Recurrence and Mortality Risks in Patients with First Incident Acute Stroke or Myocardial Infarction: A Longitudinal Study Using the Korean National Health Insurance Service Database
Abstract
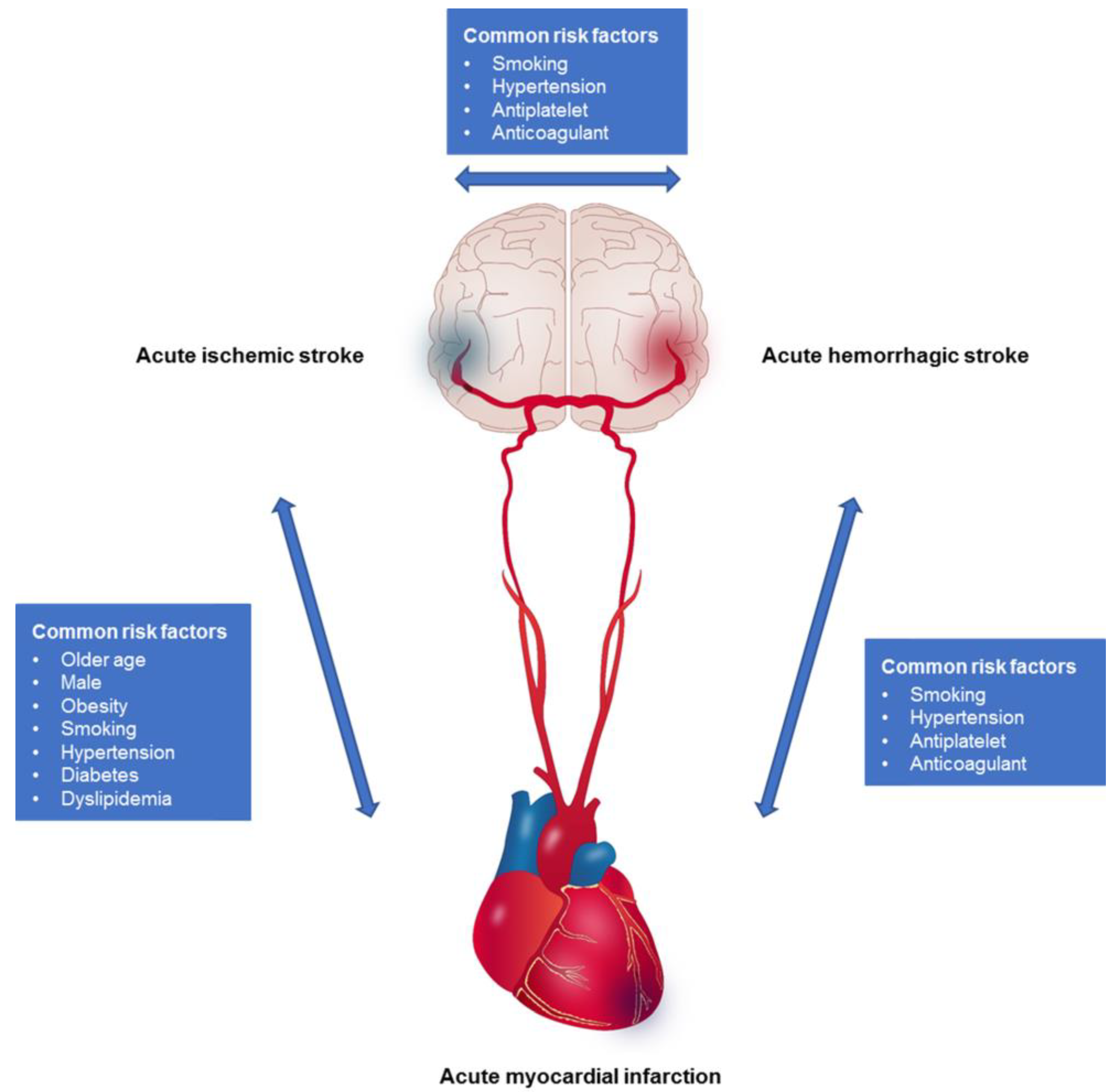
1. Introduction
2. Materials and Methods
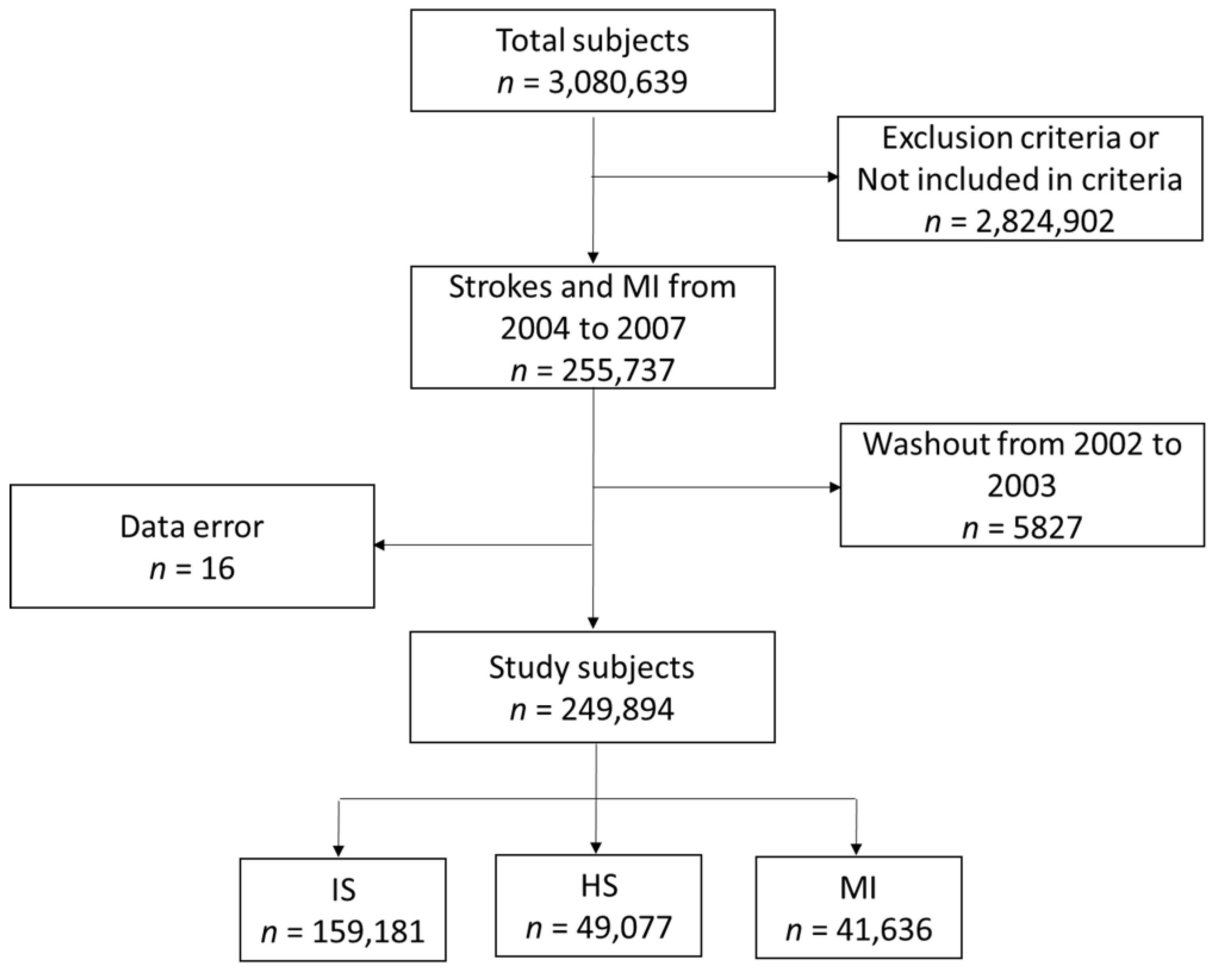
2.1. Participants and Study Design
2.2. Variable Definitions
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cox Proportional Hazards Models
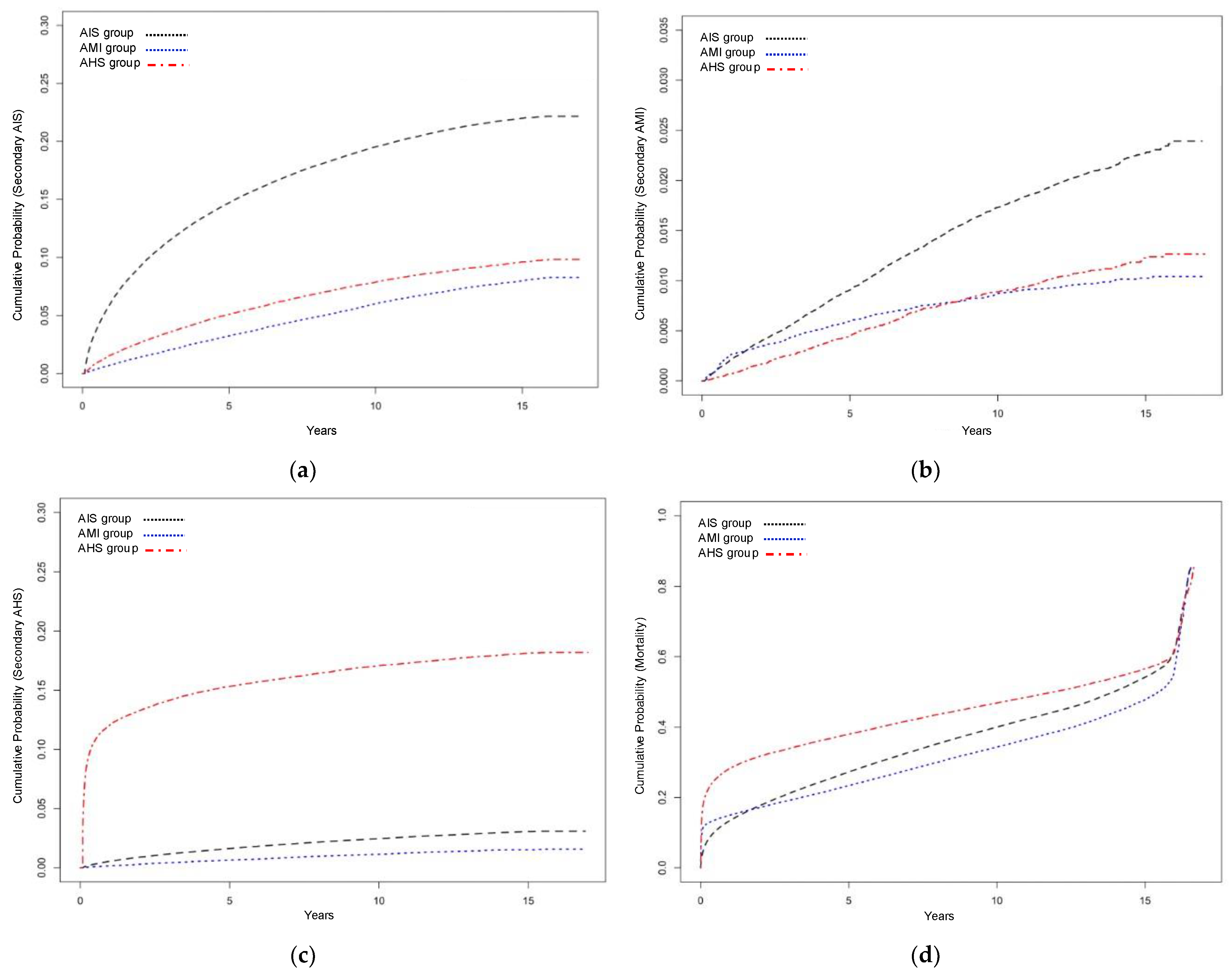
3.2.1. Secondary Acute Ischemic Stroke
3.2.2. Secondary Acute Myocardial Infarction
3.2.3. Secondary Acute Hemorrhagic Stroke
3.2.4. Long-Term Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wright, N.; Guo, Y.; Turnbull, I.; Kartsonaki, C.; Yang, L.; Bian, Z.; Pei, P.; Pan, D.; Zhang, Y.; et al. Mortality and recurrent vascular events after first incident stroke: A 9-year community-based study of 0.5 million Chinese adults. Lancet Glob. Health 2020, 8, e580–e590. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. Overview of Cardiac Rehabilitation and Current Situations in Korea. Ann. Cardiopulm. Rehabil. 2021, 1, 6–16. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, K.; Kang, J.; Koo, J.; Kim, D.H.; Kim, B.J.; Kim, W.J.; Kim, E.G.; Kim, J.G.; Kim, J.M.; et al. Executive summary of stroke statistics in Korea 2018: A report from the Epidemiology Research Council of the Korean Stroke society. J. Stroke 2019, 21, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Papi, G.; Insalata, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Comparison between ischemic and hemorrhagic strokes in functional outcome at discharge from an intensive rehabilitation hospital. Diagnostics 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Cerniauskaite, M.; Quintas, R.; Koutsogeorgou, E.; Meucci, P.; Sattin, D.; Leonardi, M.; Raggi, A. Quality-of-life and disability in patients with stroke. Am. J. Phys. Med. Rehabil. 2012, 91, S39–S47. [Google Scholar] [CrossRef]
