The Effect of Latent Tuberculosis Infection on Ovarian Reserve and Pregnancy Outcomes among Infertile Women Undergoing Intrauterine Insemination: A Retrospective Cohort Study with Propensity Score Matching
Abstract
:1. Introduction
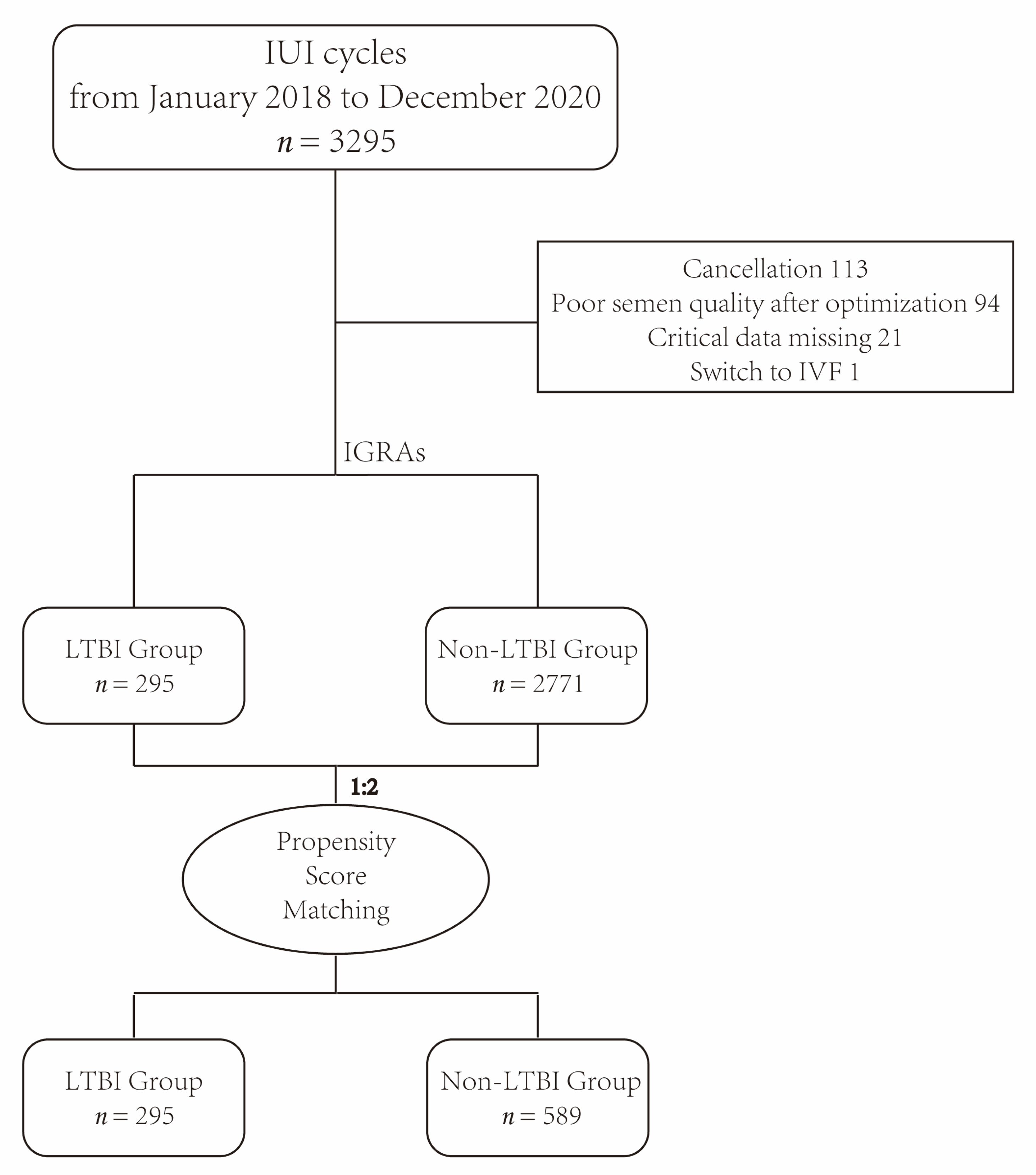
2. Materials and Methods
2.1. Study Design
2.2. Etiologies of Infertility
2.3. Ovulation Induction Protocols and Management in the IUI Program
2.4. Semen Preparation and Insemination
2.5. Luteal Phase Support
2.6. Primary and Secondary Outcomes
2.7. Statistical Analysis
2.8. Ethics Approval and Consent to Participate
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 27 October 2022).
