Abstract
Frailty is increasingly recognized as an important concept in patients with Inflammatory Bowel Disease (IBD). The aim of this scoping review is to summarize the current literature on frailty in IBD. We will discuss the definition of frailty, frailty assessment methods, the prevalence of frailty, risk factors for frailty and the prognostic value of frailty in IBD. A scoping literature search was performed using the PubMed database. Frailty prevalence varied from 6% to 53.9%, depending on the population and frailty assessment method. Frailty was associated with a range of adverse outcomes, including an increased risk for all-cause hospitalization and readmission, mortality in non-surgical setting, IBD-related hospitalization and readmission. Therefore, frailty assessment should become integrated as part of routine clinical care for older patients with IBD.
1. Introduction
The prevalence and incidence of inflammatory bowel disease (IBD) is increasing among all age groups, but especially in older people [1]. Currently, the prevalence of IBD in the population of 60 years and older is estimated to increase incrementally annually by 5.2% [2]. Older patients with IBD are generally considered a heterogenous population who are frequently affected by comorbid conditions, polypharmacy, malnutrition and sarcopenia [3]. Frailty is a concept that is increasingly used to address this heterogeneity in health status of people. Frailty represents a dynamic process of decline in functioning across multiple physiological systems, accompanied by an increased vulnerability to adverse health outcomes [4]. Increasing evidence demonstrates that this also applies for IBD, as frailty is associated with a wide range of adverse outcomes in patients with IBD [5].
Currently, there is no consensus on a standardized definition or measure of frailty. In fact, a variety of definitions and methods to asses frailty are used in the literature [6,7]. Some frailty assessment methods rely on data from medical records, while others measure components of frailty directly in patients (such as handgrip strength and walking speed, components of Fried’s Frailty criteria) [7,8]. The lack of a uniform definition and assessment method can impede the implementation of frailty in clinical care for patients with IBD and in guidelines.
The aim of this review is to summarize current literature on frailty in IBD. First, we will discuss what frailty is, the frailty assessment methods and review the prevalence of frailty in patients with IBD. Second, we will discuss risk factors for frailty in patients with IBD. We will outline the association between frailty and generic outcomes (e.g., mortality) and IBD-specific clinical outcomes (e.g., surgery, hospitalizations).
2. Materials and Methods
The study design of a scoping review was considered most suitable to explore multiple, emerging key concepts for frailty in IBD [9]. A literature search was conducted on the 10th of October 2022 using the electronic database PubMed to identify relevant English language articles. In addition, reference lists of the identified articles were screened for additional studies. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) extension for scoping reviews was followed. The search strategy was developed with a specialized research librarian. The following search strategy was used: (((“Frail Elderly” [Mesh]) OR (Frail Elderly)) OR ((((“Frailty” [Mesh]) OR (frail)) OR (frailty)) AND ((((old) OR (older)) OR (elderly)) OR (“Aged” [Mesh])))) AND (((((((“Inflammatory Bowel Diseases” [Mesh]) OR (inflammatory bowel disease)) OR (IBD [tiab])) OR (Crohn’s disease)) OR (ulcerative colitis)) OR (“Colitis, Ulcerative” [Mesh])) OR (“Crohn Disease” [Mesh])). This initial search identified 62 articles, of which 26 articles were read. Original articles were considered eligible if 1) frailty was assessed in patients with IBD and 2) frailty was related to clinical outcomes. Additionally, we included one submitted manuscript provided by one of the co-authors, as we considered the outcomes of this manuscript relevant to discuss in this literature review. Finally, 12 articles were included in this review (Figure 1).
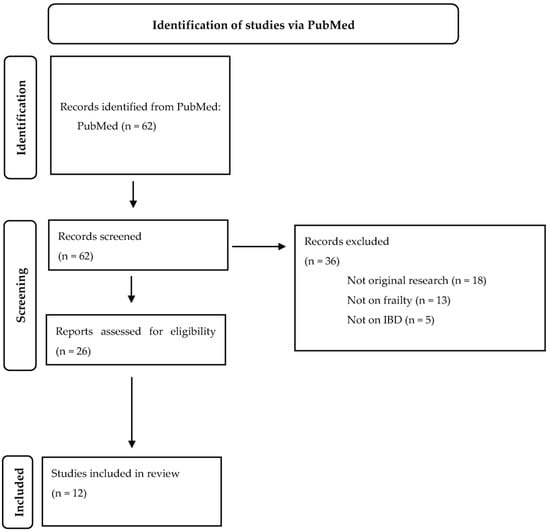
Figure 1.
Flowchart.
3. Results
3.1. What Is Frailty?
Frailty is a condition that is characterized by a decline in multiple physiologic systems resulting in a state of increased vulnerability to adverse health outcomes [10]. In literature, there is no consensus on a standardized operational definition of frailty [11]. Definitions differ based on the construct that is chosen [12]. Two definitions are predominantly used in the literature [6]. ‘’Phenotypic frailty’’ or physical frailty, proposed by Fried et al. [4], is defined as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.’’ [13,14]. Physical frailty is identified when at least three out of the following five key clinical symptoms are present: involuntary weight loss, weakness, fatigue, low levels of physical activity and reduced walking speed [4,6]. Physical frailty is considered preventable and reversible by intervention(s), as will be discussed later in this review.
