Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-Day Mortality in Patients with COVID-19
Abstract
1. Introduction
2. Methods
2.1. Patients and Sample Collection
2.2. Plasma from Healthy Controls
2.3. Assay for Plasma Levels of Syndecan-1
2.4. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Laboratory Characteristics of the Patients
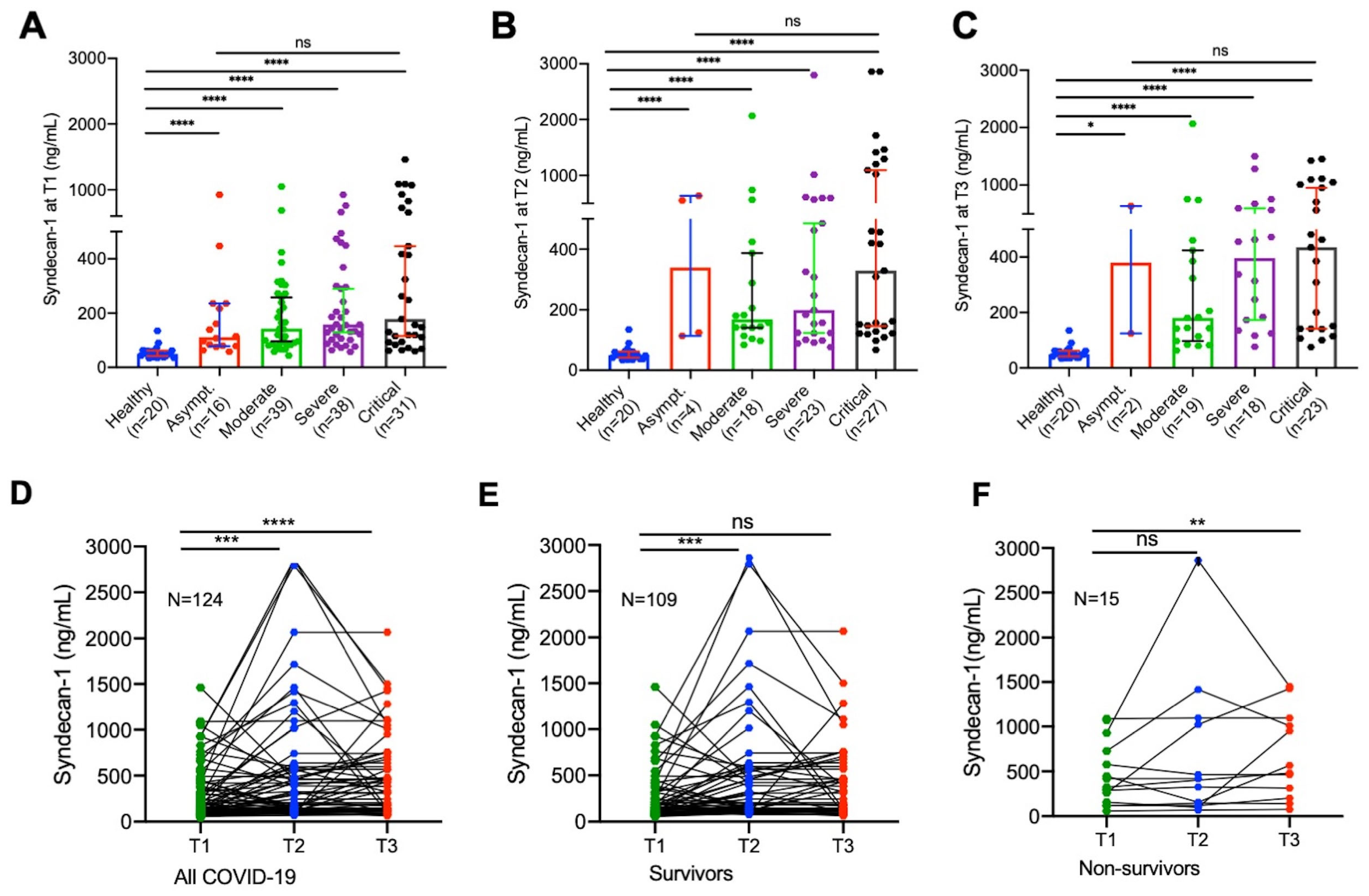
3.2. Hospitalized Patients with SARS-CoV-2 Infection Exhibited Significantly Elevated Plasma Levels of Syndecan-1
3.3. Longitudinal Changes of Plasma Syndecan-1 in Hospitalized Patients with SARS-CoV-2 Infection
3.4. Elevated Plasma Levels of Syndecan-1 Are Associated with Mortality in Patients with COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.-W.; Ilyas, I.; Weng, J.-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms, and potential therapies. Acta Pharmacol. Sin. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelium as an organ system. Crit. Care Med. 2004, 32, S271–S279. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, K.E.M.; Brabenec, L.; Wagner, N.-M. Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation. Cells 2022, 11, 1935. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello-Pellegrini, T.N.; Moslemi-Naeini, M.; Marsden, P.A. New insights into Shiga toxin-mediated endothelial dysfunction in hemolytic uremic syndrome. Virulence 2013, 4, 556–563. [Google Scholar] [CrossRef]
- Russell, R.T.; McDaniel, J.K.; Cao, W.; Shroyer, M.; Wagener, B.M.; Pittet, J.-F.; Zheng, X.L. Low Plasma ADAMTS13 Activity Is Associated with Coagulopathy, Endothelial Cell Damage and Mortality after Severe Paediatric Trauma. Thromb. Haemost. 2018, 118, 676–687. [Google Scholar] [CrossRef]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- Van Ierssel, S.H.; Conraads, V.M.; Van Craenenbroeck, E.M.; Liu, Y.; Maas, A.I.; Parizel, P.M.; Hoymans, V.Y.; Vrints, C.J.; Jorens, P.G. Endothelial dysfunction in acute brain injury and the development of cerebral ischemia. J. Neurosci. Res. 2015, 93, 866–872. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Orea-Tejada, A.; Sánchez-Moreno, C.; Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; González-Islas, D.; Debray-García, Y.; Ortega-Romero, M.S.; Keirns-Davis, C.; Cornejo-Cornejo, L.; Aguilar-Meza, J. Plasma Endothelial and Oxidative Stress Biomarkers Associated with Late Mortality in Hospitalized COVID-19 Patients. J. Clin. Med. 2022, 11, 3950. [Google Scholar] [CrossRef]
