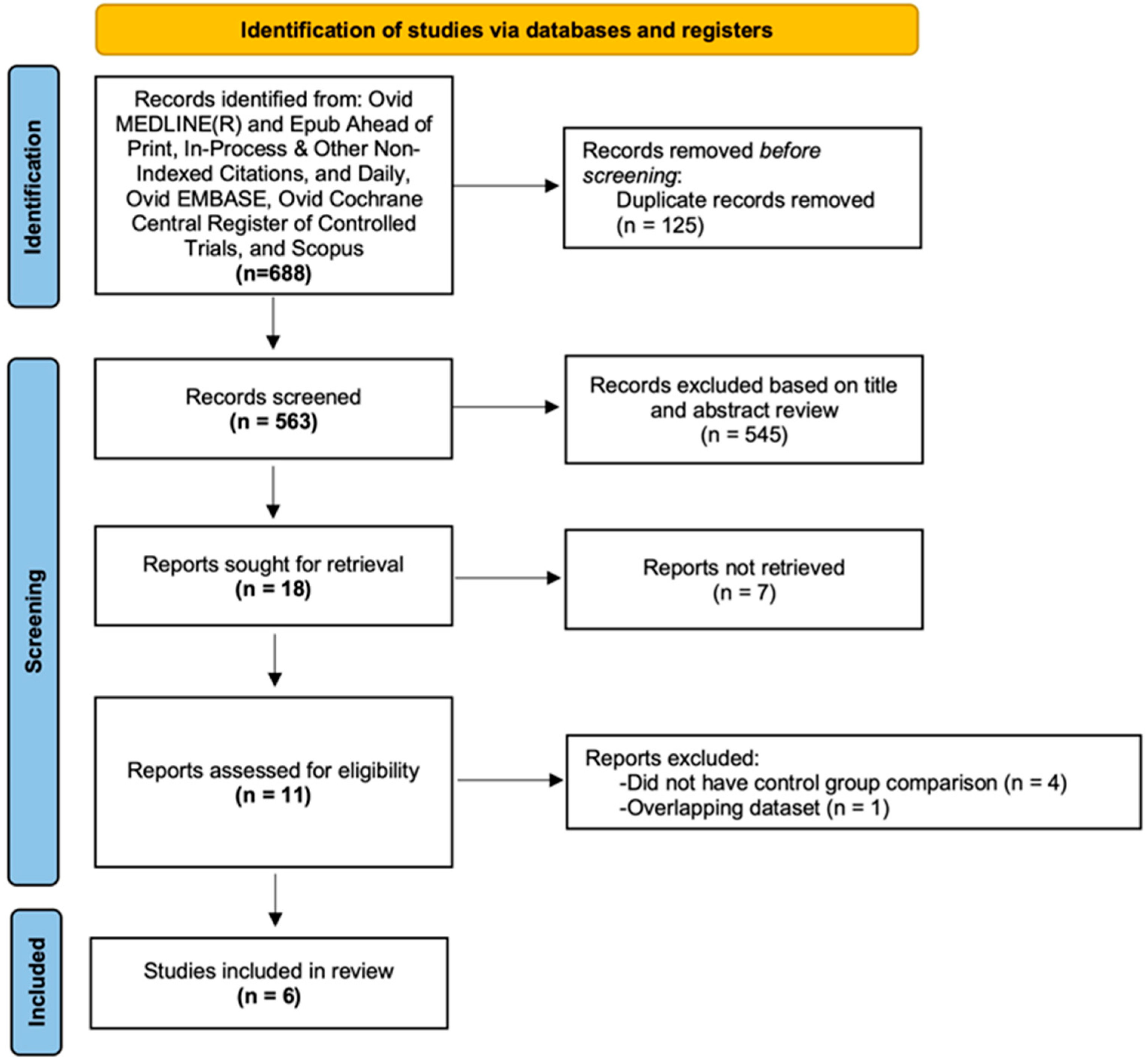
2.1. Search Strategy and Study Selection
2.2. Data Abstraction and Quality Assessment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Postoperative Complications
3.2. Impact of Each Antifibrotic Treatment on Postoperative Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.F.; Luppi, F. Diagnosis and Management of Fibrotic Interstitial Lung Diseases. Clin. Chest Med. 2021, 42, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.E.; Glaspole, I.; Grainge, C.; Goh, N.; Hopkins, P.M.; Moodley, Y.; Reynolds, P.N.; Chapman, S.; Walters, E.H.; Zappala, C.; et al. Baseline characteristics of idiopathic pulmonary fibrosis: Analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur. Respir. J. 2017, 49, 1601592. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Vasakova, M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.R.; Lancaster, L.H. Management of Progressive Fibrosing Interstitial Lung Diseases (PF-ILD). Front. Med. 2021, 8, 743977. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Delanote, I.; Wuyts, W.A.; Yserbyt, J.; Verbeken, E.K.; Verleden, G.M.; Vos, R. Safety and efficacy of bridging to lung transplantation with antifibrotic drugs in idiopathic pulmonary fibrosis: A case series. BMC Pulm. Med. 2016, 16, 156. [Google Scholar] [CrossRef]
- Wong, A.W.; Ryerson, C.J.; Guler, S.A. Progression of fibrosing interstitial lung disease. Respir. Res. 2020, 21, 32. [Google Scholar] [CrossRef]
- Crespo, M.M.; McCarthy, D.P.; Hopkins, P.M.; Clark, S.C.; Budev, M.; Bermudez, C.A.; Benden, C.; Eghtesady, P.; Lease, E.D.; Leard, L.; et al. ISHLT Consensus Statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. J. Heart Lung Transplant. 2018, 37, 548–563. [Google Scholar] [CrossRef]
- George, P.M.; Patterson, C.M.; Reed, A.K.; Thillai, M. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir. Med. 2019, 7, 271–282. [Google Scholar] [CrossRef]
- Miyahara, S.; Waseda, R.; Tokuishi, K.; Sato, T.; Iwasaki, A.; Shiraishi, T. Elucidation of prognostic factors and the effect of anti-fibrotic therapy on waitlist mortality in lung transplant candidates with idiopathic interstitial pneumonias. Respir. Investig. 2021, 59, 428–435. [Google Scholar] [CrossRef]
- Valapour, M.; Lehr, C.J.; Skeans, M.A.; Smith, J.M.; Miller, E.; Goff, R.; Foutz, J.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2019 Annual Data Report: Lung. Am. J. Transplant. 2021, 21 (Suppl. S2), 441–520. [Google Scholar] [CrossRef]
- Singer, J.P.; Katz, P.P.; Soong, A.; Shrestha, P.; Huang, D.; Ho, J.; Mindo, M.; Greenland, J.R.; Hays, S.R.; Golden, J.; et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am. J. Transplant. 2017, 17, 1334–1345. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Leuschner, G.; Stocker, F.; Veit, T.; Kneidinger, N.; Winter, H.; Schramm, R.; Weig, T.; Matthes, S.; Ceelen, F.; Arnold, P.; et al. Outcome of lung transplantation in idiopathic pulmonary fibrosis with previous anti-fibrotic therapy. J. Heart Lung Transplant. 