The accumulation deficits model considers frailty as a multidimensional state of risk which results from acquired, accumulated deficits across multiple domains [15]. This model assumes that the number rather than the nature of health problems leads to an increased state of risk or frailty [6]. Both models are predictive for adverse outcomes in a diverse pallet of medical conditions, however they probably identify significantly different patient populations as a consequence of different theoretical frameworks and frailty measures [14,16]. Several frailty assessment methods have been developed based on these two models [17]. Both models are alternately used in the articles referred to in this literature review. Therefore, if not specified otherwise, when we refer to frailty we imply the global concept of a multidimensional decline associated with increased vulnerability to adverse health outcomes.
When examining these two models in context of IBD, both are relevant and can be applied. For example, multiple components of the frailty phenotype such as fatigue, weight loss and weakness are commonly seen in IBD [4,5]. On the other hand, extra-intestinal disease manifestations, but also other comorbid conditions such as cardiovascular disease and neuropsychological disorders, are prevalent in patients with IBD and contribute to the ‘’accumulation of deficits’’ [18].
Although frailty is an ageing-related syndrome and its prevalence increases with age, it does not only manifest in older patients [19]. Frailty could be considered as a proxy for accelerated biological ageing, irrespective of chronological age [20]. This can be explained by the significant heterogeneity that exists in the rate of biological ageing between patients [21]. Several processes, including low-grade inflammation, are linked to this acceleration of biological ageing [22]. As a result, the biological age can exceed the chronological age, thereby increasing the risk for ageing-related diseases in chronologically younger patients. This effect of biological ageing might also be observed in patients with IBD, where the onset of several geriatric syndromes, including osteoporosis, are seen in relatively young patients [5,23].
Frailty shares a significant overlap with several conditions, including sarcopenia [24,25]. Sarcopenia can occur as a distinct clinical entity, but can also be causally related to frailty [14]. We will briefly outline the characteristics of sarcopenia and its relationship with frailty. Sarcopenia is defined as “a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality” [26]. A diagnosis of sarcopenia is confirmed by the presence of low muscle strength and low muscle mass or quality [26]. Several assessment methods exist to measure sarcopenia, and the choice of instrument depends on the purpose, patient population and setting [26]. Methods can roughly be divided into techniques that measure muscle mass (e.g., Computed Tomography, Magnetic Resonance Imaging and Bioelectrical Impedance Assessment) and techniques that measure muscle function (e.g., handgrip strength, walking speed) [26]. The etiology of sarcopenia can be ageing-related, but also secondary to other conditions, for example to malignancy [26]. Sarcopenia is also prevalent in patients with IBD—a recent systematic review found a prevalence of 42%—and is associated with adverse outcomes such as adverse events following surgery [27]. Although sarcopenia and frailty share a significant overlap, they are two distinct concepts [28]. Low physical function is the key characteristic that is shared by sarcopenia and frailty. However, frailty represents a broader, more multifaceted concept than sarcopenia [28].
3.2. How to Assess Frailty?
Multiple frailty assessment methods have been developed and validated to identify frailty in both clinical and research settings [29,30]. The Comprehensive Geriatric Assessment (CGA) is considered as the ‘’gold standard’’ to assess the presence of frailty [31]. The CGA is a multidisciplinary, diagnostic and treatment process that systematically assesses four geriatric domains: the somatic, mental, physical and social domain [32]. The CGA comprises both the detection of deficits in geriatric domains and subsequently the initiation of tailored intervention strategies [31]. However, the performance of a CGA in all older patients is not time or cost efficient. Therefore, frailty screening can be performed to identify patients at an increased risk for frailty, requiring referral to a geriatrician for a CGA [33]. Frailty screening methods can be categorized into two types: direct and indirect. Direct screening methods include the performance of screening questionnaires or tests directly on a patient. Commonly used screenings indicators include the FRAIL scale, Clinical Frailty Scale (CFS), Vulnerable Elders Survey 13 (VES-13) and the Geriatric-8 (G8) [29]. The CFS has gained considerable attention during the COVID-19 pandemic, when screening for frailty had to be feasible and simple to perform [10]. The CFS ranges from 1 (very fit) to 9 (terminally ill) [34]. One of the advantages of the CFS is that it incorporates components of three geriatric domains: the somatic domain (comorbidity), the functional domain (functional level) and the mental domain (cognition) [34]. Patients with scores of 5 or higher are at an increased risk for frailty and require further evaluation of the frailty status [34]. The CFS and the G8 (Table 1) are increasingly adopted as they have been consistently predictive of adverse outcomes and mortality in different settings [34,35].

Table 1.
An overview of two commonly used frailty screening methods: the Clinical Frailty Scale and the Geriatric-8.
Indirect frailty screening methods use clinical data from Electronic Health Records (EHR), also called administrative frailty tools [37]. The methodology of these assessment methods is often based on the model of ‘’accumulation of deficits’’, as previously described [38]. An advantage of these administrative assessment methods is that they allow frailty screening in large cohorts of patients.