- Lu, C.; Fang, H.; Lu, P.; Ji, L. The global tuberculosis report 2021: Key data analysis for China and the global world. Electron. J. Emerg. Infect. Dis. 2021, 6, 368–372. [Google Scholar] [CrossRef]
- Haas, M.K.; Belknap, R.W. Diagnostic Tests for Latent Tuberculosis Infection. Clin. Chest Med. 2019, 40, 829–837. [Google Scholar] [CrossRef]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef]
- Gao, L.; Lu, W.; Bai, L.; Wang, X.; Xu, J.; Catanzaro, A.; Cárdenas, V.; Li, X.; Yang, Y.; Du, J.; et al. Latent tuberculosis infection in rural China: Baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect. Dis. 2015, 15, 310–319. [Google Scholar] [CrossRef]
- Zhang, H.C.; Ruan, Q.L.; Wu, J.; Zhang, S.; Yu, S.L.; Wang, S.; Gao, Y.; Wang, F.F.; Shao, L.Y.; Zhang, W.H. Serial T-SPOT.TB in household contacts of tuberculosis patients: A 6-year observational study in China. Int. J. Tuberc. Lung Dis. 2019, 23, 989–995. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Q.; Graviss, E.A.; Jiang, W.; Yuan, Z.; Xu, B. Use of the T-SPOT.TB assay to screen latent tuberculosis infection among the TB contacts in Shanghai, China. J. Infect. 2012, 65, 39–48. [Google Scholar] [CrossRef]
- Guo, L.P.; Jiang, Y.; Liu, Y.M.; Cao, B. First assessment of interferon gamma release assay results among healthcare workers at a general hospital in China. Clin. Respir. J. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Geng, M.J.; Song, Y.D.; Xiong, Y.C.; Zhao, F.; Hu, D.M.; Li, M.; Hou, Y.Y.; Cheng, J.; He, G.X. Comparison and analysis of TST and IGRAs for testing tuberculosis infection of health care workers. Chin. J. Antitubercul. 2014, 36, 121–125. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.; Qian, S.; Li, X.; Zhang, H.; Li, M.; Feng, B.; Jin, Q.; Gao, L. Testing of tuberculosis infection among Chinese adolescents born after terminating the Bacillus Calmette-Guerin booster vaccination: Subgroup analysis of a population-based cross-sectional study. Front. Med. 2017, 11, 528–535. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Q.; Wu, L.; Wang, W.; Yuan, Z.; Xu, B. Prevalence of latent tuberculosis infection and its risk factors in schoolchildren and adolescents in Shanghai, China. Eur. J. Public Health 2013, 23, 1064–1069. [Google Scholar] [CrossRef]
- Mir, N.; Pal, L. Genital tuberculosis, infertility and assisted reproduction. Curr. Opin. Obstet. Gynecol. 2023, 35, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Grace, G.A.; Devaleenal, D.B.; Natrajan, M. Genital tuberculosis in females. Indian J. Med. Res. 2017, 145, 425–436. [Google Scholar]
