Management of Open Pediatric Fractures: Proposal of a New Multidisciplinary Algorithm
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. First Evaluation
3.2. Antibiotic Therapy
3.3. Cultures
3.4. Secondary Survey
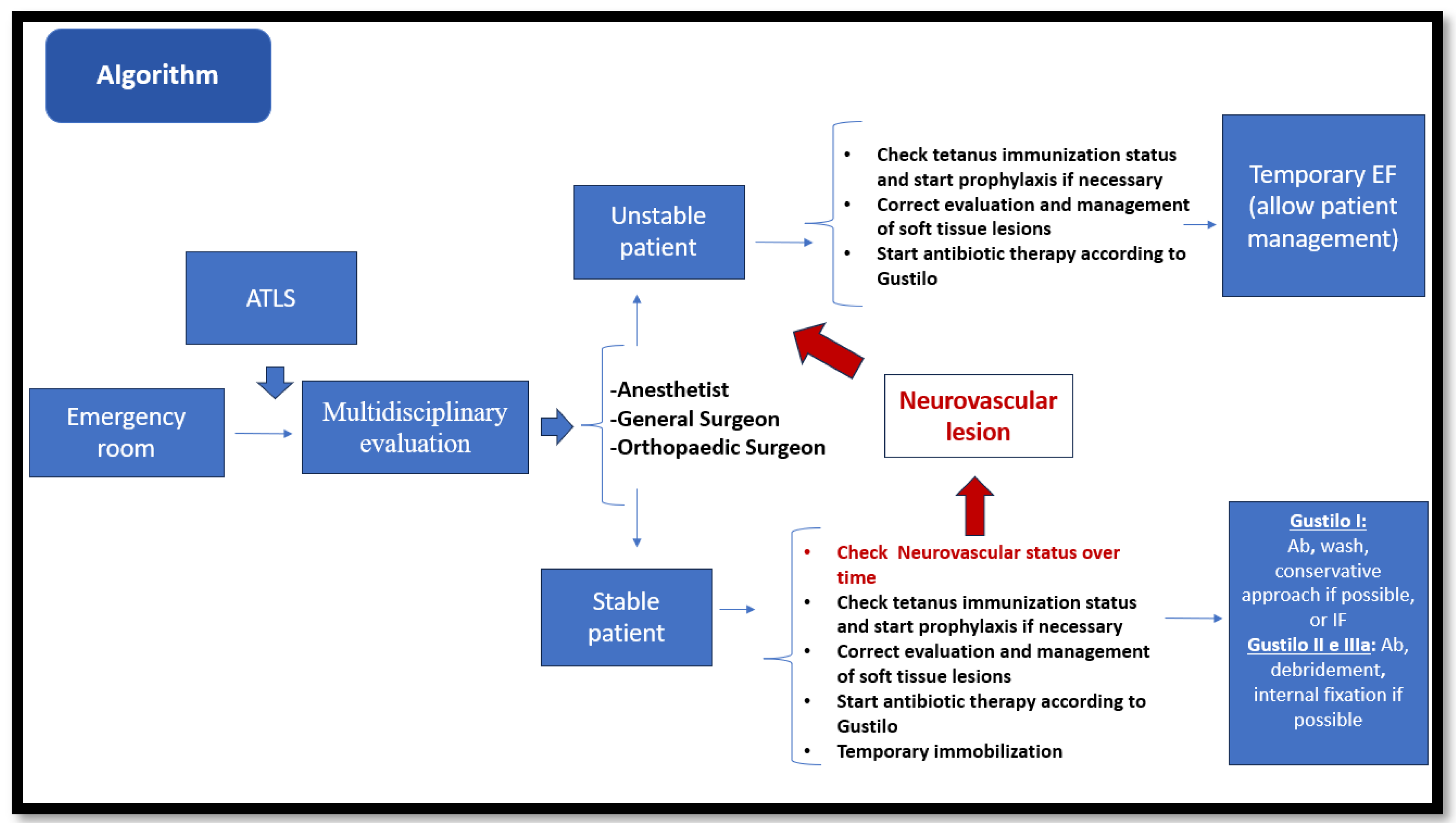
3.5. Trauma Team: Management in the Emergency Department
- -
- The excision of necrotic tissue from the margins of the wound.
- -
- The extension of the wound to adequately explore the fracture ends.
- -
- The debridement of the wound margins to the bleeding tissue.
- -
- The resection of the skin, adipose tissue, muscle tissue, and fascia contaminated in necrosis.
- -
- Fasciotomies, if necessary.
- -
- The meticulous irrigation of fracture and wound heads.