- Johnson, C.O.; Kandel, H.; Nguyen, T.H.; Yadav, L.; GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Kim, R.B.; Kim, H.S.; Kang, D.R.; Choi, J.Y.; Choi, N.C.; Hwang, S.; Hwang, J.Y. The trend in incidence and case-fatality of hospitalized acute myocardial infarction patients in Korea, 2007 to 2016. J. Korean Med. Sci. 2019, 34, e322. [Google Scholar] [CrossRef]
- Kim, Y.; Ahn, Y.; Cho, M.C.; Kim, C.J.; Kim, Y.J.; Jeong, M.H. Current status of acute myocardial infarction in Korea. Korean J Intern Med 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Urbinati, S.; Perugini, E.; Gambetti, S. Interactions between cardiovascular and cerebrovascular disease. Curr. Treat. Options Neurol. 2012, 14, 557–593. [Google Scholar] [CrossRef]
- Boulanger, M.; Li, L.; Lyons, S.; Lovett, N.G.; Kubiak, M.M.; Silver, L.; Touze, E.; Rothwell, P.M. Essen risk score in prediction of myocardial infarction after transient ischemic attack or ischemic stroke without prior coronary artery disease. Stroke 2019, 50, 3393–3399. [Google Scholar] [CrossRef]
- Otite, F.O.; Liaw, N.; Khandelwal, P.; Malik, A.M.; Romano, J.G.; Rundek, T.; Sacco, R.L.; Chaturvedi, S. Increasing prevalence of vascular risk factors in patients with stroke: A call to action. Neurology 2017, 89, 1985–1994. [Google Scholar] [CrossRef]
- Caldwell, M.; Martinez, L.; Foster, J.G.; Sherling, D.; Hennekens, C.H. Prospects for the primary prevention of myocardial infarction and stroke. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 207–214. [Google Scholar] [CrossRef]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Grysiewicz, R.A.; Thomas, K.; Pandey, D.K. Epidemiology of ischemic and hemorrhagic stroke: Incidence, prevalence, mortality, and risk factors. Neurol. Clin. 2008, 26, 871–895. [Google Scholar] [CrossRef]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef]
- Chen, P.C.; Lip, G.Y.; Yeh, G.; Lin, H.J.; Chien, K.L. Risk of bleeding and stroke with oral anticoagulation and antiplatelet therapy in patients with atrial fibrillation in Taiwan: A nationwide cohort study. PLoS ONE 2015, 10, e0125257. [Google Scholar] [CrossRef]