The majority of the included studies used an administrative frailty assessment method (Table 2). Six studies used the Hospital Frailty Risk Score (HFRS) [39,40,41,42,43,44]. The HFRS is a frailty screening method that is based on the International Statistical Classification of Diseases and Health-Related Problems, tenth revision (ICD-10) coding systems [45]. This is a computerized method that generates a score based on the type and number of ICD-10 diagnoses generated from the medical record of a patient [45]. One other study used a similar administrative assessment method: the Johns Hopkins ACG frailty-defining diagnoses indicator [46]. Three studies used a comorbidity-based frailty assessment method using ICD data abstracted from medical records. One study used the Modified Frailty Index (mFI) [47], and one used a simplified version [48]. The Simplified Frailty Index (sFI) contains five selected items of the twelve in the mFI [48]. Another comorbidity-based tool is the ‘’frailty trait count’’, which consists of the five items in the sFI with one additional item [49]. Two studies measured frailty using a geriatric assessment in patients with IBD aged 65 years and older [50,51]. They conducted a geriatric assessment that explored five geriatric domains: the somatic domain (multimorbidity, malnutrition, polypharmacy), activities of daily living (ADL, IADL), physical capacity (handgrip strength, gait speed), the mental domain (depression, cognitive function) and the social domain (presence of a life partner) [50].

Table 2.
Study characteristics, frailty measurement methods and frailty prevalence.
3.3. What Is the Prevalence of Frailty in Patients with IBD?
We identified twelve studies that examined frailty in patients with IBD. Details about the included studies are shown in Table 2. The prevalence of (high risk of) frailty appears to be higher in patients with IBD compared to a matched non-IBD control population (6% vs. 12%, respectively) [42].
The presence of frailty in the study by Asscher et al. [50] was defined as deficits in two or more geriatric domains and was established in 47.4% of older patients with IBD. This study has provided insight in which geriatric domains are most often affected in older patients with IBD. The somatic domain was most often affected (51.6%), followed by impaired activities of daily living (43.0%), the social domain (23.7%), physical activity (22.7%) and lastly the mental domain (16.5%) (Table 3). Another paper by Asscher et al. [51] reported the use of a frailty screening tool, the Geriatric-8 (G8). This tool classified 48% of patients with IBD 65 years or older at risk of being frail [51].

Table 3.
Prevalence and type of frailty defining diagnosis per study.
In the studies that used an administrative frailty assessment method, the prevalence of frailty varied between 6% and 39.3%. An important caveat is that in some of these studies, no age threshold was applied. This is reflected in the mean age of the participants (Table 2), and will have led to a lower prevalence of frailty in these studies. Prevalence of frailty in surgical patients was slightly higher, probably reflecting a patient population with a higher IBD disease burden. However, again no age thresholds were used in these studies. Although frailty is not limited to a certain age threshold and can occasionally be found in younger patients, the multidimensional decline that contributes to development of frailty is ageing-related, increasing the prevalence of frailty with ageing. Therefore, these studies do not tell us about the prevalence in older patients with IBD.
3.4. What Factors Increase the Risk for the Onset or Progression of Frailty in Community Dwelling Older Adults?
Risk factors for frailty are patient characteristics or conditions that are known to attribute to the multidimensional process of physical decline, eventually leading to the condition frailty. The number and type of risk factors can vary depending on the characteristics of the investigated population. Some risk factors consistently associate with an increased risk of frailty in different settings, while other risk factors are unique for a specific condition or population. For example, the presence of disease activity in chronic inflammatory diseases such as rheumatoid arthritis or IBD has been associated with increased risk of frailty [50,52].
Previous studies identified risk factors for the onset or progression of frailty in community-dwelling older adults [12,53]. They reported a broad range of risk factors categorized in sociodemographic factors, clinical factors, lifestyle factors and biological factors. Alternatively, these risk factors can be stratified based on the geriatric domain they affect (Table 4), enabling easier identification of geriatric domains at risk of frailty.

Table 4.
Risk factors for frailty in community-dwelling older adults categorized by geriatric domain.
3.5. What Factors Increase the Risk for the Onset or Progression Frailty in Patients with IBD?
Three papers have examined risk factors that are associated with frailty in patients with IBD [43,44,50]. Increasing age was reported as a risk factor for frailty in patients with IBD [43,50]. The role of female sex as a risk factor for frailty in patients is less clear: one study demonstrated a strong association between female sex and risk for geriatric deficits (adjusted Odds Ratio (aOR) 1.94, [95% confidence interval (CI) 1.26–2.98], p-value 0.002) [50], whereas another study did not (aOR 1.17, [95% CI 0.99–1.38], p-value 0.060) [43].
Older patients with IBD are frequently affected by multimorbidity and polypharmacy [54,55]. Kochar et al. [43] found that the presence of ≥1 comorbidity was the strongest predictor of frailty in a multivariable analysis, as it was associated with an 17.23 odds of frailty ([95% CI 8.11–36.63], p-value < 0.001).
3.6. Which IBD-Specific Factors Are Associated with an Increased Risk of Frailty?
CD and UC have distinct disease behavior and characteristics and some disease characteristics may contribute to the development of frailty. Two studies found that the presence of CD (compared to UC) was associated with an increased odds of frailty (aOR 1.35, [95% CI 1.14–1.61], p-value < 0.001) (aOR 1.80 [95% CI 1.18–2.74], p-value 0.006) [43,50].
