Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related?
Abstract
1. Introduction
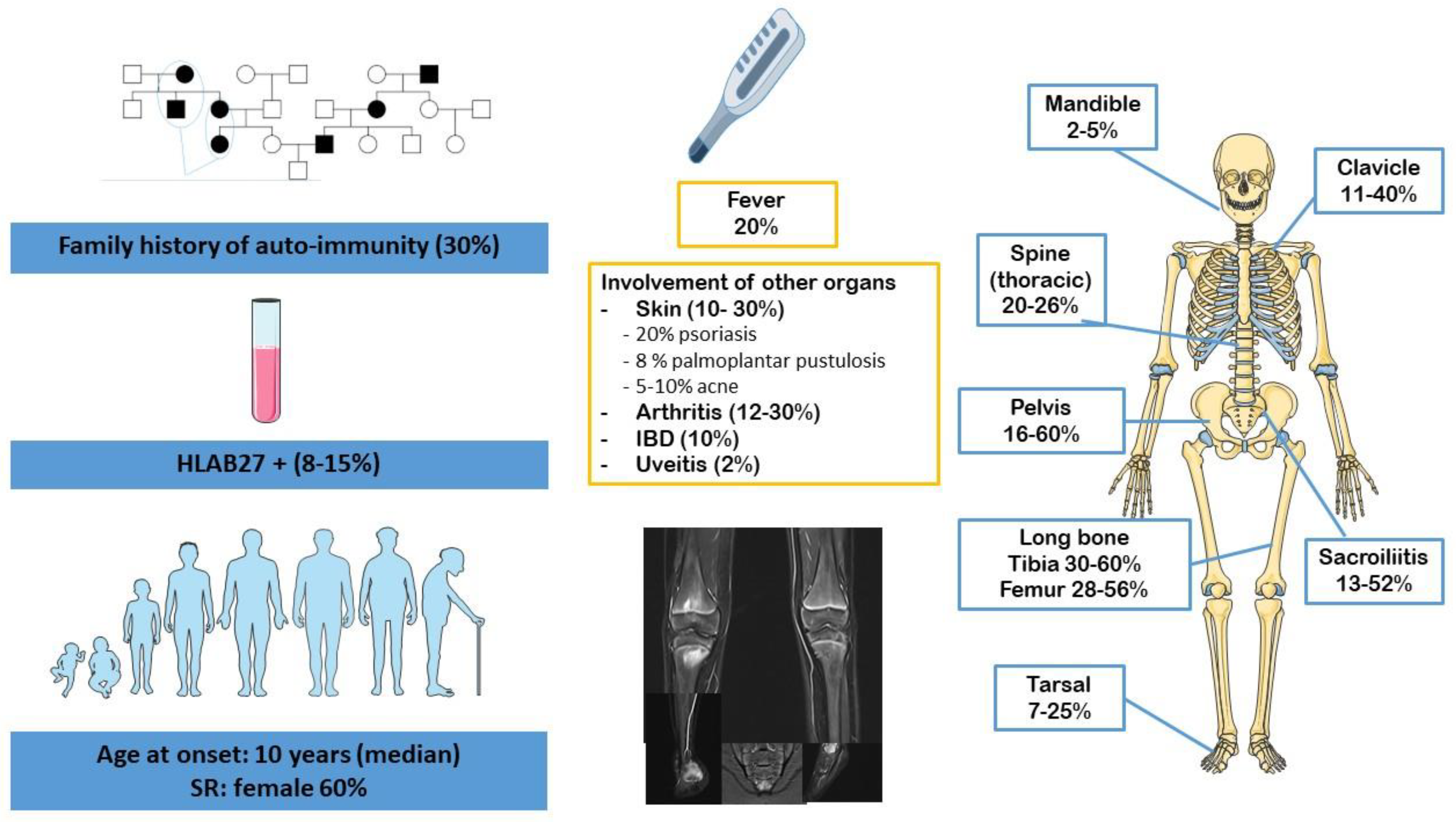
2. CRMO
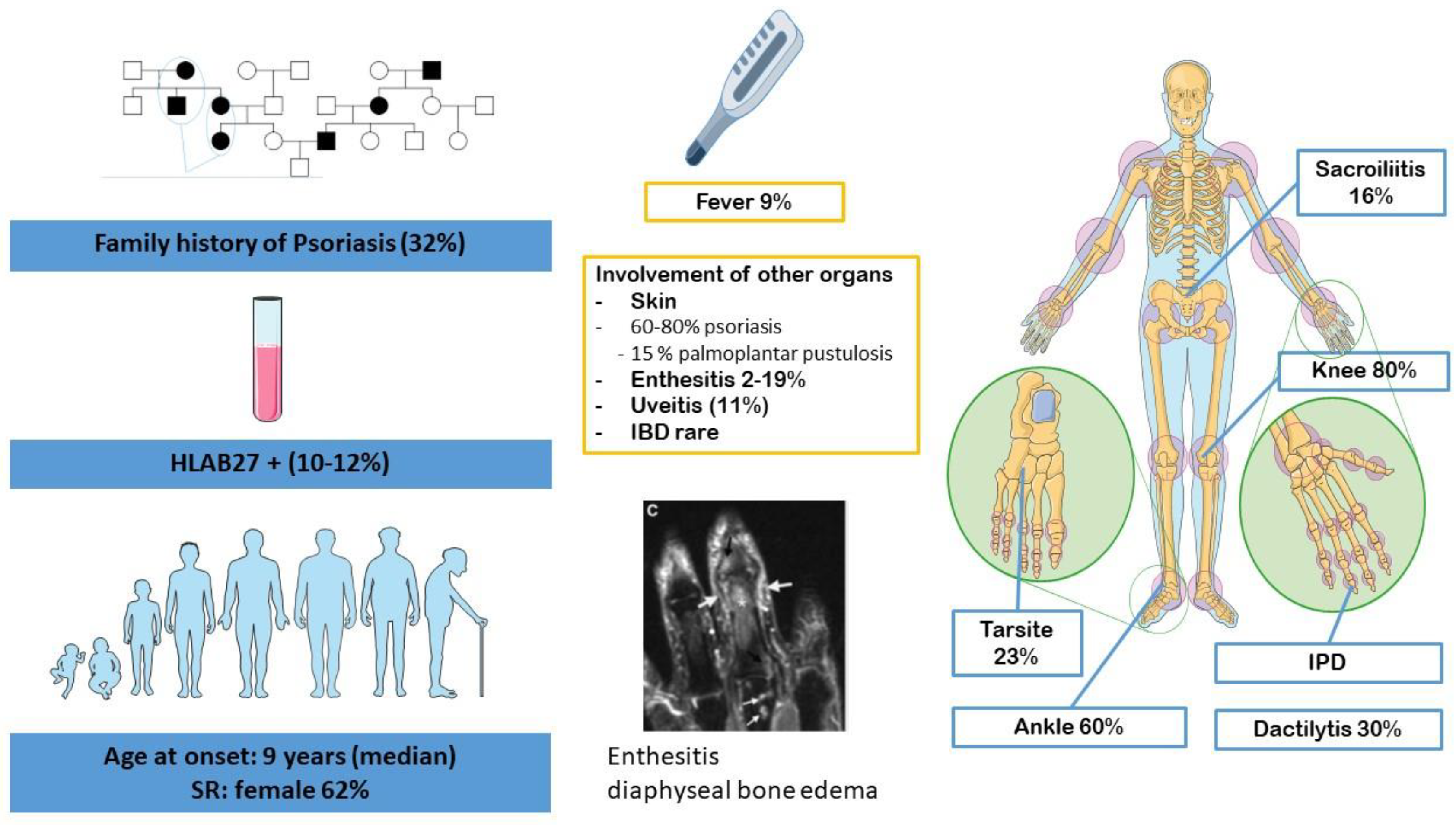
3. Juvenile Spondyloarthritis
4. Links between CRMO and JSpA
4.1. Clinical Overlap
4.1.1. Bone Inflammation, Joint Involvement and Enthesitis
4.1.2. Gender, Genetic Background and Familial History
4.1.3. Skin Involvement
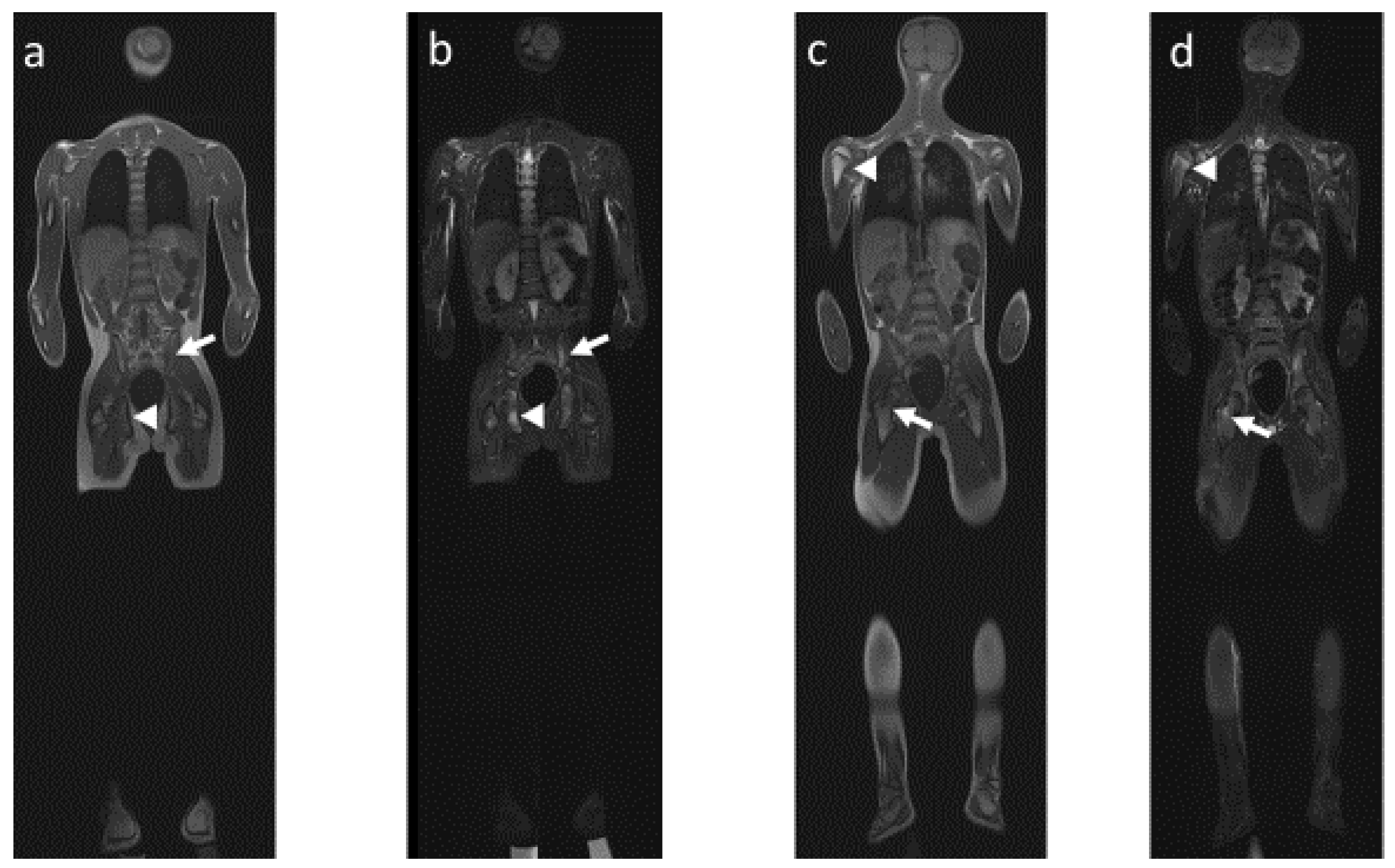
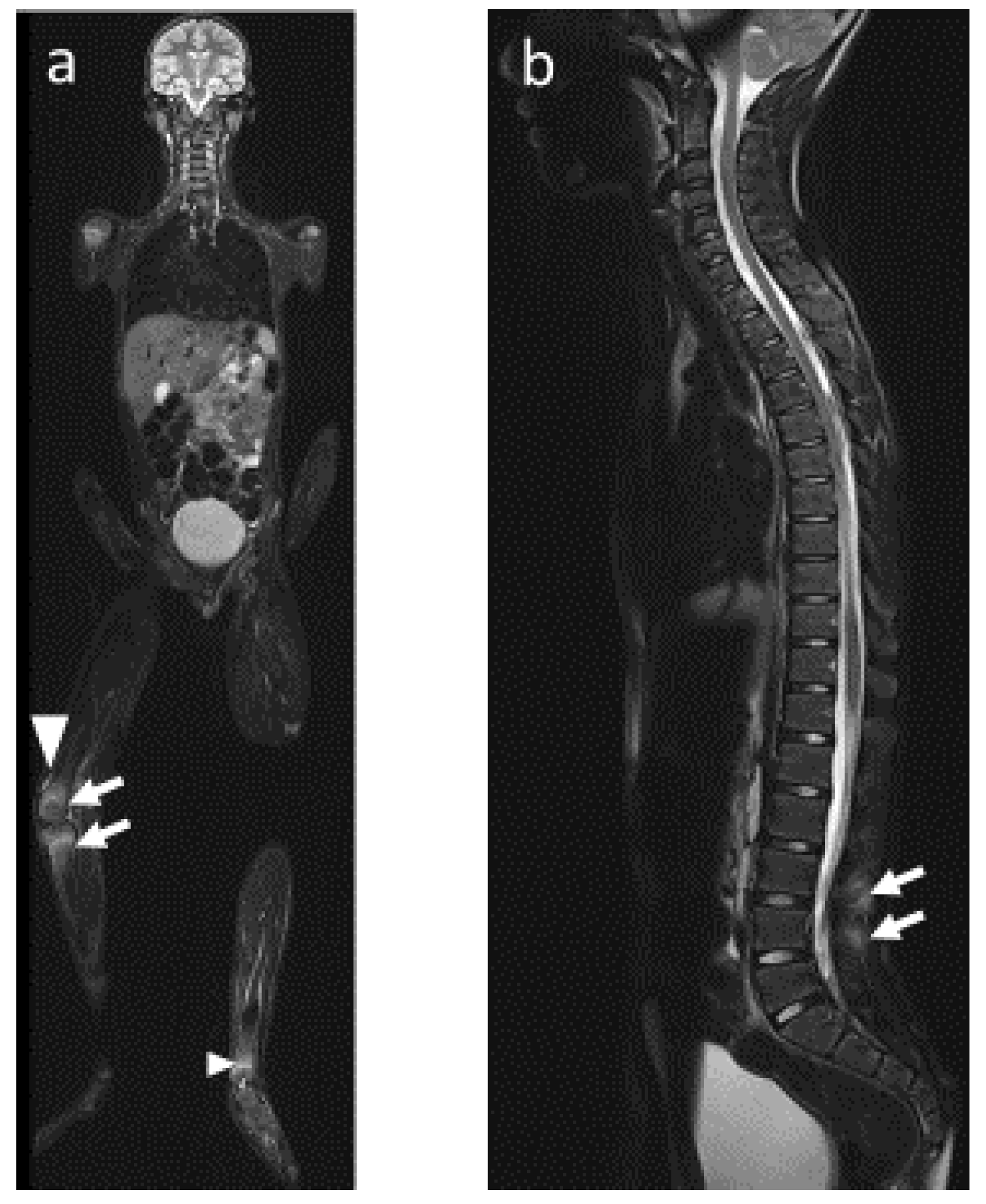
4.2. Radiological Particularities of Each Entity
- (1)
- Bone lesions
- (2)
- Vertebral and paravertebral involvement
- (3)
- Sacroiliac involvement
- (4)
- Mandibular involvement
4.3. Pathophysiological Particularity: Differences and Similarities
4.3.1. IL17/23 Axis
Spondyloarthritis
CRMO
4.3.2. Intestinal Inflammation: The Common Link between CRMO and JSpA?
4.3.3. Innate Immunity: A Common Thread?
4.3.4. What about the HLAB27
4.4. Similar Therapeutic Approaches
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansson, A.; Renner, E.D.; Ramser, J.; Mayer, A.; Haban, M.; Meindl, A.; Grote, V.; Diebold, J.; Jansson, V.; Schneider, K.; et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatol. Oxf. Engl. 2007, 46, 154–160. [Google Scholar] [CrossRef]
- Jansson, A.F.; Grote, V.; ESPED Study Group. Nonbacterial osteitis in children: Data of a German Incidence Surveillance Study. Acta Paediatr. 2011, 100, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; McCann, L.; Hahn, G.; Hedrich, C.M. Chronic nonbacterial osteomyelitis (CNO) and chronic recurrent multifocal osteomyelitis (CRMO). J. Transl. Autoimmun. 2021, 4, 100095. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Tzaribatchev, N.; Claussen, C.D.; Carrino, J.A.; Horger, M.S. Chronic recurrent multifocal osteomyelitis: Comparison of whole-body MR imaging with radiography and correlation with clinical and laboratory data. Radiology 2009, 252, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.F.; Khan, M.A. The SAPHO syndrome. Baillieres Clin. Rheumatol. 1994, 8, 333–362. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.F. Proposed classification criteria of SAPHO syndrome. In Proceedings of the American College of Rheumatology 67th Annual Scientific Meeting, Orlando, FL, USA, 23–28 October 2003. [Google Scholar]