- Jirge, P.R.; Chougule, S.M.; Keni, A.; Kumar, S.; Modi, D. Latent genital tuberculosis adversely affects the ovarian reserve in infertile women. Hum. Reprod. 2018, 33, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, R.; Li, R.; Wang, H.; Qiao, J.; Mol, B.W.J. Ovarian stimulation in infertile women treated with the use of intrauterine insemination: A cohort study from China. Fertil. Steril. 2018, 109, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Merviel, P.; Heraud, M.H.; Grenier, N.; Lourdel, E.; Sanguinet, P.; Copin, H. Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature. Fertil. Steril. 2010, 93, 79–88. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Gelbaya, T.A.; Potdar, N.; Jeve, Y.B.; Nardo, L.G. Definition and epidemiology of unexplained infertility. Obstet. Gynecol. Surv. 2014, 69, 109–115. [Google Scholar] [CrossRef]
- Chen, Z.J.; Liu, J.Y.; Huang, H.F.; Qiao, J.; Zhou, C.Q.; Huang, G.N.; Sun, Y.P.; Yang, D.Z.; Liang, X.Y.; Yu, Q.; et al. Guideline on diagnosis of infertility. Chin. J. Obstet. Gynecol. 2019, 54, 505–511. [Google Scholar] [CrossRef]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, S. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3129. [Google Scholar] [CrossRef]
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: Relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962. [Google Scholar] [CrossRef]
- Dai, W.; Ma, L.; Cao, Y.; Wu, D.; Yu, T.; Zhai, J. In vitro fertilization outcome in women with endometrial tuberculosis and tubal tuberculosis. Gynecol. Endocrinol. 2020, 36, 819–823. [Google Scholar] [CrossRef]
- Bhanothu, V.; Theophilus, J.P.; Reddy, P.K.; Rozati, R. Occurrence of female genital tuberculosis among infertile women: A study from a tertiary maternal health care research centre in South India. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Li, R. Progress in diagnosis and treatment of female genital tuberculosis induced infertility. Chin. J. Obstet. Gynecol. 2015, 50, 954–956. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Hao, C.; Zhang, H.; Wei, D.; Zhang, Y.; Zhu, Y.; Deng, X.; Qi, X.; Li, H.; et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N. Engl. J. Med. 2018, 378, 126–136. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ 2019, 367, l5770. [Google Scholar] [CrossRef]
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef]
- Tam, J.K. Latent Mycobacterium tuberculosis Infection. N. Engl. J. Med. 2015, 373, 1178–1179. [Google Scholar] [CrossRef]
- Lalvani, A.; Pareek, M. Interferon gamma release assays: Principles and practice. Enferm. Infecc. Microbiol. Clin. 2010, 28, 245–252. [Google Scholar] [CrossRef]
- Pai, M.; Zwerling, A.; Menzies, D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: An update. Ann. Intern. Med. 2008, 149, 177–184. [Google Scholar] [CrossRef]
- Farhat, M.; Greenaway, C.; Pai, M.; Menzies, D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 2006, 10, 1192–1204. [Google Scholar] [PubMed]