- Cha, Y.J. The Economic burden of stroke based on South Korea's National Health Insurance Claims database. Int. J. Health Policy Manag. 2018, 7, 904–909. [Google Scholar] [CrossRef]
- Seo, H.; Yoon, S.J.; Yoon, J.; Kim, D.; Gong, Y.; Kim, A.R.; Oh, I.H.; Kim, E.J.; Lee, Y.H. Recent trends in economic burden of acute myocardial infarction in South Korea. PLoS ONE 2015, 10, e0117446. [Google Scholar] [CrossRef]
- Chan, S.; Hemphill, J.C., 3rd. Critical care management of intracerebral hemorrhage. Crit. Care Clin. 2014, 30, 699–717. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; Tai, W.; Boden-Albala, B.; Rundek, T.; Paik, M.C.; Sacco, R.L.; Elkind, M.S. Risk of myocardial infarction or vascular death after first ischemic stroke: The Northern Manhattan Study. Stroke 2007, 38, 1752–1758. [Google Scholar] [CrossRef]
- Touze, E.; Varenne, O.; Chatellier, G.; Peyrard, S.; Rothwell, P.M.; Mas, J.L. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: A systematic review and meta-analysis. Stroke 2005, 36, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- Calvet, D.; Song, D.; Yoo, J.; Turc, G.; Sablayrolles, J.L.; Choi, B.W.; Heo, J.H.; Mas, J.L. Predicting asymptomatic coronary artery disease in patients with ischemic stroke and transient ischemic attack: The PRECORIS score. Stroke 2014, 45, 82–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J.; Chimowitz, M.I.; Alpert, J.S.; Awad, I.A.; Cerqueria, M.D.; Fayad, P.; Taubert, K.A. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Stroke 2003, 34, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Lavallee, P.C.; Labreuche, J.; Ducrocq, G.; Juliard, J.M.; Feldman, L.; Cabrejo, L.; Meseguer, E.; Guidoux, C.; Adrai, V.; et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke 2013, 44, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.; MacGregor, L.; Lees, K.R.; Diener, H.C.; Hacke, W.; Davis, S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007, 38, 2295–2302. [Google Scholar] [CrossRef]