- Andrianto; Al-Farabi, M.J.; Nugraha, R.A.; Marsudi, B.A.; Azmi, Y. Biomarkers of endothelial dysfunction and outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. Microvasc. Res. 2021, 138, 104224. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Chen, Y.; Ma, J.; Yang, Y.; Aodeng, S.; Cui, Q.; Wen, K.; Xiao, M.; Xie, J.; et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021, 27, 151. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.J.H. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Ramnath, R.; Kadoya, H.; Desposito, D.; Riquier-Brison, A.; Ferguson, J.K.; Onions, K.L.; Ogier, A.S.; ElHegni, H.; Coward, R.; et al. Aldosterone induces albuminuria via matrix metalloproteinase–dependent damage of the endothelial glycocalyx. Kidney Int. 2019, 95, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.-F.; Aquino, R.S.; Park, P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mobayen, G.; Dhutia, A.; Clarke, C.; Prendecki, M.; McAdoo, S.; Keniyopoullos, R.; Malik, T.; Laffan, M.; Willicombe, M.; McKinnon, T. Severe COVID-19 is associated with endothelial activation and abnormal glycosylation of von Willebrand factor in patients undergoing hemodialysis. Res. Pract. Thromb. Haemost. 2021, 5, e12582. [Google Scholar] [CrossRef] [PubMed]
- Beurskens, D.M.; Bol, M.E.; Delhaas, T.; Van de Poll, M.; Reutelingsperger, C.P.; Nicolaes, G.A.; Sels, J.-W.E. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth. Intensiv. Care 2020, 48, 221–228. [Google Scholar] [CrossRef]
- Ogawa, F.; Oi, Y.; Nakajima, K.; Matsumura, R.; Nakagawa, T.; Miyagawa, T.; Sakai, K.; Saji, R.; Taniguchi, H.; Takahashi, K.; et al. Temporal change in Syndecan-1 as a therapeutic target and a biomarker for the severity classification of COVID-19. Thromb. J. 2021, 19, 55. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- Lu, R.; Sui, J.; Zheng, X.L. Elevated plasma levels of syndecan-1 and soluble thrombomodulin predict adverse outcomes in thrombotic thrombocytopenic purpura. Blood Adv. 2020, 4, 5378–5388. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 2021, 11, 8076–8091. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Qian, Y.; Lei, T.; Patel, P.S.; Lee, C.H.; Monaghan-Nichols, P.; Xin, H.-B.; Qiu, J.; Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J. Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Thymis, J.; Katogiannis, K.; Korakas, E.; Varlamos, C.; Andreadou, I.; Tsoumani, M.; Triantafyllidi, H.; et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021, 23, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Chioh, F.W.; Fong, S.