- Andreasen, C.M.; Jurik, A.G.; Glerup, M.B.; Høst, C.; Mahler, B.T.; Hauge, E.-M.; Herlin, T. Response to Early-onset Pamidronate Treatment in Chronic Nonbacterial Osteomyelitis: A Retrospective Single-center Study. J. Rheumatol. 2019, 46, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Girschick, H.; Finetti, M.; Orlando, F.; Schalm, S.; Insalaco, A.; Ganser, G.; Nielsen, S.; Herlin, T.; Koné-Paut, I.; Martino, S.; et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: A series of 486 cases from the Eurofever international registry. Rheumatol. Oxf. Engl. 2018, 57, 1203–1211. [Google Scholar] [CrossRef]
- Tay, S.H.; Yeo, J.G.; Leong, J.Y.; Albani, S.; Arkachaisri, T. Juvenile Spondyloarthritis: What More Do We Know About HLA-B27, Enthesitis, and New Bone Formation? Front. Med. 2021, 8, 666772. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.M.L.; Laxer, R.M. Juvenile spondyloarthropathy. Curr. Opin. Rheumatol. 2003, 15, 374–379. [Google Scholar] [CrossRef]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef]
- Srinivasalu, H.; Sikora, K.A.; Colbert, R.A. Recent Updates in Juvenile Spondyloarthritis. Rheum. Dis. Clin. N. Am. 2021, 47, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Gensler, L.S.; Ward, M.M.; Reveille, J.D.; Learch, T.J.; Weisman, M.H.; Davis, J.C. Clinical, radiographic and functional differences between juvenile-onset and adult-onset ankylosing spondylitis: Results from the PSOAS cohort. Ann. Rheum. Dis. 2008, 67, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Zisman, D.; Gladman, D.D.; Stoll, M.L.; Strand, V.; Lavi, I.; Hsu, J.J.; Mellins, E.D.; CARRA Legacy Registry Investigators. The Juvenile Psoriatic Arthritis Cohort in the CARRA Registry: Clinical Characteristics, Classification, and Outcomes. J. Rheumatol. 2017, 44, 342–351. [Google Scholar] [CrossRef]
- Rumsey, D.G.; Lougee, A.; Matsouaka, R.; Collier, D.H.; Schanberg, L.E.; Schenfeld, J.; Shiff, N.J.; Stoll, M.L.; Stryker, S.; Weiss, P.F.; et al. Juvenile Spondyloarthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry: High Biologic Use, Low Prevalence of HLA-B27, and Equal Sex Representation in Sacroiliitis. Arthritis Care Res. 2021, 73, 940–946. [Google Scholar] [CrossRef]
- Aoust, L.; Rossi-Semerano, L.; Koné-Paut, I.; Dusser, P. Time to diagnosis in juvenile idiopathic arthritis: A french perspective. Orphanet J. Rare Dis. 2017, 12, 43. [Google Scholar] [CrossRef]
- Stoll, M.L.; Bhore, R.; Dempsey-Robertson, M.; Punaro, M. Spondyloarthritis in a pediatric population: Risk factors for sacroiliitis. J. Rheumatol. 2010, 37, 2402–2408. [Google Scholar] [CrossRef]