- Kang, K.; Park, T.H.; Kim, N.; Jang, M.U.; Park, S.S.; Park, J.M.; Ko, Y.; Lee, S.; Lee, K.B.; Lee, J.; et al. Recurrent stroke, myocardial infarction, and major vascular events during the first year after acute ischemic stroke: The multicenter prospective observational study about recurrence and its determinants after acute ischemic stroke I. J. Stroke Cerebrovasc. Dis. 2016, 25, 656–664. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, S.E.; Kim, J.Y.; Kang, J.; Kim, B.J.; Han, M.K.; Choi, K.H.; Kim, J.T.; Shin, D.I.; Cha, J.K.; et al. Five-year risk of acute myocardial infarction after acute ischemic stroke in Korea. J. Am. Heart Assoc. 2021, 10, e018807. [Google Scholar] [CrossRef]
- Boulanger, M.; Bejot, Y.; Rothwell, P.M.; Touze, E. Long-term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: Systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e007267. [Google Scholar] [CrossRef]
- Gentil, A.; Bejot, Y.; Lorgis, L.; Durier, J.; Zeller, M.; Osseby, G.V.; Dentan, G.; Beer, J.C.; Moreau, T.; Giroud, M.; et al. Comparative epidemiology of stroke and acute myocardial infarction: The Dijon Vascular project (Diva). J. Neurol. Neurosurg. Psychiatry 2009, 80, 1006–1011. [Google Scholar] [CrossRef]
- Millett, E.R.C.; Peters, S.A.E.; Woodward, M. Sex differences in risk factors for myocardial infarction: Cohort study of UK Biobank participants. BMJ 2018, 363, k4247. [Google Scholar] [CrossRef]
- Merkler, A.E.; Diaz, I.; Wu, X.; Murthy, S.B.; Gialdini, G.; Navi, B.B.; Yaghi, S.; Weinsaft, J.W.; Okin, P.M.; Safford, M.M.; et al. Duration of heightened ischemic stroke risk after acute myocardial infarction. J. Am. Heart Assoc. 2018, 7, e010782. [Google Scholar] [CrossRef]
- Putaala, J.; Nieminen, T. Stroke risk period after acute myocardial infarction revised. J. Am. Heart Assoc. 2018, 7, e011200. [Google Scholar] [CrossRef]
- Brammas, A.; Jakobsson, S.; Ulvenstam, A.; Mooe, T. Mortality after ischemic stroke in patients with acute myocardial infarction: Predictors and trends over time in Sweden. Stroke 2013, 44, 3050–3055. [Google Scholar] [CrossRef]