- Cattamanchi, A.; Ssewenyana, I.; Davis, J.L.; Huang, L.; Worodria, W.; den Boon, S.; Yoo, S.; Andama, A.; Hopewell, P.C.; Cao, H. Role of interferon-gamma release assays in the diagnosis of pulmonary tuberculosis in patients with advanced HIV infection. BMC Infect. Dis. 2010, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Lawal, T.; Granger, E.; Simoni, M.; Hui, P.; Buza, N.; Pal, L. Genital tuberculosis screening at an academic fertility center in the United States. Am. J. Obstet. Gynecol. 2020, 223, 737.e1–737.e10. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Sharma, V.; Bahadur, A.; Sharma, J.B.; Roy, K.K.; Kumar, S. The effect of tuberculosis on ovarian reserve among women undergoing IVF in India. Int. J. Gynaecol. Obstet. 2012, 117, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Gurgan, T.; Urman, B.; Yarali, H. Results of in vitro fertilization and embryo transfer in women with infertility due to genital tuberculosis. Fertil. Steril. 1996, 65, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Efferen, L.S. Tuberculosis and pregnancy. Curr. Opin. Pulm. Med. 2007, 13, 205–211. [Google Scholar] [CrossRef]
- Miele, K.; Morris, S.B.; Tepper, N.K. Tuberculosis in Pregnancy. Obstet. Gynecol. 2020, 135, 1444–1453. [Google Scholar] [CrossRef]
- Gai, X.Y.; Chi, H.B.; Zeng, L.; Cao, W.L.; Chen, L.X.; Zhang, C.; Lu, M.; Ning, L.D.; Chang, C.; Zhang, W.X.; et al. Untreated Prior Pulmonary Tuberculosis Adversely Affects Pregnancy Outcomes in Infertile Women Undergoing in vitro Fertilization and Embryo Transfer: A Large Retrospective Cohort Study. Biomed. Environ. Sci. 2021, 34, 130–138. [Google Scholar] [CrossRef]
- Diedrich, K.; Fauser, B.C.; Devroey, P.; Griesinger, G.; Evian Annual Reproduction Workshop, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 2007, 13, 365–377. [Google Scholar] [CrossRef]
- Mekinian, A.; Cohen, J.; Alijotas-Reig, J.; Carbillon, L.; Nicaise-Roland, P.; Kayem, G.; Darai, E.; Fain, O.; Bornes, M. Unexplained Recurrent Miscarriage and Recurrent Implantation Failure: Is There a Place for Immunomodulation? Am. J. Reprod. Immunol. 2016, 76, 8–28. [Google Scholar] [CrossRef]
- Dam, P.; Shirazee, H.H.; Goswami, S.K.; Ghosh, S.; Ganesh, A.; Chaudhury, K.; Chakravarty, B. Role of latent genital tuberculosis in repeated IVF failure in the Indian clinical setting. Gynecol. Obstet. Investig. 2006, 61, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, N.; Gennarelli, G.; Vanni, V.S.; Ferrari, S.; Ruffa, A.; Rovere-Querini, P.; Pagliardini, L.; Candiani, M.; Papaleo, E. Concomitant Autoimmunity in Endometriosis Impairs Endometrium-Embryo Crosstalk at the Implantation Site: A Multicenter Case-Control Study. J. Clin. Med. 2023, 12, 3557. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, U.; Baloni, P.; Singh, A.; Chandra, N. Immune Subtyping in Latent Tuberculosis. Front. Immunol. 2021, 12, 595746. [Google Scholar] [CrossRef] [PubMed]