- Romero-López, J.P.; Elewaut, D.; Pacheco-Tena, C.; Burgos-Vargas, R. Inflammatory Foot Involvement in Spondyloarthritis: From Tarsitis to Ankylosing Tarsitis. Front. Med. 2021, 8, 730273. [Google Scholar] [CrossRef]
- Weiss, P.F.; Xiao, R.; Biko, D.M.; Chauvin, N.A. Assessment of Sacroiliitis at Diagnosis of Juvenile Spondyloarthritis by Radiography, Magnetic Resonance Imaging, and Clinical Examination. Arthritis Care Res. 2016, 68, 187–194. [Google Scholar] [CrossRef]
- Lin, C.; MacKenzie, J.D.; Courtier, J.L.; Gu, J.T.; Milojevic, D. Magnetic resonance imaging findings in juvenile spondyloarthropathy and effects of treatment observed on subsequent imaging. Pediatr. Rheumatol. Online J. 2014, 12, 25. [Google Scholar] [CrossRef]
- Vittecoq, O.; Said, L.A.; Michot, C.; Mejjad, O.; Thomine, J.M.; Mitrofanoff, P.; Lechevallier, J.; Ledosseur, P.; Gayet, A.; Lauret, P.; et al. Evolution of chronic recurrent multifocal osteitis toward spondylarthropathy over the long term. Arthritis Rheum. 2000, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Goirand, M.; Breton, S.; Chevallier, F.; Duong, N.-P.; Uettwiller, F.; Melki, I.; Mouy, R.; Wouters, C.; Bader-Meunier, B.; Job-Deslandre, C.; et al. Clinical features of children with enthesitis-related juvenile idiopathic arthritis/juvenile spondyloarthritis followed in a French tertiary care pediatric rheumatology centre. Pediatr. Rheumatol. Online J. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Jadon, D.R.; Van den Bosch, F.; Mease, P.J.; Gladman, D.D. Axial involvement in psoriatic arthritis: An update for rheumatologists. Semin. Arthritis Rheum. 2021, 51, 880–887. [Google Scholar] [CrossRef]
- Hofmann, S.R.; Kapplusch, F.; Girschick, H.J.; Morbach, H.; Pablik, J.; Ferguson, P.J.; Hedrich, C.M. Chronic Recurrent Multifocal Osteomyelitis (CRMO): Presentation, Pathogenesis, and Treatment. Curr. Osteoporos. Rep. 2017, 15, 542–554. [Google Scholar] [CrossRef]
- Benjamin, M.; Moriggl, B.; Brenner, E.; Emery, P.; McGonagle, D.; Redman, S. The “enthesis organ” concept: Why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004, 50, 3306–3313. [Google Scholar] [CrossRef]
- Mathew, A.J.; Østergaard, M. Magnetic Resonance Imaging of Enthesitis in Spondyloarthritis, Including Psoriatic Arthritis-Status and Recent Advances. Front. Med. 2020, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Jurik, A.G.; Klicman, R.F.; Simoni, P.; Robinson, P.; Teh, J. SAPHO and CRMO: The Value of Imaging. Semin. Musculoskelet. Radiol. 2018, 22, 207–224. [Google Scholar] [CrossRef]