- Devgun, J.K.; Gul, S.; Mohananey, D.; Jones, B.M.; Hussain, M.S.; Jobanputra, Y.; Kumar, A.; Svensson, L.G.; Tuzcu, E.M.; Kapadia, S.R. Cerebrovascular events after cardiovascular procedures: Risk factors, recognition, and prevention strategies. J. Am. Coll. Cardiol. 2018, 71, 1910–1920. [Google Scholar] [CrossRef]
- Rymer, M.M. Hemorrhagic stroke: Intracerebral hemorrhage. Mo. Med. 2011, 108, 50–54. [Google Scholar]
- Murthy, S.B.; Diaz, I.; Wu, X.; Merkler, A.E.; Iadecola, C.; Safford, M.M.; Sheth, K.N.; Navi, B.B.; Kamel, H. Risk of arterial ischemic events after intracerebral hemorrhage. Stroke 2020, 51, 137–142. [Google Scholar] [CrossRef]
- Casolla, B.; Moulin, S.; Kyheng, M.; Hénon, H.; Labreuche, J.; Leys, D.; Bauters, C.; Cordonnier, C. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke 2019, 50, 1100–1107. [Google Scholar] [CrossRef]
- Binsell-Gerdin, E.; Graipe, A.; Ögren, J.; Jernberg, T.; Mooe, T. Hemorrhagic stroke the first 30 days after an acute myocardial infarction: Incidence, time trends and predictors of risk. Int. J. Cardiol. 2014, 176, 133–138. [Google Scholar] [CrossRef]
- Weimar, C.; Kleine-Borgmann, J. Epidemiology, prognosis and prevention of non-traumatic intracerebral hemorrhage. Curr. Pharm. Des. 2017, 23, 2193–2196. [Google Scholar] [CrossRef]
- Diener, H.C.; Hankey, G.J. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef] [PubMed]
- Poortaghi, S.; Raiesifar, A.; Bozorgzad, P.; Golzari, S.E.; Parvizy, S.; Rafii, F. Evolutionary concept analysis of health seeking behavior in nursing: A systematic review. BMC Health Serv. Res. 2015, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Moledina, A.; Tang, K.L. Socioeconomic status, mortality, and access to cardiac services after acute myocardial infarction in Canada: A systematic review and meta-analysis. CJC Open 2021, 3, 950–964. [Google Scholar] [CrossRef]
| Variables | AIS Group (n = 159,181) | AMI Group (n = 41,636) | AHS Group (n = 49,077) | p Value |
|---|---|---|---|---|
| Age, years | 68.5 ± 11.5 | 64.3 ± 12.2 | 67.6 ± 11.2 | <0.001 |
| Male, n (%) | 81,047 (50.9) | 28,008 (67.3) | 23,064 (47.0) | <0.001 |