- Latorre, I.; Fernandez-Sanmartin, M.A.; Muriel-Moreno, B.; Villar-Hernandez, R.; Vila, S.; Souza-Galvao, M.L.; Stojanovic, Z.; Jimenez-Fuentes, M.A.; Centeno, C.; Ruiz-Manzano, J.; et al. Study of CD27 and CCR4 Markers on Specific CD4(+) T-Cells as Immune Tools for Active and Latent Tuberculosis Management. Front. Immunol. 2018, 9, 3094. [Google Scholar] [CrossRef]
- Shah, D.K. Diminished ovarian reserve and endometriosis: Insult upon injury. Semin. Reprod. Med. 2013, 31, 144–149. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Wang, K.; Ren, D.; Qiu, Z.; Li, W. Clinical analysis of pregnancy complicated with miliary tuberculosis. Ann. Med. 2022, 54, 71–79. [Google Scholar] [CrossRef]
- Xia, L.; Mijiti, P.; Liu, X.H.; Hu, Z.D.; Fan, X.Y.; Lu, S.H. Association of in vitro fertilization with maternal and perinatal outcomes among pregnant women with active tuberculosis: A retrospective hospital-based cohort study. Front. Public Health 2022, 10, 1021998. [Google Scholar] [CrossRef]
- Zhuang, G.; Yang, L.; Qu, L.; Liu, W.; Zhu, H. Congenital tuberculosis in a neonate following in vitro fertilization-embryo transfer: A case report. Front. Pediatr. 2022, 10, 985707. [Google Scholar] [CrossRef]
- Samedi, V.; Field, S.K.; Al Awad, E.; Ratcliffe, G.; Yusuf, K. Congenital tuberculosis in an extremely preterm infant conceived after in vitro fertilization: Case report. BMC Pregnancy Childbirth 2017, 17, 66. [Google Scholar] [CrossRef]
- McLaughlin, S.E.; Vora, S.B.; Church, E.C.; Spitters, C.; Thyer, A.; LaCourse, S.; Herndon, C.N. Adverse pregnancy outcomes after in vitro fertilization due to undiagnosed urogenital tuberculosis and proposed screening algorithm for patients from tuberculosis-endemic countries. F&S Rep. 2022, 3, 285–291. [Google Scholar] [CrossRef]
- Gai, X.; Chi, H.; Cao, W.; Zeng, L.; Chen, L.; Zhang, W.; Song, D.; Wang, Y.; Liu, P.; Li, R.; et al. Acute miliary tuberculosis in pregnancy after in vitro fertilization and embryo transfer: A report of seven cases. BMC Infect. Dis. 2021, 21, 913. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Wang, C.; Zhao, L.; Wu, X.; Gao, Y.; Liu, H. Characteristics of miliary tuberculosis in pregnant women after in vitro fertilisation and embryo transfer. Int. J. Tuberc. Lung Dis. 2019, 23, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Froberg, G.; Jansson, L.; Nyberg, K.; Obasi, B.; Westling, K.; Berggren, I.; Bruchfeld, J. Screening and treatment of tuberculosis among pregnant women in Stockholm, Sweden, 2016–2017. Eur. Respir. J. 2020, 55, 1900851. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.R.; Winters, N.; Menzies, D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: Systematic review and meta-analysis. BMJ 2020, 368, m549. [Google Scholar] [CrossRef] [PubMed]
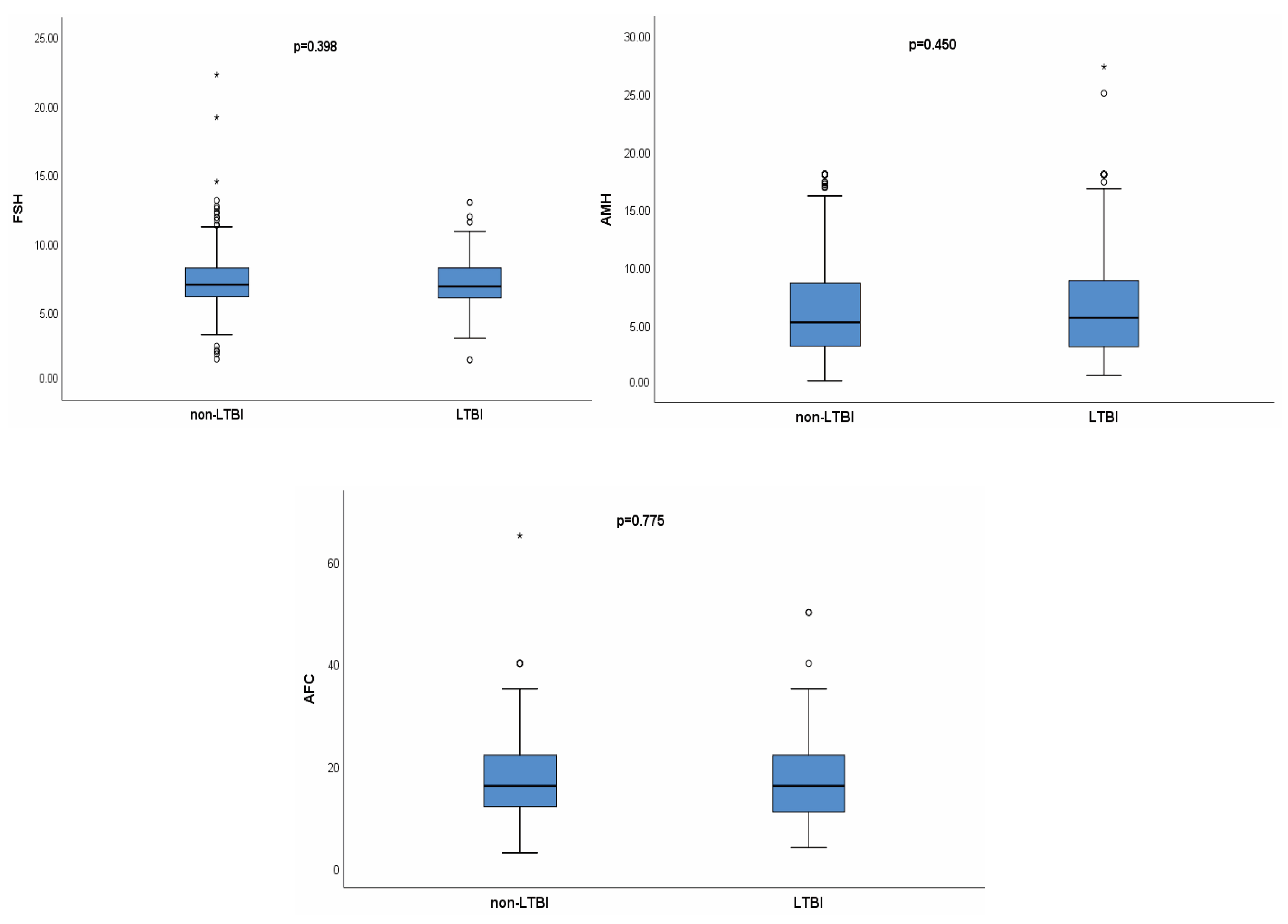
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Non-LTBI (n = 2771) | LTBI (n = 295) | p-Value | Non-LTBI (n = 589) | LTBI (n = 295) | p-Value | |