- Rohekar, G.; Inman, R.D. Conundrums in nosology: Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome and spondylarthritis. Arthritis Rheum. 2006, 55, 665–669. [Google Scholar] [CrossRef]
- Borzutzky, A.; Stern, S.; Reiff, A.; Zurakowski, D.; Steinberg, E.A.; Dedeoglu, F.; Sundel, R.P. Pediatric chronic nonbacterial osteomyelitis. Pediatrics 2012, 130, e1190–e1197. [Google Scholar] [CrossRef]
- Weiss, P.F.; Chauvin, N.A. Imaging in the diagnosis and management of axial spondyloarthritis in children. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101596. [Google Scholar] [CrossRef]
- Felbo, S.K.; Terslev, L.; Østergaard, M. Imaging in peripheral and axial psoriatic arthritis: Contributions to diagnosis, follow-up, prognosis and knowledge of pathogenesis. Clin. Exp. Rheumatol. 2018, 36, 24–34. [Google Scholar] [PubMed]
- Queiro, R.; Tejón, P.; Alonso, S.; Alperi, M.; Ballina, J. Erosive discovertebral lesion (Andersson lesion) as the first sign of disease in axial psoriatic arthritis. Scand. J. Rheumatol. 2013, 42, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Mathew, A.J.; Koppikar, S.; Eder, L.; Østergaard, M. Imaging in the diagnosis and management of peripheral psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101594. [Google Scholar] [CrossRef] [PubMed]
- Khanna, G.; Sato, T.S.P.; Ferguson, P. Imaging of chronic recurrent multifocal osteomyelitis. Radiogr. Rev. Publ. Radiol. Soc. North Am. Inc. 2009, 29, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Earwaker, J.W.S.; Cotten, A. SAPHO: Syndrome or concept? Imaging findings. Skeletal Radiol. 2003, 32, 311–327. [Google Scholar] [CrossRef]
- Aquino, M.R.; Tse, S.M.L.; Gupta, S.; Rachlis, A.C.; Stimec, J. Whole-body MRI of juvenile spondyloarthritis: Protocols and pictorial review of characteristic patterns. Pediatr. Radiol. 2015, 45, 754–762. [Google Scholar] [CrossRef]
- Falip, C.; Alison, M.; Boutry, N.; Job-Deslandre, C.; Cotten, A.; Azoulay, R.; Adamsbaum, C. Chronic recurrent multifocal osteomyelitis (CRMO): A longitudinal case series review. Pediatr. Radiol. 2013, 43, 355–375. [Google Scholar] [CrossRef]
- Hospach, T.; Langendoerfer, M.; von Kalle, T.; Maier, J.; Dannecker, G.E. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur. J. Pediatr. 2010, 169, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, P.; de Horatio, L.T.; Toma, P.; Ording Müller, L.-S.; Avenarius, D.; von Brandis, E.; Zadig, P.; Casazza, I.; Pardeo, M.; Pires-Marafon, D.; et al. Chronic nonbacterial osteomyelitis—Clinical and magnetic resonance imaging features. Pediatr. Radiol. 2021, 51, 282–288. [Google Scholar] [CrossRef]
