Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer
Abstract
1. Introduction
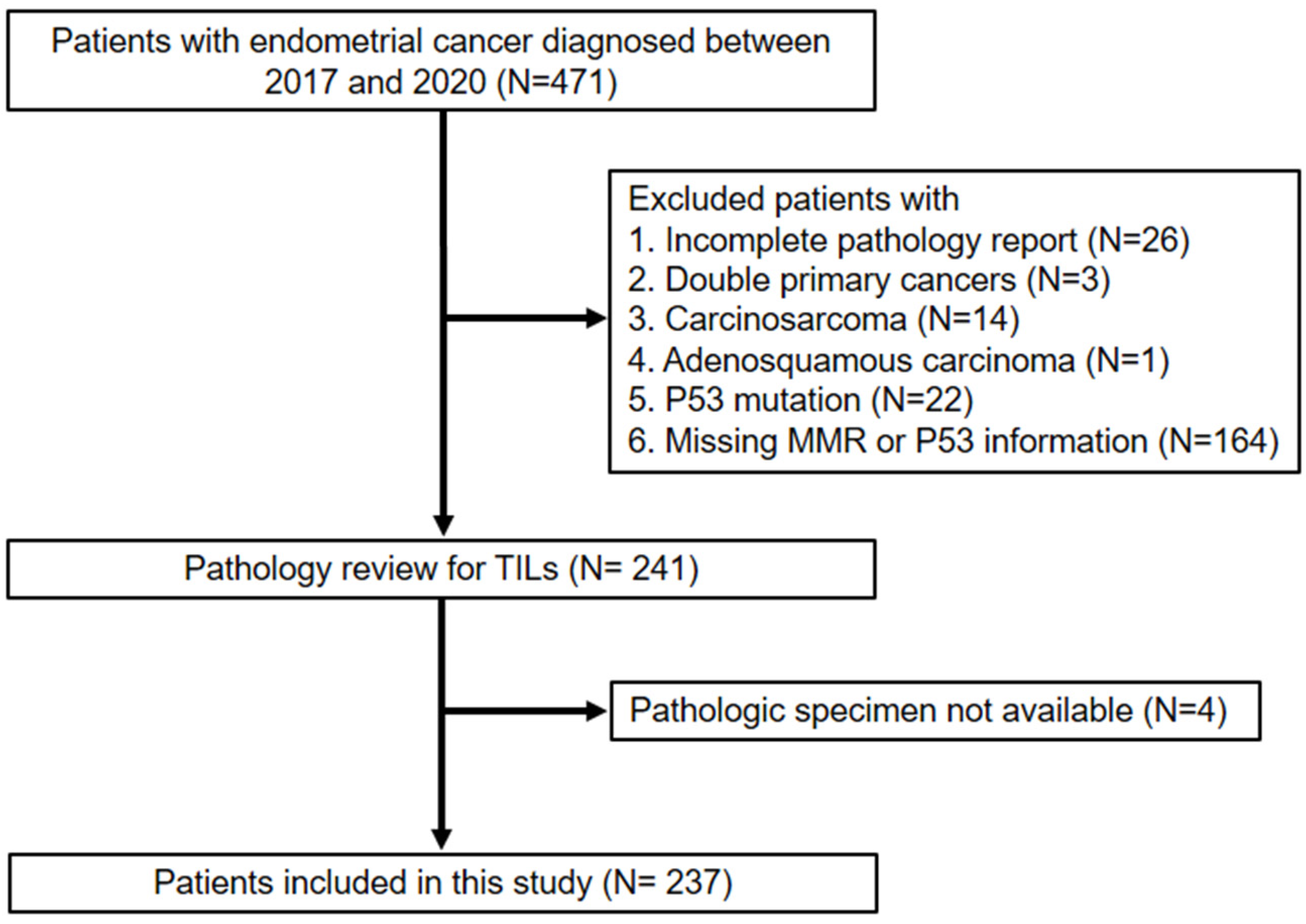
2. Materials and Methods
Statistical Analysis
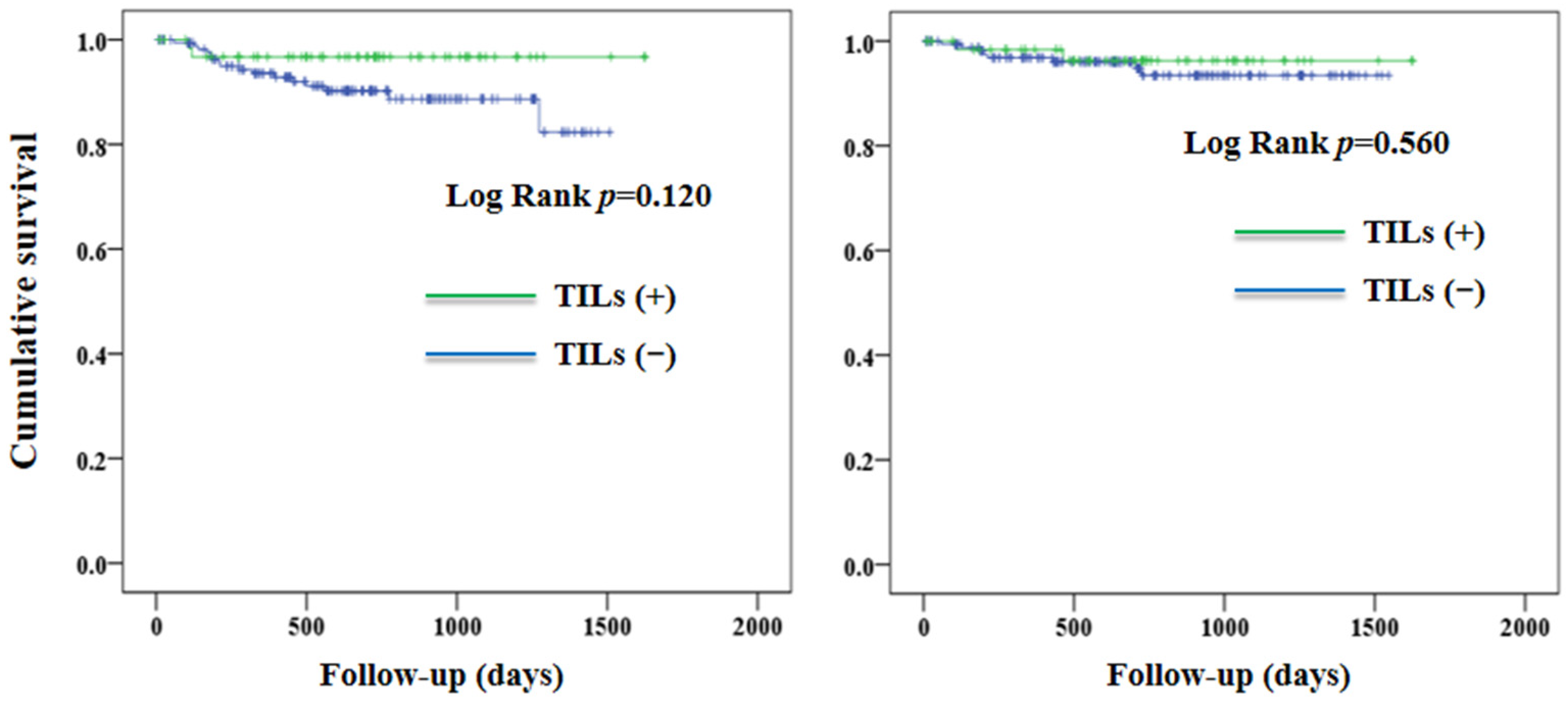
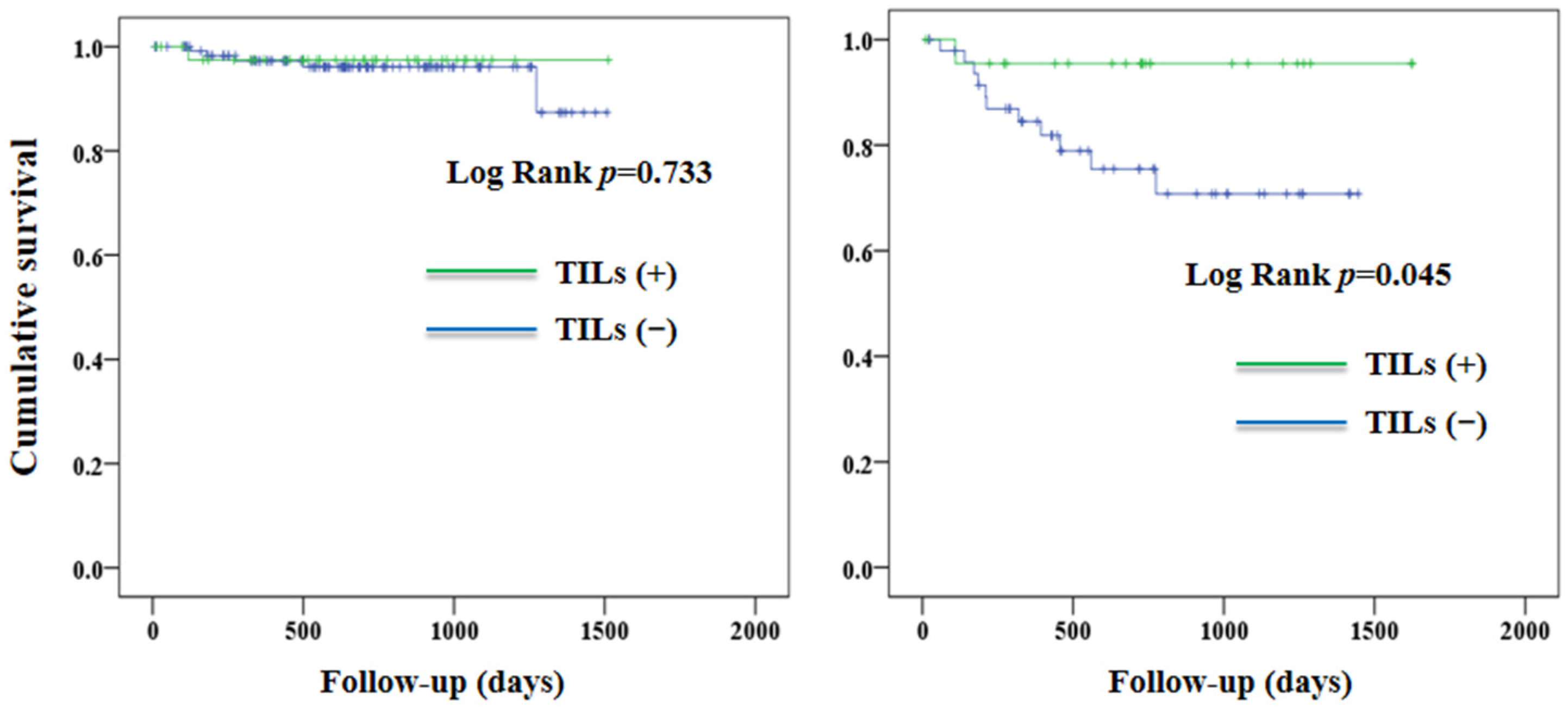
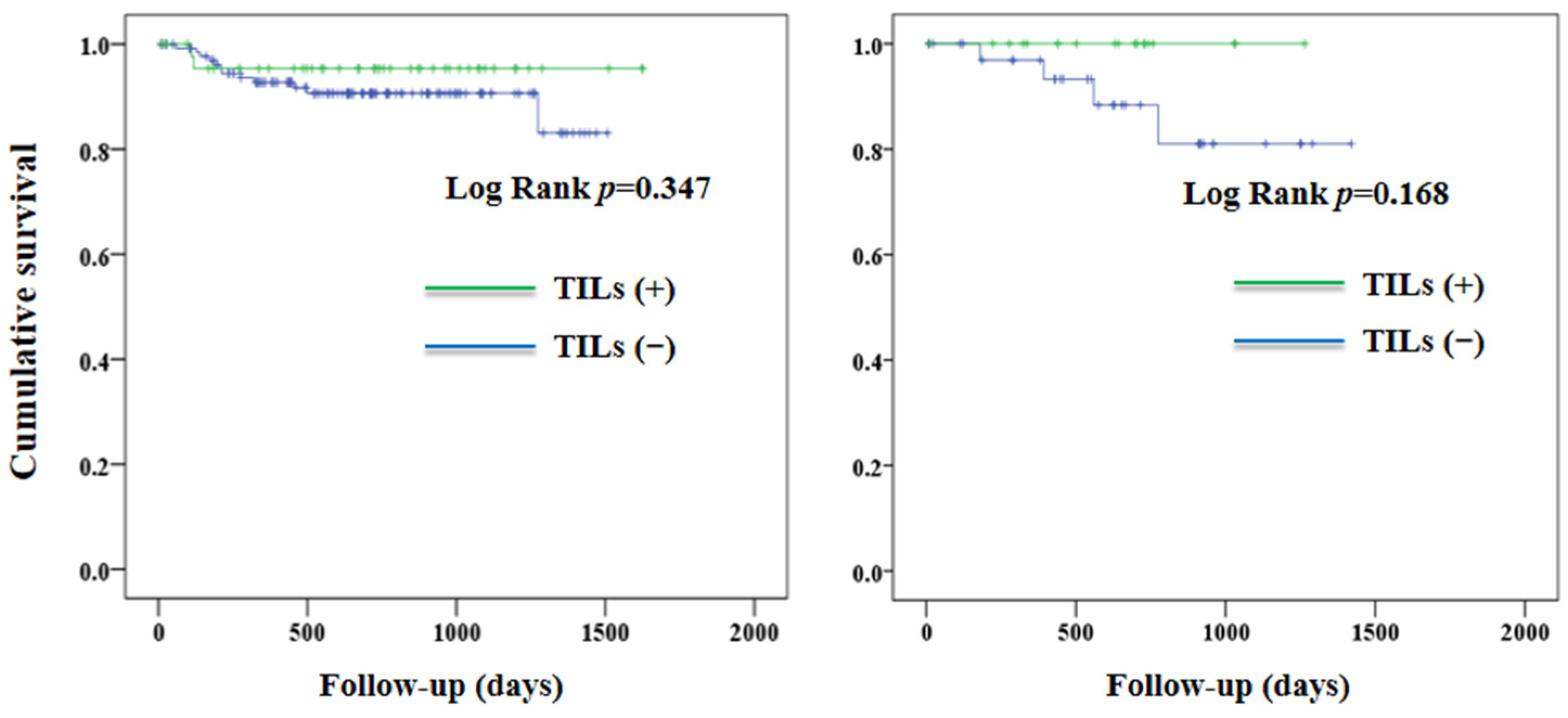
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Takenaka, K.; Olzomer, E.M.; Hoehn, K.L.; Curry-Hyde, A.; Jun Chen, B.; Farrell, R.; Byrne, F.L.; Janitz, M. Investigation of circular RNA transcriptome in obesity-related endometrial cancer. Gene 2022, 855, 147125. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Chatterji, S.; Byles, J.; Cutler, D.; Seeman, T.; Verdes, E. Health, functioning, and disability in older adults--present status and future implications. Lancet 2015, 385, 563–575. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Rodríguez-Palacios, D.Á.; Colorado-Yohar, S.M.; Velten, M.; Vaamonde-Martín, R.J.; Ballesta, M.; Chirlaque, M.D. Incidence and Trend of Type I and II Endometrial Cancer in Women from Two Population-Based European Cancer Registries (1998–2012). Int. J. Environ. Res. Public Health 2022, 19, 3789. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Talhouk, A.; McAlpine, J.N. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol. Oncol. Res. Pract. 2016, 3, 14. [Google Scholar] [CrossRef]