Disease activity was found as an important factor in relation to frailty. Asscher et al. [50] reported an independent association between disease activity and the presence of deficits in the geriatric assessment, this was seen for both biochemical (aOR 3.36, [95% CI 1.94–5.83], p-value 0.000) and clinical disease activity (aOR 2.19, [95% CI 1.28–3.74], p-value 0.004). Disease activity was the strongest associated factor in relation to frailty in this study. Conversely, treating disease activity in frail, older patients with IBD was associated with reduced post-treatment frailty [44], especially in those patients with a higher baseline level of frailty. Inflammatory activity can introduce potential risk factors for frailty into different geriatric domains. For example, inflammation can induce loss of skeletal muscle and may eventually lead to sarcopenia [27]. In addition, disease activity is described as a risk factor for malnutrition and polypharmacy [56,57]. Active disease can also impact the mental domain as both mood disorders and cognitive performance have been associated with disease activity over time in IBD [58,59]. These data reflect the important role of disease activity contributing to frailty in patients with IBD and the dynamic character of frailty: the degree of frailty can vary over time depending on the presence or absence of contributing risk factors (Figure 2).
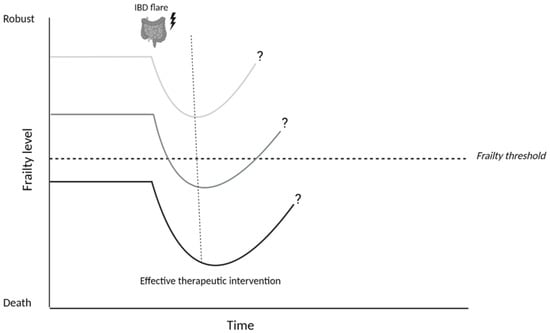
Figure 2.
Hypothesized effect of an IBD flare on frailty in patients with Inflammatory Bowel Disease. An IBD flare should be considered as a potential stressor that can cause a temporary or definite decline in the degree of frailty (level). The impact of this stressor depends on the frailty level prior to the IBD flare, which consists of existing risk factors for frailty in a patient. Achieving disease remission and thereby eliminating the stressor, enables (partial) recovery of frailty levels to baseline. It is likely that this recovery phase takes longer in patients who were already frail prior to the IBD-flare, because they already had a reduced reserve capacity. Images were created using biorender.com.
Therapeutic strategies for the management of patients with IBD often consist of corticosteroids, immunomodulators or biologicals [3]. Older patients are susceptible to developing a range of potential adverse outcomes, especially related to long-term use of corticosteroids (e.g., diabetes, sarcopenia, glucocorticoid-induced osteoporosis) [60,61]. These adverse outcomes could function as risk factors to the onset or progression of frailty. In the IBD literature, one study reported an association between corticosteroid use at baseline and risk of frailty (aOR 1.45, [95% CI 1.21–1.75], p-value < 0.001) [43], whereas another study did not find this association [50]. No association was found between the use of immunomodulators or biologicals and an increased risk of frailty [43,50].
Kochar et al. [43] reported both previous IBD-related hospitalization and previous IBD-related surgery to be associated with an increased risk of frailty, whereas Asscher et al. [50] did not. Both studies found previous all-cause hospitalization as a risk factor for frailty in patients with IBD [43,50]. The association between frailty and hospitalization appears bidirectional, as frailty is established as a predictor for adverse outcomes such as mortality and hospitalizations, and previous hospitalizations also associate with risk of frailty. During a hospital admission, frailty is associated with functional decline, increased vulnerability to complications and other adverse health outcomes [62,63].
3.7. What Is the Prognostic Value of Frailty in Patients with IBD?
In this section, we will review the impact of frailty on generic outcomes and IBD-specific outcomes (Figure 3). Frailty was consistently associated with an increased risk for all-cause hospitalization and all-cause re-admission. Kochar et al. [42] reported a HR 2.42 ([95% CI 2.24–2.61]) for all-cause hospitalization in their cohort. In addition, Asscher et al. [51] reported a positive association between severe geriatric deficits and increased risk for all-cause hospitalizations. In addition, deficits in geriatric domains were associated with acute and IBD-related hospitalizations [51]. They also examined the association between an increased risk of frailty at baseline and the occurrence of all-cause and acute hospitalizations at follow-up. Risk of frailty was associated with acute hospitalizations (aHR 2.21, [95% CI 1.27–3.87], p-value 0.005), but not with all-cause hospitalizations (aHR 1.53, [95% CI 0.96–2.44], p-value 0.074) [51]. Frailty was associated with an increased risk for all-cause readmission in the papers of Faye et al. [46] (adjusted risk ratio (aRR) 1.16, [95% CI 1.14–1.17], p-value < 0.01) and Qian et al. [40] (aHR 1.21, [95% CI 1.17–1.25], p-value < 0.01).