-W.; Young, B.E.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L.L.; Gao, F.; et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife 2021, 10, e64909. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Zaza, G.; Arduini, A.; Onisto, M.; Gambaro, G. Endothelial Glycocalyx as a Regulator of Fibrotic Processes. Int. J. Mol. Sci. 2021, 22, 2996. [Google Scholar] [CrossRef]
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. Ann. Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar] [CrossRef]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.; Martins, R.B.; Benatti, M.N.; Almado, C.E.; De Sá, K.S.; Bonato, V.L.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef]
- Yuan, L.; Cheng, S.; Sol, W.M.; Van der Velden, A.I.; Vink, H.; Rabelink, T.J.; Berg, B.M.V.D. Heparan sulfate mimetic fucoidan restores the endothelial glycocalyx and protects against dysfunction induced by serum of COVID-19 patients in the intensive care unit. ERJ Open Res. 2022, 8, 00652-2021. [Google Scholar] [CrossRef]
- Drost, C.C.; Rovas, A.; Osiaevi, I.; Rauen, M.; Van der Vlag, J.; Buijsers, B.; Salmenov, R.; Lukasz, A.; Pavenstädt, H.; Linke, W.A.; et al. Heparanase Is a Putative Mediator of Endothelial Glycocalyx Damage in COVID-19—A Proof-of-Concept Study. Front. Immunol. 2022, 13, 916512. [Google Scholar] [CrossRef]
- Rodriguez, E.G.; Ostrowski, S.R.; Cardenas, J.C.; Baer, L.A.; Tomasek, J.S.; Henriksen, H.H.; Stensballe, J.; Cotton, B.A.; Holcomb, J.B.; Johansson, P.I.; et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J. Am. Coll. Surg. 2017, 225, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H.; Sah, R.; Lescanic, A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am. J. Physiol. Circ. Physiol. 2011, 300, H415–H422. [Google Scholar] [CrossRef]
- Popova, T.G.; Millis, B.; Bailey, C.; Popov, S.G. Platelets, inflammatory cells, von Willebrand factor, syndecan-1, fibrin, fibronectin, and bacteria co-localize in the liver thrombi of Bacillus anthracis-infected mice. Microb. Pathog. 2012, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smart, L.; Bosio, E.; Macdonald, S.; Dull, R.; Fatovich, D.; Neil, C.; Arendts, G. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J. Crit. Care 2018, 47, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Zahednasab, H.; Farahmand, M.; Mirzaei, R.; Zamani, F.; Tabibzadeh, A.; Bouzari, B.; Ajdarkosh, H.; Nikkhah, M.; Hashemi, M.R.; et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef]