- Zal, T.; Chodaczek, G. Intravital imaging of anti-tumor immune response and the tumor microenvironment. Semin. Immunopathol. 2010, 32, 305–317. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Tan, Q.; Kong, J.; Yu, B. Tissue Infiltrating Immune Cells as Prognostic Biomarkers in Endometrial Cancer: A Meta-Analysis. Dis. Markers 2020, 2020, 1805764. [Google Scholar] [CrossRef]
- Willvonseder, B.; Stögbauer, F.; Steiger, K.; Jesinghaus, M.; Kuhn, P.H.; Brambs, C.; Engel, J.; Bronger, H.; Schmidt, G.P.; Haller, B.; et al. The immunologic tumor microenvironment in endometrioid endometrial cancer in the morphomolecular context: Mutual correlations and prognostic impact depending on molecular alterations. Cancer Immunol. Immunother. 2021, 70, 1679–1689. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hada, T.; Ishibashi, H.; Iwahashi, H.; Kakimoto, S.; Suzuki, R.; Sakamoto, T.; Matsuura, H.; Tsuda, H.; Takano, M. A New Model to Improve the Prediction of Prognosis of Endometrial Carcinoma by Combining Traditional Classification with the Presence of Tumor-infiltrating Lymphocytes. Anticancer Res. 2021, 41, 1047–1053. [Google Scholar] [CrossRef]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Gilks, C.B.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van De Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar]