- McGonagle, D.G.; McInnes, I.B.; Kirkham, B.W.; Sherlock, J.; Moots, R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: Recent advances and controversies. Ann. Rheum. Dis. 2019, 78, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; van der Heijde, D.; Landewé, R.; Mpofu, S.; Rahman, P.; Tahir, H.; Singhal, A.; Boettcher, E.; Navarra, S.; Meiser, K.; et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann. Rheum. Dis. 2018, 77, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Nash, P.; Kirkham, B.; Okada, M.; Rahman, P.; Combe, B.; Burmester, G.-R.; Adams, D.H.; Kerr, L.; Lee, C.; Shuler, C.L.; et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: Results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017, 389, 2317–2327. [Google Scholar] [CrossRef]
- Mease, P.J.; van der Heijde, D.; Ritchlin, C.T.; Okada, M.; Cuchacovich, R.S.; Shuler, C.L.; Lin, C.-Y.; Braun, D.K.; Lee, C.H.; Gladman, D.D.; et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017, 76, 79–87. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.R.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef]
- Dick, A.D.; Tugal-Tutkun, I.; Foster, S.; Zierhut, M.; Melissa Liew, S.H.; Bezlyak, V.; Androudi, S. Secukinumab in the treatment of noninfectious uveitis: Results of three randomized, controlled clinical trials. Ophthalmology 2013, 120, 777–787. [Google Scholar] [CrossRef]
- Sharma, S.M.; Fu, D.J.; Xue, K. A Review of the Landscape of Targeted Immunomodulatory Therapies for Non-Infectious Uveitis. Ophthalmol. Ther. 2018, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.R.; Morbach, H.; Schwarz, T.; Rösen-Wolff, A.; Girschick, H.J.; Hedrich, C.M. Attenuated TLR4/MAPK signaling in monocytes from patients with CRMO results in impaired IL-10 expression. Clin. Immunol. 2012, 145, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J.; Paul, D.; Gahr, M.; Hedrich, C.M. Pilot study: Possible association of IL10 promoter polymorphisms with CRMO. Rheumatol. Int. 2012, 32, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.R.; Kubasch, A.S.; Ioannidis, C.; Rösen-Wolff, A.; Girschick, H.J.; Morbach, H.; Hedrich, C.M. Altered expression of IL-10 family cytokines in monocytes from CRMO patients result in enhanced IL-1β expression and release. Clin. Immunol. 2015, 161, 300–307. [Google Scholar] [CrossRef]