| Diabetes, n (%) | 46,152 (29.0) | 12,976 (31.2) | 7547 (15.4) | <0.001 |
| Hypertension, n (%) | 149,622 (94.0) | 40,013 (96.1) | 41,557 (84.7) | <0.001 |
| Dyslipidemia, n (%) | 76,647 (48.2) | 32,643 (78.4) | 13,177 (26.8) | <0.001 |
| SES, n (%) | <0.001 | |||
| Low | 53,184 (33.4) | 11,971 (28.8) | 15,442 (31.5) | |
| Middle | 47,244 (29.7) | 13,209 (31.7) | 15,665 (31.9) | |
| High | 58,753 (36.9) | 16,456 (39.5) | 17,970 (36.6) |
| Variables | Adjusted HR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Initial event | |||
| AIS | 1.000 | ||
| AMI | 0.318 | 0.306–0.330 | <0.001 |
| AHS a | 0.489 | 0.472–0.506 | <0.001 |
| Comorbidities | |||
| Diabetes | 1.310 | 1.283–1.338 | <0.001 |
| Hypertension | 1.026 | 0.970–1.085 | 0.377 |
| Dyslipidemia | 0.928 | 0.909–0.948 | <0.001 |
| SES | |||
| Low | 1.000 | ||
| Middle | 0.883 | 0.862–0.905 | <0.001 |
| High | 0.837 | 0.817–0.857 | <0.001 |
| Age (per year) | 1.018 | 1.017–1.019 | <0.001 |
| Female | 0.865 | 0.847–0.883 | <0.001 |
| Variables | Adjusted HR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Initial event | |||
| AIS | 1.000 | ||
| AMI | 0.388 | 0.348–0.433 | <0.001 |
| AHS a | 0.711 | 0.640–0.790 | <0.001 |
| Comorbidities | |||
| Diabetes | 1.666 | 1.555–1.785 | <0.001 |
| Hypertension | 0.796 | 0.657–0.965 | 0.020 |
| Dyslipidemia | 1.221 | 1.136–1.313 | <0.001 |
| SES | |||
| Low | 1.000 | ||
| Middle | 0.939 | 0.864–1.020 | 0.137 |
| High | 0.901 | 0.832–0.976 | 0.011 |
| Age (per year) | 1.013 | 1.010–1.017 | <0.001 |
| Female | 0.625 | 0.582–0.671 | <0.001 |
| Variables | Adjusted HR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Initial event | |||
| AIS | 1.000 | ||
| AMI | 0.555 | 0.508–0.607 | <0.001 |
| AHS | 8.546 | 8.218–8.887 | <0.001 |
| Comorbidities | |||
| Diabetes | 0.965 | 0.923–1.009 | 0.120 |
| Hypertension | 0.687 | 0.646–0.731 | <0.001 |
| Dyslipidemia | 0.842 | 0.811–0.875 | <0.001 |
| SES | |||
| Low | 1.000 | ||
| Middle | 1.005 | 0.962–1.049 | 0.838 |
| High | 1.013 | 0.971–1.056 | 0.550 |