2017, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Boehm, P.M.; Lee, S.; Ius, F.; Jaksch, P.; Klepetko, W.; Tudorache, I.; Ristl, R.; Welte, T.; Gottlieb, J. Effect of antifibrotics on short-term outcome after bilateral lung transplantation: A multicentre analysis. Eur. Respir. J. 2018, 51, 1800503. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, J.A.; Munsif, M.; Ranzenbacher, L.; Thomson, C.; Musk, M.; Snell, G.; Glanville, A.; Chambers, D.C.; Hopkins, P. Risk of anastomotic dehiscence in patients with pulmonary fibrosis transplanted while receiving anti-fibrotics: Experience of the Australian Lung Transplant Collaborative. J. Heart Lung Transplant. 2019, 38, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyoshi, K.; Higo, H.; Kurosaki, T.; Otani, S.; Sugimoto, S.; Yamane, M.; Kiura, K.; Toyooka, S.; Oto, T. Lung transplant candidates with idiopathic pulmonary fibrosis and long-term pirfenidone therapy: Treatment feasibility influences waitlist survival. Respir. Investig. 2019, 57, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dorey-Stein, Z.L.; Shapiro, W.; Zhao, H.; Cordova, F.C.; Criner, G.J.; Galli, J.A. Effect of antifibrotic therapy in patients with idiopathic pulmonary fibrosis undergoing lung transplant in the peri and post-operative period. Respir. Med. 2021, 190, 106599. [Google Scholar] [CrossRef]
- Graham, C.N.; Watson, C.; Barlev, A.; Stevenson, M.; Dharnidharka, V.R. Mean lifetime survival estimates following solid organ transplantation in the US and UK. J. Med. Econ. 2022, 25, 230–237. [Google Scholar] [CrossRef]
- Zhu, M.Z.L.; Huang, J.Y.; Liu, D.H.; Snell, G.I. Does continuation of antifibrotics before lung transplantation influence post-transplant outcomes in patients with idiopathic pulmonary fibrosis? Interact. Cardiovasc. Thorac. Surg. 2022, 34, 250–254. [Google Scholar] [CrossRef]
- Malas, J.; Ranganath, N.K.; Phillips, K.G.; Bittle, G.J.; Hisamoto, K.; Smith, D.E.; Lesko, M.B.; Angel, L.F.; Lonze, B.E.; Kon, Z.N. Early airway dehiscence: Risk factors and outcomes with the rising incidence of extracorporeal membrane oxygenation as a bridge to lung transplantation. J. Card. Surg. 2019, 34, 933–940. [Google Scholar] [CrossRef]
- Yserbyt, J.; Dooms, C.; Vos, R.; Dupont, L.J.; Van Raemdonck, D.E.; Verleden, G.M. Anastomotic airway complications after lung transplantation: Risk factors, treatment modalities and outcome-a single-centre experience. Eur. J. Cardiothorac. Surg. 2016, 49, e1–e8. [Google Scholar] [CrossRef]
- Wind, S.; Schmid, U.; Freiwald, M.; Marzin, K.; Lotz, R.; Ebner, T.; Stopfer, P.; Dallinger, C. Clinical Pharmacokinetics and Pharmacodynamics of Nintedanib. Clin. Pharmacokinet. 2019, 58, 1131–1147. [Google Scholar] [CrossRef]
- Meng, H.; Xu, Y. Pirfenidone-loaded liposomes for lung targeting: Preparation and in vitro/in vivo evaluation. Drug Des. Devel. Ther. 2015, 9, 3369–3376. [Google Scholar] [CrossRef]
- Saito, M.; Chen-Yoshikawa, T.F.; Suetsugu, K.; Okabe, R.; Takahagi, A.; Masuda, S.; Date, H. Pirfenidone alleviates lung ischemia-reperfusion injury in a rat model. J. Thorac. Cardiovasc. Surg. 2019, 158, 289–296. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]