- Shia, J.; Black, D.; Hummer, A.J.; Boyd, J.; Soslow, R.A. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum. Pathol. 2008, 39, 116–125. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.; de Jong, R.A.; Hoogeboom, B.N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- de Jong, R.A.; Leffers, N.; Boezen, H.M.; ten Hoor, K.A.; van der Zee, A.G.; Hollema, H.; Nijman, H.W. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol. Oncol. 2009, 114, 105–110. [Google Scholar] [CrossRef]
- Čermáková, P.; Melichar, B.; Tomšová, M.; Zoul, Z.; Kalábová, H.; Spaček, J.; Doležel, M. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Res. 2014, 34, 5555–5561. [Google Scholar]
- Kono-Sato, T.; Miyai, K.; Yamagishi, Y.; Miyamoto, M.; Takano, M.; Matsukuma, S.; Sato, K.; Tsuda, H. Intraepithelial lymphocytes are indicators of better prognosis in surgically resected endometrioid-type endometrial carcinomas at early and advanced stages. BMC Cancer 2022, 22, 361. [Google Scholar] [CrossRef]
- Bounous, V.E.; Ferrero, A.; Campisi, P.; Fuso, L.; Pezua Sanjinez, J.O.S.; Manassero, S.; De Rosa, G.; Biglia, N. Immunohistochemical Markers and TILs Evaluation for Endometrial Carcinoma. J. Clin. Med. 2022, 11, 5678. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; Loiero, D.; Duiker, E.W.; Bart, J.; Hendriks, A.M.; Jalving, M.; Workel, H.H.; Hollema, H.; Werner, N.; et al. Prognostic image-based quantification of CD8CD103 T cell subsets in high-grade serous ovarian cancer patients. Oncoimmunology 2021, 10, 1935104. [Google Scholar] [CrossRef]
- Kondratiev, S.; Sabo, E.; Yakirevich, E.; Lavie, O.; Resnick, M.B. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin. Cancer Res. 2004, 10, 4450–4456. [Google Scholar] [CrossRef]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D'Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Hussein, Y.R.; Weigelt, B.; Levine, D.A.; Schoolmeester, J.K.; Dao, L.N.; Balzer, B.L.; Liles, G.; Karlan, B.; Köbel, M.; Lee, C.H.; et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod. Pathol. 2015, 28, 505–514. [Google Scholar] [CrossRef]
- Ino, K.; Yamamoto, E.; Shibata, K.; Kajiyama, H.; Yoshida, N.; Terauchi, M.; Nawa, A.; Nagasaka, T.; Takikawa, O.; Kikkawa, F. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: Its association with disease progression and survival. Clin. Cancer Res. 2008, 14, 2310–2317. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin. Cancer Biol. 2006, 16, 3–15. [Google Scholar] [CrossRef]

| Variables | TILs (−) | TILs (+) | p |
|---|---|---|---|
| Number of patients | 172 | 65 | |