- Hofmann, S.R.; Schwarz, T.; Möller, J.C.; Morbach, H.; Schnabel, A.; Rösen-Wolff, A.; Girschick, H.J.; Hedrich, C.M. Chronic non-bacterial osteomyelitis is associated with impaired Sp1 signaling, reduced IL10 promoter phosphorylation, and reduced myeloid IL-10 expression. Clin. Immunol. 2011, 141, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Chitu, V.; Marquardt, A.; Hanke, P.; Schmittwolf, C.; Zeitlmann, L.; Schropp, P.; Barth, B.; Yu, P.; Paffenholz, R.; et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood 2006, 107, 3350–3358. [Google Scholar] [CrossRef]
- Lamot, L.; Miler, M.; Vukojević, R.; Vidović, M.; Lamot, M.; Trutin, I.; Gabaj, N.N.; Harjaček, M. The Increased Levels of Fecal Calprotectin in Children With Active Enthesitis Related Arthritis and MRI Signs of Sacroiliitis: The Results of a Single Center Cross-Sectional Exploratory Study in Juvenile Idiopathic Arthritis Patients. Front. Med. 2021, 8, 650619. [Google Scholar] [CrossRef]
- Mielants, H.; Veys, E.M.; Cuvelier, C.; De Vos, M.; Goemaere, S.; Maertens, M.; Joos, R. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy--A prospective study. J. Rheumatol. 1993, 20, 1567–1572. [Google Scholar]
- Rausch, P.; Hartmann, M.; Baines, J.F.; von Bismarck, P. Analysis of the fecal and oral microbiota in chronic recurrent multifocal osteomyelitis. Arthritis Res. Ther. 2022, 24, 54. [Google Scholar] [CrossRef]
- Lukens, J.R.; Gurung, P.; Vogel, P.; Johnson, G.R.; Carter, R.A.; McGoldrick, D.J.; Bandi, S.R.; Calabrese, C.R.; Vande Walle, L.; Lamkanfi, M.; et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014, 516, 246–249. [Google Scholar] [CrossRef]
- Cassel, S.L.; Janczy, J.R.; Bing, X.; Wilson, S.P.; Olivier, A.K.; Otero, J.E.; Iwakura, Y.; Shayakhmetov, D.M.; Bassuk, A.G.; Abu-Amer, Y.; et al. Inflammasome-independent IL-1β mediates autoinflammatory disease in Pstpip2-deficient mice. Proc. Natl. Acad. Sci. USA 2014, 111, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Chan, Y.; Kheur, S.; Kheur, M.; Gopalakrishnan, D.; Watt, R.M.; Mattheos, N. Salivary microbiome of an urban Indian cohort and patterns linked to subclinical inflammation. Oral Dis. 2017, 23, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Colina, M.; Lo Monaco, A.; Khodeir, M.; Trotta, F. Propionibacterium acnes and SAPHO syndrome: A case report and literature review. Clin. Exp. Rheumatol. 2007, 25, 457–460. [Google Scholar] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Watad, A.; Bridgewood, C.; Russell, T.; Marzo-Ortega, H.; Cuthbert, R.; McGonagle, D. The Early Phases of Ankylosing Spondylitis: Emerging Insights from Clinical and Basic Science. Front. Immunol. 