| Studies | Number of Participants | Duration of Antifibrotic Treatment (Days) | LAS | Waitlist Time | Bilateral Transplantation | Intra-Operative ECMO | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antifibrotic | No Antifibrotic | Antifibrotic | No Antifibrotic | Antifibrotic | No Antifibrotic | Antifibrotic | No Antifibrotic | |||
| Delanote [12] | 7 ¶ 2 † | 6 | 419 ± 315 | 32.4 ± 2.8 ¶ 31.5 ± 0.7 † | 31.5 ± 3.4 | 155 (40–299) | 203 (88–275) | 6 ¶ 2 † | 5 | 1(7%) ¶ |
| Leuschner [21] | 23 ¶ 7 † | 32 | 591 ± 402 | 52.1 ± 15.5 ¶ 50.3 ± 18.8 † | 54.5 ± 16.7 | 122±369 | NA | 13 ¶ 1 † | 20 | 30 (48%) |
| Lambers [22] | 23 ¶ 13 † | 96 | NA | 38 (26–50) ¶ 37 (33–40) † | 39 (34–45) ‡ 37 (33–40) § | NA | NA | 23 ¶ 13 † | 96 | 74 (56%) |
| Mackintosh [23] | 29 ¶ 11 † | 186 | NA | NA | NA | 53 (6–655) * | 77 (0–1195) * | 34 | 159 | 146 (65%) |
| Tanaka [24] | 4 ¶ | 10 | 1356 (558–2004) | 35.2 (34–39) ¶ | 42.5 (33–56) | 438 (241–910) | 389 (8–1366) | 2 ¶ | 5 | NA |
| Dorey-Stein [25] | 28 ¶ 14 † | 52 | 579 ± 597 | 45.1 ± 14.1 | 53.9 ± 20.4 | 166 | 150 | 16 | 16 | 5 (5%) |
| Studies | Selection | Comparability | Outcome |
|---|---|---|---|
| Delanote et al. [12] | ★★★ | - | ★★★ |
| Leuschner et al. [21] | ★★★★ | - | ★★ |
| Lambers et al. [22] | ★★★★ | - | ★★ |
| Mackintosh et al. [23] | ★★★★ | - | ★★ |
| Tanaka et al. [24] | ★★★★ | - | ★★ |
| Dorey-Stein et al. [25] | ★★★★ | - | ★★★ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taweesedt, P.; Lertjitbanjong, P.; Eksombatchai, D.; Charoenpong, P.; Moua, T.; Thongprayoon, C.; Tangpanithandee, S.; Petnak, T. Impact of Antifibrotic Treatment on Postoperative Complications in Patients with Interstitial Lung Diseases Undergoing Lung Transplantation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 655. https://doi.org/10.3390/jcm12020655
Taweesedt P, Lertjitbanjong P, Eksombatchai D, Charoenpong P, Moua T, Thongprayoon C, Tangpanithandee S, Petnak T. Impact of Antifibrotic Treatment on Postoperative Complications in Patients with Interstitial Lung Diseases Undergoing Lung Transplantation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(2):655. https://doi.org/10.3390/jcm12020655
Chicago/Turabian StyleTaweesedt, Pahnwat, Ploypin Lertjitbanjong, Dararat Eksombatchai, Prangthip Charoenpong, Teng Moua, Charat Thongprayoon, Supawit Tangpanithandee, and Tananchai Petnak. 2023. "Impact of Antifibrotic Treatment on Postoperative Complications in Patients with Interstitial Lung Diseases Undergoing Lung Transplantation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 2: 655. https://doi.org/10.3390/jcm12020655
APA StyleTaweesedt, P., Lertjitbanjong, P., Eksombatchai, D., Charoenpong, P., Moua, T., Thongprayoon, C., Tangpanithandee, S., & Petnak, T. (2023). Impact of Antifibrotic Treatment on Postoperative Complications in Patients with Interstitial Lung Diseases Undergoing Lung Transplantation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(2), 655. https://doi.org/10.3390/jcm12020655