| Age, years | 54.4 ± 10.5 | 55.7 ± 9.5 | 0.391 |
| Body mass index, kg/m2 | 26.6 ± 5.9 | 25.6 ± 5.9 | 0.257 |
| Nulliparous, n (%) | 50 (29.1) | 21 (32.3) | 0.627 |
| Cell type, n (%) | 0.353 | ||
| Endometrioid | 168 (97.7) | 62 (95.4) | |
| Others | 4 (2.3) | 3 (4.6) | |
| High-grade, n (%) a | 49 (28.5) | 23 (35.4) | 0.303 |
| Tumor size ≥ 20 mm, n (%) | 133 (77.3) | 56 (86.2) | 0.131 |
| Myometrial infiltration ≥ 50%, n (%) | 51 (29.7) | 17 (26.2) | 0.595 |
| Lymphovascular space invasion, n (%) | 50 (29.1) | 21 (32.3) | 0.652 |
| Lymph node metastasis, n (%) | 18 (10.5) | 10 (15.4) | 0.218 |
| Pathology stage, n (%) | 0.795 | ||
| Stage 1 | 135 (78.5) | 50 (76.9) | |
| Stage 2–4 | 37 (21.5) | 15 (23.1) | |
| Estrogen receptor-positive, n (%) | 164 (95.3) | 59 (90.8) | 0.168 |
| Progesterone receptor-positive, n (%) | 151 (87.8) | 55 (84.6) | 0.123 |
| MMR-deficient, n (%) | 36 (20.9) | 19 (29.2) | 0.177 |
| Radiotherapy, n (%) | 41 (23.8) | 17 (26.2) | 0.711 |
| Chemotherapy, n (%) | 33 (19.2) | 16 (24.6) | 0.357 |
| Independent variable: TILs (+ vs. −) | Hazard Ratio (95% CI) a | p |
|---|---|---|
| Overall | ||
| Disease progression or death | 0.358 (0.076, 1.684) | 0.193 |
| All-cause mortality | 0.907 (0.128, 6.423) | 0.922 |
| In high-grade subgroup | ||
| Disease progression or death | 0.265 (0.027, 2.553) | 0.250 |