- Kozłowski, P.; Śmiarowski, M.; Przyborska, W.; Zemlik, K.; Małecka-Giełdowska, M.; Leszczyńska, A.; Garley, M.; Ciepiela, O. Mild-to-Moderate COVID-19 Convalescents May Present Pro-Longed Endothelium Injury. J. Clin. Med. 2022, 11, 6461. [Google Scholar] [CrossRef]

| Characteristics | Asymptomatic (n = 16) | Moderate (n = 39) | Severe (n = 38) | Critical (n = 31) | p-Value |
|---|---|---|---|---|---|
| Gender (male/female) | 9/7 | 16/23 | 23/15 | 17/14 | 0.36 |
| Age, year (mean ± SD) | 56.2 ± 15.5 | 63.3 ± 16.5 | 63.9 ± 15.1 | 67.1 ± 13.3 | 0.15 |
| BMI, kg/m2, median (IQR) | 26.0 (24.4, 31.5) # | 29.1 (23.1, 33.5) | 27.8 (23.4, 31.6) | 28.9 (25.0, 32.3) | 0.82 |
| Races, n (%) | 0.45 | ||||
| White | 11 (68.8) | 24 (61.5) | 28 (73.7) | 19 (61.3) | |
| Black | 1 (6.2) | 9 (23.1) | 8 (21.1) | 4 (12.9) | |
| Asian | 1 (6.2) | 2 (5.1) | 0 (0.0) | 2 (6.5) | |
| Others | 3 (18.8) | 4 (10.3) | 2 (5.3) | 6 (19.3) | |
| Comorbidities, n (%) | |||||
| Diabetes mellitus | 2 (12.5) | 9 (23.1) | 8 (21.1) | 14 (45.2) | 0.06 |
| Hypertension | 2 (12.5) | 19 (48.7) | 18 (47.3) | 18 (58.1) | 0.03 |
| CVD | 2 (12.5) | 8 (20.5) | 8 (21.1) | 11 (35.5) | 0.31 |
| COPD | 0 (0.0) | 5 (12.8) | 6 (15.8) | 5 (16.1) | 0.40 |
| Hyperlipidemia | 1 (6.2) | 12 (30.8) | 8 (21.1) | 12 (38.7) | 0.08 |
| CKI | 1 (6.2) | 7 (17.9) | 5 (13.2) | 3 (9.7) | 0.69 |
| History of malignancy | 1 (6.2) | 7 (17.9) | 11 (28.9) | 7 (22.6) | 0.29 |
| History of thrombotic events, n (%) | 1 (6.2) | 5 (12.8) | 3 (7.9) | 5 (16.1) | 0.70 |
| DVT | 0 (0.0) | 3 (7.7) | 1 (2.6) | 1 (3.2) | n.d. |
| PE | 0 (0.0) | 2 (5.1) | 0 (0.0) | 2 (6.4) | n.d. |
| DVT+PE | 0 (0.0) | 0 (0.0) | 1 (2.6) | 1 (3.2) | n.d. |
| Stroke | 1 (6.3) | 0 (0.0) | 1 (2.6) | 0 (0.0) | n.d. |
| MI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.2) | n.d. |
| Organ transplanted, n (%) | 0 (0.0) | 3 (7.7) | 5 (13.2) | 6 (19.3) | 0.21 |
| ICU admission, n (%) | 2 (12.5) | 3 (7.7) | 10 (26.3) | 29 (93.5) | 0.00 |
| Oxygen support, n (%) | |||||
| Nasal cannula | 0 (0.0) | 0 (0.0) | 33 (86.8) | 2 (6.5) | n.d. |
| Non-invasive ventilation | 0 (0.0) | 0 (0.0) | 2 (5.3) | 4 (12.9) | n.d. |
| High-flow oxygen | 0 (0.0) | 0 (0.0) | 3 (7.9) | 11 (35.5) | n.d. |
| Intubation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 14 (45.2) | n.d. |