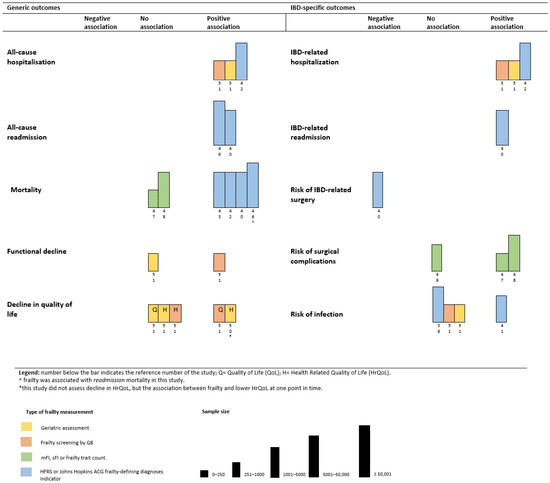
Figure 3.
Harvest plot of the association between frailty and (1) generic outcomes and (2) IBD-specific outcomes.
Findings from studies on the association between frailty and mortality differed depending on the setting and the frailty tool used. Two studies in a surgical IBD-setting that used a comorbidity-based frailty tool found no association between frailty and mortality [47,48]. Four studies that used the HFRS, all reported a strong association between frailty and mortality ((aOR 2.90 [95% CI 2.29–3.68); (HR of 3.22 [95% CI 2.86–3.61]); (aHR 1.57 [95% CI 1.34–1.83], p-value < 0.01); (aRR 1.12 ([95% CI 1.02–1.23], p-value 0.02)) [40,42,43,46]. The role of frailty in the surgical IBD setting as a predictor for mortality was not demonstrated, yet there is consistent evidence that frailty is associated with an increased risk for mortality in non-surgical IBD setting when the HFRS is applied.
Functional decline and decreased quality of life were evaluated as outcomes of frailty by Asscher et al. [50,51]. Frailty, reflected by the number of geriatric deficits, was associated with lower health-related quality of life (HRQoL) [50]. In their paper with follow-up data, they demonstrated that risk of frailty at baseline was associated with a decline in the quality of life (QoL) (aOR 2.14 [95% CI 1.26–3.62], p-value 0.005) and functional status (IADL) (aOR 3.64 [95% CI 1.65–8.00], p-value 0.001) after 18 months [51]. Interestingly, frailty measured by a geriatric assessment at baseline was not associated with a decline in QoL or functional status at follow-up [50].
The results on the association between frailty and an increased risk of infections in IBD were inconclusive. Two studies reported no association between frailty or risk of frailty and increased risk of infections [39,51], whereas one study found frailty associated with an increased risk of infections in both anti-tumor necrosis factor and immunomodulator therapy [41]. Three studies assessed the association between frailty and risk for morbidity following surgery. Telemi et al. [47] (aOR 25.5; p-value ≤ 0.001) and Wolf et al. [48] (aOR 2.59 [95% CI 1.84–3.63], p-value < 0.0001) reported a positive association between frailty and overall morbidity following surgery. However, Cohan et al. [49] did not find this association.
4. Conclusions and Future Directions
In this scoping review we aimed to summarize the current literature on frailty in IBD. We aimed to describe (1) frailty assessment methods, (2) the prevalence of frailty in IBD, (3) risk factors for frailty in IBD and (4) the prognostic value of frailty in IBD. The majority of the studies that were included in this review used an administrative frailty assessment method, most commonly the HFRS. Only two studies used a geriatric assessment to measure frailty. Overall, the prevalence of frailty greatly varied and depended on the population and frailty assessment method. In addition, as expected, frailty was more prevalent in older patients. The risk factors for frailty that were found in patients with IBD include increasing age, presence of comorbidity, Crohn’s disease (CD), clinical and biochemical disease activity and previous all-cause hospitalization. Frailty was linked to a range of adverse outcomes that include an increased risk for all-cause hospitalization, all-cause readmission, mortality in non-surgical setting and IBD-related hospitalization and readmission.
Implementing frailty into clinical care for patients with IBD does not have to be complicated or time-consuming. Identification of those patients at risk of frailty is the most important aspect, as this allows for further assessment and intervention. There is growing evidence that frailty is most amendable to intervention in the early stages, urging early identification in the beginning of the disease process of frailty [64]. Therefore, periodic evaluation of frailty status should be part of clinical care, especially in older patients with IBD, because frailty is a dynamic condition that can fluctuate depending on the presence of risk factors and stressors. We propose the Clinical Frailty Scale (CFS) as the frailty screening method in all patients with IBD, although this scale is not yet validated in this population. The CFS has shown excellent performance in a wide variety of patient populations and is recommended as frailty screening instrument by the International Conference of Frailty and Sarcopenia Research (ICFSR) [34,64].
There is increasing evidence that physical frailty can be prevented or reversed by the application of combined nutritional and physical exercise intervention programs [65]. To date, no studies have been conducted on the impact of these interventional programs on frailty in patients with IBD. However, in other medical fields the efficacy of interventional programs has already been demonstrated [66]. Interventional programs might not only improve physical frailty, but also positively impact on therapy outcomes [67]. For example, a randomized controlled trial investigated the impact of CGA-based interventions (medication changes, nutritional therapy and physiotherapy) in frail patients receiving adjuvant chemotherapy for colorectal carcinoma [67]. More patients in the intervention group completed planned chemotherapy without further dose reductions or delay compared to patients receiving standard of care [67].