| All-cause mortality | 0.682 (0.051, 9.106) | 0.772 |
| In MMR-deficient subgroup | ||
| Disease progression or death | 0.012 (0, >999) | 0.775 |
| All-cause mortality | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.-T.; Hsu, S.-T.; Sun, L.; Hwang, S.-F.; Liu, C.-K.; Shih, Y.-H.; Chen, M.-J.; Li, H.-N.; Wang, J.-S.; Wen, M.-C.; et al. Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer. J. Clin. Med. 2023, 12, 603. https://doi.org/10.3390/jcm12020603
Fan C-T, Hsu S-T, Sun L, Hwang S-F, Liu C-K, Shih Y-H, Chen M-J, Li H-N, Wang J-S, Wen M-C, et al. Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer. Journal of Clinical Medicine. 2023; 12(2):603. https://doi.org/10.3390/jcm12020603
Chicago/Turabian StyleFan, Chun-Ting, Shih-Tien Hsu, Lou Sun, Sheau-Feng Hwang, Chih-Ku Liu, Yu-Hsiang Shih, Ming-Jer Chen, Hsin-Ni Li, Jun-Sing Wang, Mei-Chin Wen, and et al. 2023. "Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer" Journal of Clinical Medicine 12, no. 2: 603. https://doi.org/10.3390/jcm12020603
APA StyleFan, C.-T., Hsu, S.-T., Sun, L., Hwang, S.-F., Liu, C.-K., Shih, Y.-H., Chen, M.-J., Li, H.-N., Wang, J.-S., Wen, M.-C., & Lu, C.-H. (2023). Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer. Journal of Clinical Medicine, 12(2), 603. https://doi.org/10.3390/jcm12020603