2018, 9, 2668. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Liava, C.; Daoussis, D.; Akriviadis, E.; Garyfallos, A.; Dimitroulas, T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. 2019, 25, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Harjacek, M. Immunopathophysiology of Juvenile Spondyloarthritis (jSpA): The “Out of the Box” View on Epigenetics, Neuroendocrine Pathways and Role of the Macrophage Migration Inhibitory Factor (MIF). Front. Med. 2021, 8, 700982. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Vanaki, N.; Aslani, S.; Jamshidi, A.; Mahmoudi, M. Role of innate immune system in the pathogenesis of ankylosing spondylitis. Biomed. Pharmacother. 2018, 105, 130–143. [Google Scholar] [CrossRef]
- Assassi, S.; Reveille, J.D.; Arnett, F.C.; Weisman, M.H.; Ward, M.M.; Agarwal, S.K.; Gourh, P.; Bhula, J.; Sharif, R.; Sampat, K.; et al. Whole-blood gene expression profiling in ankylosing spondylitis shows upregulation of toll-like receptor 4 and 5. J. Rheumatol. 2011, 38, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Xie, Z.; Huang, L.; Yang, R.; Gao, L.; Tang, Y.; Zhang, X.; Ye, J.; Chen, K.; et al. Whole Genome Expression Profiling and Signal Pathway Screening of MSCs in Ankylosing Spondylitis. Stem Cells Int. 2014, 2014, 913050. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Q.; Swart, D.; Alcorn, D.; Tocker, J.; Elkon, K.B. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007, 56, 1145–1151. [Google Scholar] [CrossRef]
- Mao, H.; Pan, F.; Guo, H.; Bu, F.; Xin, T.; Chen, S.; Guo, Y. Feedback mechanisms between M2 macrophages and Th17 cells in colorectal cancer patients. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 12223–12230. [Google Scholar] [CrossRef]
- Weiss, P.F.; Roth, J. Juvenile-Versus Adult-Onset Spondyloarthritis: Similar, but Different. Rheum. Dis. Clin. N. Am. 2020, 46, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Reiser, C.; Klotsche, J.; Hospach, A.; Berendes, R.; Schnabel, A.; Jansson, A.F.; Hufnagel, M.; Grösch, N.; Niewerth, M.; Minden, K.; et al. First-year follow-up of children with chronic nonbacterial osteomyelitis-an analysis of the German National Pediatric Rheumatologic Database from 2009 to 2018. Arthritis Res. Ther. 2021, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Laxer, R.M.; Manson, D.; Gold, R. Chronic recurrent multifocal osteomyelitis: A noninfectious inflammatory process. Pediatr. Infect. Dis. J. 1987, 6, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Skrabl-Baumgartner, A.; Singer, P.; Greimel, T.; Gorkiewicz, G.; Hermann, J. Chronic non-bacterial osteomyelitis: A comparative study between children and adults. Pediatr. Rheumatol. Online J. 2019, 17, 49. [Google Scholar] [CrossRef]