The effective management of patients with increased risk of frailty or frailty also includes incorporating frailty in clinical decision making and therapeutic management strategies. The use of frailty as a risk construct instead of age and comorbidity allows for the better selection of patients who are ‘fit’ for a certain surgical or pharmacological treatment. To date, evidence-based guidelines for treatment of older patients with IBD are lacking and older patients are often excluded from clinical trials, as recently stressed by Vieujean et al. [68]. Frailty could be integrated into treatment selection to tailor pharmaceutical management, for example, by adjusting therapy regimens or reducing dose therapy in patients with frailty. A positive effect of these ‘’tailored’’ approaches in patients with frailty were demonstrated in oncology with adjusted chemotherapy regimens [69].
Future research on frailty in IBD should focus on the identification of the optimal frailty screening method to identify patients with IBD at increased risk of frailty. We demonstrated that frailty outcomes varied depending on the frailty assessment method and the age of the investigated population. Different frailty risk factors and outcomes may apply depending on age and setting. Reanalyzing previous data, stratified by patients’ age specifically, might provide more insight into the frailty syndrome in younger and older patients. Moreover, tailored treatment strategies, such as adjusted therapy regimens or multicomponent intervention programs for patients with frailty in IBD are imaginable, however, research is needed on this topic.
Author Contributions
A.F.: study concept and design, assessing eligibility of studies, data analysis and interpretation of data, drafting of the manuscript; K.K.: study concept and design, critical revision of manuscript, supervision of study; J.M.: study concept and design, critical revision of manuscript, supervision of study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Taleban, S.; Colombel, J.F.; Mohler, M.J.; Fain, M.J. Inflammatory bowel disease and the elderly: A review. J. Crohn’s Colitis 2015, 9, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Nimmons, D.; Limdi, J.K. Elderly patients and inflammatory bowel disease. World J. Gastrointest. Pharm. 2016, 7, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Orkaby, A.R.; Ananthakrishnan, A.N.; Ritchie, C.S. Frailty in inflammatory bowel diseases: An emerging concept. Ther. Adv. Gastroenterol. 2021, 14, 17562848211025474. [Google Scholar] [CrossRef]
- Theou, O.; Walston, J.; Rockwood, K. Operationalizing Frailty Using the Frailty Phenotype and Deficit Accumulation Approaches. Interdiscip. Top. Gerontol. Geriatr. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- Huang, E.Y.; Lam, S.C. Review of frailty measurement of older people: Evaluation of the conceptualization, included domains, psychometric properties, and applicability. Aging Med. 2021, 4, 272–291. [Google Scholar] [CrossRef]
- Bouillon, K.; Kivimaki, M.; Hamer, M.; Sabia, S.; Fransson, E.I.; Singh-Manoux, A.; Gale, C.R.; Batty, G.D. Measures of frailty in population-based studies: An overview. BMC Geriatr. 2013, 13, 64. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Cmaj 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Rockwood, K.; Howlett, S.E. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Cohen, A.A.; Xue, Q.-L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K. Conceptual Models of Frailty: Accumulation of Deficits. Can. J. Cardiol. 2016, 32, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Massa, M.S.; Potter, C.M.; Clarke, R.; Bennett, D.A. Systematic review of the utility of the frailty index and frailty phenotype to predict all-cause mortality in older people. Syst. Rev. 2022, 11, 187. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Argollo, M.; Gilardi, D.; Peyrin-Biroulet, C.; Chabot, J.-F.; Peyrin-Biroulet, L.; Danese, S. Comorbidities in inflammatory bowel disease: A call for action. Lancet Gastroenterol. Hepatol. 2019, 4, 643–654. [Google Scholar] [CrossRef]
- Loecker, C.; Schmaderer, M.; Zimmerman, L. Frailty in Young and Middle-Aged Adults: An Integrative Review. J. Frailty Aging 2021, 10, 327–333. [Google Scholar] [CrossRef]
- Ji, L.; Jazwinski, S.M.; Kim, S. Frailty and Biological Age. Ann. Geriatr. Med. Res. 2021, 25, 141–149. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.S.; Colombel, J.-F. Aging and IBD: A New Challenge for Clinicians and Researchers. Inflamm. Bowel Dis. 2021, 28, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in inflammatory bowel disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J. Gerontol. Ser. A 2004, 59, M255–M263. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Keevil, V.L.; Romero-Ortuno, R. Ageing well: A review of sarcopenia and frailty. Proc. Nutr. Soc. 2015, 74, 337–347. [Google Scholar] [CrossRef]