| Age (years) | 29.47 ± 3.44 | 29.95 ± 3.37 | 0.021 * | 29.76 ± 3.47 | 29.95 ± 3.37 | 0.437 |
| Etiology of Infertility | ||||||
| PCOS (%) | 27.9 (774/2771) | 35.6 (105/295) | 0.006 * | 34.3 (202/589) | 35.6 (105/295) | 0.702 |
| Other ovary disorder (%) | 6.4 (176/2771) | 5.4 (16/295) | 0.532 | 5.8 (34/589) | 5.4 (16/295) | 0.832 |
| Fallopian tube and pelvic factor (%) | 16.1 (446/2771) | 12.5 (37/295) | 0.111 | 17.1 (101/589) | 12.5 (37/295) | 0.075 |
| Endometriosis (%) | 3.1 (86/2771) | 0.3 (1/295) | 0.007 * | 0.3 (2/589) | 0.3 (1/295) | >0.999 |
| Male factor (%) | 56.4 (1562/2771) | 52.5 (155/295) | 0.208 | 54.2 (319/589) | 52.5 (155/295) | 0.649 |
| Uterine factor (%) | 8.8 (244/2771) | 6.8 (20/295) | 0.238 | 7.5 (44/589) | 6.8 (20/295) | 0.709 |
| Chromosome factor (%) | 11.2 (309/2771) | 7.1 (21/295) | 0.034 * | 7.8 (46/589) | 7.1 (21/295) | 0.714 |
| Unexplained (%) | 22.5 (624/2771) | 24.7 (73/295) | 0.386 | 21.2 (125/589) | 24.7 (73/295) | 0.236 |
| Type of IUI | 0.078 | 0.965 | ||||
| AIH (%) | 72.5 (2009/2771) | 77.3 (228/295) | 77.4 (456/589) | 77.3 (228/295) | ||
| AID (%) | 27.5 (762/2771) | 22.7 (67/295) | 22.6 (133/589) | 22.7 (67/295) | ||
| Type of Infertility | 0.013 * | 0.191 | ||||
| Primary infertility (%) | 84.8 (2351/2771) | 79.3 (234/295) | 75.4 (444/589) | 79.3 (234/295) | ||
| Secondary infertility (%) | 15.2 (420/2771) | 20.7 (61/295) | 24.6 (157/912) | 20.7 (61/295) | ||
| Duration of Infertility (years) | 3.00 (2.00, 4.00) | 2.00 (2.00, 4.00) | 0.845 | 3.00 (2.00, 4.00) | 2.00 (2.00, 4.00) | 0.740 |
| BMI (kg/m2) | 21.71 ± 3.01 | 21.77 ± 2.67 | 0.691 | 21.99 ± 3.10 | 21.77 ± 2.67 | 0.278 |
| Ovulation Protocol | 0.295 | 0.648 | ||||
| Ovarian stimulation (%) | 86.6 (2401/2771) | 88.8 (262/295) | 89.8 (529/589) | 88.8 (262/295) | ||
| Natural ovulation (%) | 13.4 (370/2771) | 11.2 (33/295) | 10.2 (60/589) | 11.2 (33/295) | ||
| Endometrial Thickness on HCG day (mm) | 9.30 ± 2.04 | 9.32 ± 2.13 | 0.898 | 9.22 ± 2.04 | 9.32 ± 2.13 | 0.524 |
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Non-LTBI (n = 2771) | LTBI (n = 295) | p-Value | Non-LTBI (n = 589) | LTBI (n = 295) | p-Value | |
| Biochemical pregnancy (%) | 15.1 (419/2771) | 12.9 (38/295) | 0.305 | 17.7 (104/589) | 12.9 (38/295) | 0.068 |
| Clinical pregnancy (%) | 12.7 (352/2771) | 10.8 (32/295) | 0.360 | 15.1 (89/589) | 10.8 (32/295) | 0.082 |
| Live birth (%) | 10.6 (294/2771) | 8.1 (24/295) | 0.185 | 12.1 (71/589) | 8.1 (24/295) | 0.076 |
| Ectopic pregnancy (%) | 0.3 (8/2771) | 0.3 (1/295) | 0.598 | 0.3 (2/589) | 0.3 (1/295) | >0.999 |
| Miscarriage (%) | 16.5 (58/352) | 25.0 (8/32) | 0.221 | 20.2 (18/89) | 25.0 (8/32) | 0.573 |
| Multiple pregnancy (%) | 4.8 (17/352) | 3.1 (1/32) | >0.999 | 1.1 (1/89) | 3.1 (1/32) | 0.461 |
| Gestational age (weeks) | 38.58 ± 1.59 | 38.88 ± 1.26 | 0.373 | 38.77 ± 1.47 | 38.88 ± 1.26 | 0.765 |