- Hedrich, C.M.; Morbach, H.; Reiser, C.; Girschick, H.J. New Insights into Adult and Paediatric Chronic Non-bacterial Osteomyelitis CNO. Curr. Rheumatol. Rep. 2020, 22, 52. [Google Scholar] [CrossRef]


| CRMO | SpA | |
|---|---|---|
| SR (boys/girls) | 0/5 | 1/7 |
| Age at onset (years) | 10 | 9, 5 |
| Fever | 17–20% | 9% |
| Axial involvement | 60% | 63% |
| Dorsal spine | 24% | 24% |
| Lumbar spine | rare | 44% |
| Sacroiliac joints | 13–52% | 47% |
| Enthesis | 18–33% | 86% |
| Peripheral arthritis | 12–30% | 87% |
| HLAB27 | 10% | 38–68% |
| JSpA | SAPHO | CRMO | ||
|---|---|---|---|---|
| ERA | JPsA | |||
| Clinical Manifestations | ||||
| Axial | Inflammatory back pain | Inflammatory back pain Asymptomatic | Anterior chest wall pain Soft tissue swelling Pain in the spine or gluteal region | Musculoskeletal complaints such as pain, tenderness and/or swelling plus hyperostosis of the medial end of the clavicle |
| Peripherical | Arthritis of the large joints | Polyarticular involvement | Long bone pain (30%) Peripheral arthritis (12–60%) | Long bone pain (30%) Peripheral arthritis (12%) Clavicular lesion |
| Enthesitis | 64–72% | 18–33% | 13–50% | 18–33% |
| Skin involvement | Psoriasis 2% | Psoriasis 60–80% PPP 14.6% | PPP 90% Psoriasis 8–16% Acne 5–10% | Psoriasis 20% Acne 5% |
| Imaging |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koné-Paut, I.; Mannes, I.; Dusser, P. Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related? J. Clin. Med. 2023, 12, 453. https://doi.org/10.3390/jcm12020453
Koné-Paut I, Mannes I, Dusser P. Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related? Journal of Clinical Medicine. 2023; 12(2):453. https://doi.org/10.3390/jcm12020453
Chicago/Turabian StyleKoné-Paut, Isabelle, Inès Mannes, and Perrine Dusser. 2023. "Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related?" Journal of Clinical Medicine 12, no. 2: 453. https://doi.org/10.3390/jcm12020453
APA StyleKoné-Paut, I., Mannes, I., & Dusser, P. (2023). Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related? Journal of Clinical Medicine, 12(2), 453. https://doi.org/10.3390/jcm12020453