- Sukkriang, N.; Punsawad, C. Comparison of geriatric assessment tools for frailty among community elderly. Heliyon 2020, 6, e04797. [Google Scholar] [CrossRef]
- Oviedo-Briones, M.; Laso, Á.R.; Carnicero, J.A.; Cesari, M.; Grodzicki, T.; Gryglewska, B.; Sinclair, A.; Landi, F.; Vellas, B.; Checa-López, M.; et al. A Comparison of Frailty Assessment Instruments in Different Clinical and Social Care Settings: The Frailtools Project. J. Am. Med. Dir. Assoc. 2021, 22, 607.e607–607.e612. [Google Scholar] [CrossRef]
- Parker, S.G.; McCue, P.; Phelps, K.; McCleod, A.; Arora, S.; Nockels, K.; Kennedy, S.; Roberts, H.; Conroy, S. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Cella, A.; Pilotto, A.; Daragjati, J.; Veronese, N.; Musacchio, C.; Mello, A.M.; Logroscino, G.; Padovani, A.; Prete, C.; et al. Three Decades of Comprehensive Geriatric Assessment: Evidence Coming From Different Healthcare Settings and Specific Clinical Conditions. J. Am. Med. Dir. Assoc. 2017, 18, 192.e1–192.e11. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.E.; Jonker, J.M.; de Rooij, S.E.; Vos, A.G.; Smorenburg, C.H.; van Munster, B.C. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012, 13, e437–e444. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Kastora, S.; Kounidas, G.; Perrott, S.; Carter, B.; Hewitt, J.; Myint, P.K. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100896. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Nghiem, S.; Sajeewani, D.; Henderson, K.; Afoakwah, C.; Byrnes, J.; Moyle, W.; Scuffham, P. Development of frailty measurement tools using administrative health data: A systematic review. Arch. Gerontol. Geriatr. 2020, 89, 104102. [Google Scholar] [CrossRef]
- Levit, L.A.; Kaltenbaugh, M.W.; Magnuson, A.; Hershman, D.L.; Goncalves, P.H.; Garrett-Mayer, E.; Bruinooge, S.S.; Miller, R.S.; Klepin, H.D. Challenges and opportunities to developing a frailty index using electronic health record data. J. Geriatr. Oncol. 2021, 12, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Heien, H.C.; Sangaralingham, L.; Shah, N.D.; Lai, J.C.; Sandborn, W.J.; Moore, A.A. Frailty and Risk of Serious Infections in Biologic-treated Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 27, 1626–1633. [Google Scholar] [CrossRef]
- Qian, A.S.; Nguyen, N.H.; Elia, J.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. Frailty Is Independently Associated with Mortality and Readmission in Hospitalized Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 2054–2063.e14. [Google Scholar] [CrossRef]
- Kochar, B.; Cai, W.; Cagan, A.; Ananthakrishnan, A.N. Pretreatment Frailty Is Independently Associated with Increased Risk of Infections After Immunosuppression in Patients with Inflammatory Bowel Diseases. Gastroenterology 2020, 158, 2104–2111.e2. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Jylhävä, J.; Söderling, J.; Ritchie, C.S.; Olsson, M.; Hjortswang, H.; Myrelid, P.; Bengtsson, J.; Strid, H.; Andersson, M.; et al. Prevalence and Implications of Frailty in Older Adults with Incident Inflammatory Bowel Diseases: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2358–2365.e11. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Cai, W.; Cagan, A.; Ananthakrishnan, A.N. Frailty is independently associated with mortality in 11 001 patients with inflammatory bowel diseases. Aliment. Pharm. 2020, 52, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.D.; Cai, W.; Ananthakrishnan, A.N. Inflammatory Bowel Disease Patients Who Respond to Treatment with Anti-tumor Necrosis Factor Agents Demonstrate Improvement in Pre-treatment Frailty. Dig. Dis. Sci. 2022, 67, 622–628. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Faye, A.S.; Wen, T.; Soroush, A.; Ananthakrishnan, A.N.; Ungaro, R.; Lawlor, G.; Attenello, F.J.; Mack, W.J.; Colombel, J.-F.; Lebwohl, B. Increasing Prevalence of Frailty and Its Association with Readmission and Mortality Among Hospitalized Patients with IBD. Dig. Dis. Sci. 2021, 66, 4178–4190. [Google Scholar] [CrossRef] [PubMed]
- Telemi, E.; Trofymenko, O.; Venkat, R.; Pandit, V.; Pandian, T.K.; Nfonsam, V.N. Frailty Predicts Morbidity after Colectomy for Ulcerative Colitis. Am. Surg. 2018, 84, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.H.; Hassab, T.; D’Adamo, C.R.; Svoboda, S.; Demos, J.; Ahuja, V.; Katlic, M. Frailty is a stronger predictor than age for postoperative morbidity in Crohn’s disease. Surgery 2021, 170, 1061–1065. [Google Scholar] [CrossRef]
- Cohan, J.N.; Bacchetti, P.; Varma, M.G.; Finlayson, E. Outcomes after ileoanal pouch surgery in frail and older adults. J. Surg. Res. 2015, 198, 327–333. [Google Scholar] [CrossRef]