| Age (per year) | 0.998 | 0.996–0.999 | 0.007 |
| Female | 1.132 | 1.092–1.173 | <0.001 |
| Variables | Adjusted HR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Initial event | |||
| AIS | 1.000 | ||
| AMI a | 1.436 | 1.412–1.461 | <0.001 |
| AHS | 1.328 | 1.309–1.348 | <0.001 |
| Comorbidities | |||
| Diabetes | 1.339 | 1.321–1.357 | <0.001 |
| Hypertension | 0.406 | 0.398–0.414 | <0.001 |
| Dyslipidemia | 0.582 | 0.574–0.590 | <0.001 |
| SES | |||
| Low | 1.000 | ||
| Middle | 0.939 | 0.926–0.953 | <0.001 |
| High | 0.846 | 0.834–0.857 | <0.001 |
| Age (per year) | 1.092 | 1.091–1.093 | <0.001 |
| Female | 0.755 | 0.746–0.764 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Kim, M.-C.; Hong, D.; Jeong, Y.-S.; Kim, H.S.; Kim, J.H. Recurrence and Mortality Risks in Patients with First Incident Acute Stroke or Myocardial Infarction: A Longitudinal Study Using the Korean National Health Insurance Service Database. J. Clin. Med. 2023, 12, 568. https://doi.org/10.3390/jcm12020568
Park D, Kim M-C, Hong D, Jeong Y-S, Kim HS, Kim JH. Recurrence and Mortality Risks in Patients with First Incident Acute Stroke or Myocardial Infarction: A Longitudinal Study Using the Korean National Health Insurance Service Database. Journal of Clinical Medicine. 2023; 12(2):568. https://doi.org/10.3390/jcm12020568
Chicago/Turabian StylePark, Dougho, Mun-Chul Kim, Daeyoung Hong, Yong-Suk Jeong, Hyoung Seop Kim, and Jong Hun Kim. 2023. "Recurrence and Mortality Risks in Patients with First Incident Acute Stroke or Myocardial Infarction: A Longitudinal Study Using the Korean National Health Insurance Service Database" Journal of Clinical Medicine 12, no. 2: 568. https://doi.org/10.3390/jcm12020568
APA StylePark, D., Kim, M.-C., Hong, D., Jeong, Y.-S., Kim, H. S., & Kim, J. H. (2023). Recurrence and Mortality Risks in Patients with First Incident Acute Stroke or Myocardial Infarction: A Longitudinal Study Using the Korean National Health Insurance Service Database. Journal of Clinical Medicine, 12(2), 568. https://doi.org/10.3390/jcm12020568