| Acute events, n (%) | |||||
| Sepsis or septic shock | 0 (0.0) | 0 (0.0) | 1 (2.6) | 13 (41.9) | 0.00 |
| AKI (KDIGO) | 1 (6.2) | 3 (7.7) | 3 (7.9) | 12 (38.7) | 0.00 |
| Thrombotic events | 1(6.2) | 0 (0.0) | 6 (15.8) | 7 (22.6) | 0.007 |
| Outcomes | |||||
| Length of hospitalization, day | 3 (3, 7) | 4 (3, 7) | 7 (5, 10) | 15 (10, 26) | 0.00 |
| 60-day mortality, n (%) | 0 (0.0) | 0 (0.0) | 3 (7.9) | 12 (38.7) | 0.00 |
| Parameters | Asymptomatic (n = 16) | Moderate (n = 39) | Severe (n = 38) | Critical (n = 31) | p-Value |
|---|---|---|---|---|---|
| WBC (×109/L) | 5.8 (4.5, 8.2) # | 7.1 (5.3, 9.5) | 5.5 (3.9, 9.6) | 7.9 (5.0, 11.8) | 0.22 |
| Neutrophil (%) | 66 (57, 79) | 72 (59, 79) | 82 (68, 88) | 88 (80, 93) | 0.00 |
| Lymphocyte (×109/L) | 1.3 (1.0, 1.5) | 1.2 (0.9, 1.5) | 0.5 (0.3, 0.9) | 0.5 (0.3, 0.8) | 0.00 |
| Lymphocyte (%) | 15 (9, 28) | 15 (11, 23) | 9 (6, 17) | 5 (3, 10) | 0.00 |
| Platelet (×109/L) | 223 (173, 312) | 230 (175, 282) | 174 (122, 240) | 221 (149, 311) | 0.14 |
| CRP (mg/dL) | n.d. | 2.5 (1.1, 4.0) | 8.1 (3.9, 17.6) | 7.0 (4.4, 14.8) | 0.08 |
| D-Dimer (ng/mL FEU) | n.d. | 1014 (394, 1377) | 1544 (1014, 2999) | 1932 (1088, 5620) | 0.01 |
| Albumin (g/dL) | 4.3 (3.9, 4.6) | 4.0 (3.4, 4.2) | 3.3 (3.0, 3.7) | 3.3 (2.9, 3.7) | 0.00 |
| LDH (U/L) | 161 (135, 186) | 211 (178, 376) | 338 (209, 480) | 342 (308, 413) | 0.09 |
| Creatinine (mg/dL) | 0.8 (0.7, 1.1) | 1.2 (0.9, 2.5) | 1.2 (0.9, 2.4) | 1.2 (0.7, 1.9) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ye, Z.; Bignotti, A.; Zheng, X.L. Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-Day Mortality in Patients with COVID-19. J. Clin. Med. 2023, 12, 552. https://doi.org/10.3390/jcm12020552
Zhang Q, Ye Z, Bignotti A, Zheng XL. Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-Day Mortality in Patients with COVID-19. Journal of Clinical Medicine. 2023; 12(2):552. https://doi.org/10.3390/jcm12020552
Chicago/Turabian StyleZhang, Quan, Zhan Ye, Antonia Bignotti, and X. Long Zheng. 2023. "Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-Day Mortality in Patients with COVID-19" Journal of Clinical Medicine 12, no. 2: 552. https://doi.org/10.3390/jcm12020552
APA StyleZhang, Q., Ye, Z., Bignotti, A., & Zheng, X. L. (2023). Longitudinal Assessment of Plasma Syndecan-1 Predicts 60-Day Mortality in Patients with COVID-19. Journal of Clinical Medicine, 12(2), 552. https://doi.org/10.3390/jcm12020552