| Mean birth weight (g) | 3240.37 ± 504.02 | 3301.04 ± 509.07 | 0.571 | 3341.55 ± 492.06 | 3301.04 ± 509.07 | 0.730 |
| Caesarean section (%) | 65.0 (191/294) | 70.8 (17/24) | 0.561 | 71.8 (51/71) | 70.8 (17/24) | 0.925 |
| Maternal and neonatal complications | ||||||
| Preterm Birth (%) | 5.1 (18/352) | 3.1 (1/32) | 0.943 | 4.5 (4/89) | 3.1 (1/32) | >0.999 |
| Gestational Diabetes Mellitus (%) | 4.3 (15/352) | 3.1 (1/32) | >0.999 | 4.5 (4/89) | 3.1 (1/32) | >0.999 |
| Pregnancy-induced Hypertension (%) | 2.6 (9/352) | 3.1 (1/32) | 0.586 | 2.2 (2/89) | 3.1 (1/32) | >0.999 |
| Low Birth Weight (%) | 5.4 (19/352) | 6.3 (2/32) | >0.999 | 2.2 (2/89) | 6.3 (2/32) | 0.610 |
| Fetal Macrosomia (%) | 2.3 (8/352) | 3.1 (1/32) | 0.547 | 2.2 (2/89) | 3.1 (1/32) | >0.999 |
| Placenta Previa (%) | 1.1 (4/352) | 0 | >0.999 | 1.1 (1/89) | 0 | >0.999 |
| Premature Rupture of Membranes (%) | 1.1 (4/352) | 0 | >0.999 | 1.1 (1/89) | 0 | >0.999 |
| Intrahepatic Cholestasis of Pregnancy (%) | 0.3 (1/352) | 0 | >0.999 | 1.1 (1/89) | 0 | >0.999 |
| Fetal Growth Restriction (%) | 0.3 (1/352) | 0 | >0.999 | 0 | 0 | N/A |
| Birth Defect (%) | 0.3 (1/352) | 0 | >0.999 | 0 | 0 | N/A |
| Asphyxia of Newborn (%) | 0.3 (1/352) | 0 | >0.999 | 1.1 (1/89) | 0 | >0.999 |
| Pneumonia of Newborn (%) | 0.3 (1/352) | 0 | >0.999 | 0 | 0 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Chen, Y.; Yao, W.; Wang, L.; Zhang, B.; Jin, L.; Yue, J. The Effect of Latent Tuberculosis Infection on Ovarian Reserve and Pregnancy Outcomes among Infertile Women Undergoing Intrauterine Insemination: A Retrospective Cohort Study with Propensity Score Matching. J. Clin. Med. 2023, 12, 6398. https://doi.org/10.3390/jcm12196398
Chu Y, Chen Y, Yao W, Wang L, Zhang B, Jin L, Yue J. The Effect of Latent Tuberculosis Infection on Ovarian Reserve and Pregnancy Outcomes among Infertile Women Undergoing Intrauterine Insemination: A Retrospective Cohort Study with Propensity Score Matching. Journal of Clinical Medicine. 2023; 12(19):6398. https://doi.org/10.3390/jcm12196398
Chicago/Turabian StyleChu, Yifan, Ying Chen, Wen Yao, Luyao Wang, Bo Zhang, Lei Jin, and Jing Yue. 2023. "The Effect of Latent Tuberculosis Infection on Ovarian Reserve and Pregnancy Outcomes among Infertile Women Undergoing Intrauterine Insemination: A Retrospective Cohort Study with Propensity Score Matching" Journal of Clinical Medicine 12, no. 19: 6398. https://doi.org/10.3390/jcm12196398
APA StyleChu, Y., Chen, Y., Yao, W., Wang, L., Zhang, B., Jin, L., & Yue, J. (2023). The Effect of Latent Tuberculosis Infection on Ovarian Reserve and Pregnancy Outcomes among Infertile Women Undergoing Intrauterine Insemination: A Retrospective Cohort Study with Propensity Score Matching. Journal of Clinical Medicine, 12(19), 6398. https://doi.org/10.3390/jcm12196398