- Asscher, V.E.R.; Waars, S.N.; van der Meulen-de Jong, A.E.; Stuyt, R.J.L.; Baven-Pronk, A.M.C.; van der Marel, S.; Jacobs, R.J.; Haans, J.J.L.; Meijer, L.J.; Klijnsma-Slagboom, J.D.; et al. Deficits in Geriatric Assessment Associate with Disease Activity and Burden in Older Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2022, 20, e1006–e1021. [Google Scholar] [CrossRef]
- Asscher, V.E.R.; Rodriguez Girondo, M.D.M.; Fens, J. Frailty associates with hospitalization and decline in quality of life and functional status in older patients with inflammatory bowel disease. 2022; Submitted. [Google Scholar]
- Hanlon, P.; Morton, F.; Siebert, S.; Jani, B.D.; Nicholl, B.I.; Lewsey, J.; McAllister, D.; Mair, F.S. Frailty in rheumatoid arthritis and its relationship with disease activity, hospitalisation and mortality: A longitudinal analysis of the Scottish Early Rheumatoid Arthritis cohort and UK Biobank. RMD Open 2022, 8, e002111. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef] [PubMed]
- Bähler, C.; Schoepfer, A.M.; Vavricka, S.R.; Brüngger, B.; Reich, O. Chronic comorbidities associated with inflammatory bowel disease: Prevalence and impact on healthcare costs in Switzerland. Eur. J. Gastroenterol. Hepatol. 2017, 29, 916–925. [Google Scholar] [CrossRef]
- Mesonero, F.; Fernández, C.; Sánchez-Rodríguez, E.; García-García Paredes, A.; Senosiain, C.; Albillos, A.; López-Sanromán, A. Polypharmacy in Patients with Inflammatory Bowel Disease: Prevalence and Outcomes in a Single-center Series. J. Clin. Gastroenterol. 2022, 56, e189–e195. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Ouyang, C.; Wang, D.; Zhang, X.; Liang, J.; Zhu, W.; Cao, Q. Prevalence of Malnutrition, Its Risk Factors, and the Use of Nutrition Support in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, S59–S66. [Google Scholar] [CrossRef]
- Einav, L.; Hirsch, A.; Ron, Y.; Cohen, N.A.; Lahav, S.; Kornblum, J.; Anbar, R.; Maharshak, N.; Fliss-Isakov, N. Risk Factors for Malnutrition among IBD Patients. Nutrients 2021, 13, 4098. [Google Scholar] [CrossRef]
- Marrie, R.A.; Graff, L.A.; Fisk, J.D.; Patten, S.B.; Bernstein, C.N. The Relationship between Symptoms of Depression and Anxiety and Disease Activity in IBD Over Time. Inflamm. Bowel Dis. 2021, 27, 1285–1293. [Google Scholar] [CrossRef]
- Golan, D.; Gross, B.; Miller, A.; Klil-Drori, S.; Lavi, I.; Shiller, M.; Honigman, S.; Almog, R.; Segol, O. Cognitive Function of Patients with Crohn’s Disease is Associated with Intestinal Disease Activity. Inflamm. Bowel Dis. 2016, 22, 364–371. [Google Scholar] [CrossRef]
- Lee, M.K.; Jeong, H.H.; Kim, M.J.; Ryu, H.; Baek, J.; Lee, B. Nutrients against Glucocorticoid-Induced Muscle Atrophy. Foods 2022, 11, 687. [Google Scholar] [CrossRef]
- Yamada, Y.; Tada, M.; Mandai, K.; Hidaka, N.; Inui, K.; Nakamura, H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: From the CHIKARA study. Clin. Rheumatol. 2020, 39, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Rajabali, N.; Tandon, P.; Abraldes, J.G.; Peerani, F. Association between Frailty or Sarcopenia and Adverse Outcomes in Inflammatory Bowel Disease: A Systematic Review. Gastro Hep Adv. 2022, 1, 241–250. [Google Scholar] [CrossRef]
- Gill, T.M.; Williams, C.S.; Tinetti, M.E. The Combined Effects of Baseline Vulnerability and Acute Hospital Events on the Development of Functional Dependence among Community-Living Older Persons. J. Gerontol. Ser. A 1999, 54, M377–M383. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Travers, J.; Romero-Ortuno, R.; Bailey, J.; Cooney, M.-T. Delaying and reversing frailty: A systematic review of primary care interventions. Br. J. Gen. Pract. 2019, 69, e61–e69. [Google Scholar] [CrossRef]
- Kidd, T.; Mold, F.; Jones, C.; Ream, E.; Grosvenor, W.; Sund-Levander, M.; Tingström, P.; Carey, N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019, 19, 184. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Olsen, A.P.; Bardal, P.; Schultz, M.; Dolin, T.G.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: A randomised trial (GERICO). Br. J. Cancer 2021, 124, 1949–1958. [Google Scholar] [CrossRef]
- Vieujean, S.; Caron, B.; Jairath, V.; Benetos, A.; Danese, S.; Louis, E.; Peyrin-Biroulet, L. Is it time to include older adults in inflammatory bowel disease trials? A call for action. Lancet Healthy Longev. 2022, 3, e356–e366. [Google Scholar] [CrossRef]
- Lastra-German, I.K.; Navarrete-Reyes, A.P.; Mejía-Domínguez, N.R.; Agreda-Vásquez, G.P. Adjusted Chemotherapy According to Frailty Status in Elderly Patients with Diffuse Large B-Cell Lymphoma: Experience From a Single Referral Center in Mexico City. Clin. Lymphoma Myeloma Leuk. 2019, 19, e98–e106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).