3.6. Choice of Treatment: When and Which
3.7. Use of Vac Therapy
3.8. Post-Operative Rehabilitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rexiti, P.; Zhang, T.C.; Batuer, C.; Cao, L. Orthopedic treatment for open fracture of lower extremities and soft tissue defects in young children and rapid rehabilitation after operation. Phys. Sportsmed. 2020, 48, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.G.; Kay, R.M.; Skaggs, D.L. Open fractures in children. Principles of evaluation and management. J. Bone Joint Surg. Am. 2005, 87, 2784–2798. [Google Scholar] [CrossRef] [PubMed]
- Trionfo, A.; Cavanaugh, P.K.; Herman, M.J. Pediatric Open Fractures. Orthop. Clin. N. Am. 2016, 47, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Lahoti, O.; Arya, A. Management of Orthopaedic Injuries in Multiply Injured Child. Indian. J. Orthop. 2018, 52, 454–461. [Google Scholar] [CrossRef]
- Grisoli, A.; Dynako, J.; Zimmer, D.; Zackariya, N.; Shariff, F.; Walsh, M.; Mamczak, C.N.; Peterson, C.; Boyer, B.; Hurwich, M.; et al. Management of a Pediatric Type 3C Open Femoral Fracture Following a High-Velocity Gunshot Wound at an Adult Level II Trauma Center. Pediatr. Emerg. Care 2021, 37, e574–e578. [Google Scholar] [CrossRef]
- Erdös, J.; Dlaska, C.; Szatmary, P.; Humenberger, M.; Vécsei, V.; Hajdu, S. Acute compartment syndrome in children: A case series in 24 patients and review of the literature. Int. Orthop. 2011, 35, 569–575. [Google Scholar] [CrossRef]
- Flynn, J.M.; Bashyal, R.K.; Yeger-McKeever, M.; Garner, M.R.; Launay, F.; Sponseller, P.D. Acute traumatic compartment syndrome of the leg in children: Diagnosis and outcome. J. Bone Joint Surg. Am. 2011, 93, 937–941. [Google Scholar] [CrossRef]
- Mauffrey, C.; Bailey, J.R.; Bowles, R.J.; Price, C.; Hasson, D.; Hak, D.J.; Stahel, P.F. Acute management of open fractures: Proposal of a new multidisciplinary algorithm. Orthopedics 2012, 35, 877–881. [Google Scholar] [CrossRef]
- Faraj, A.A. The reliability of the pre-operative classification of open tibial fractures in children a proposal for a new classification. Acta Orthop. Belg. 2002, 68, 49–55. [Google Scholar]
- Elia, G.; Blood, T.; Got, C. The Management of Pediatric Open Forearm Fractures. J. Hand Surg. 2020, 45, 523–527. [Google Scholar] [CrossRef]
- Singh, A.; Bierrum, W.; Wormald, J.; Eastwood, D.M. Non-operative versus operative management of open fractures in the paediatric population: A systematic review and meta-analysis of the adverse outcomes. Injury 2020, 51, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.W.; Troyer, S.C.; Martus, J.E. Pediatric Open Long-Bone Fracture and Subsequent Deep Infection Risk: The Importance of Early Hospital Care. Child 2022, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.L.; Kocher, M.S.; Skaggs, D.L. Evidence-based review: Management of open pediatric fractures. J. Pediatr. Orthop. 2012, 32 (Suppl. 2), S123–S127. [Google Scholar] [CrossRef]
- Godfrey, J.; Pace, J.L. Type I Open Fractures Benefit From Immediate Antibiotic Administration But Not Necessarily Immediate Surgery. J. Pediatr. Orthop. 2016, 36 (Suppl. 1), S6–S10. [Google Scholar] [CrossRef]
- Lavelle, W.F.; Uhl, R.; Krieves, M.; Drvaric, D.M. Management of open fractures in pediatric patients: Current teaching in Accreditation Council for Graduate Medical Education (ACGME) accredited residency programs. J. Pediatr. Orthop. B 2008, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Valenziano, C.P.; Chattar-Cora, D.; O’Neill, A.; Hubli, E.H.; Cudjoe, E.A. Efficacy of primary wound cultures in long bone open extremity fractures: Are they of any value? Arch. Orthop. Trauma Surg. 2002, 122, 259–261. [Google Scholar] [CrossRef]
- Lingaraj, R.; Santoshi, J.A.; Devi, S.; Najimudeen, S.; Gnanadoss, J.J.; Kanagasabai, R.; Kanungo, R. Predebridement wound culture in open fractures does not predict postoperative wound infection: A pilot study. J. Nat. Sci. Biol. Med. 2015, 6 (Suppl. 1), S63–S68. [Google Scholar]
- Ryan, S.P.; Pugliano, V. Controversies in Initial Management of Open Fractures. Scand. J. Surg. 2014, 103, 132–137. [Google Scholar] [CrossRef]
- Hao, M.; Peng, A.Q. Comparison of bacteria isolated from open fractures following debridement and subsequent infection. J. Orthop. Sci. 2021, 26, 243–246. [Google Scholar] [CrossRef]
- Black, K.D.; Cico, S.J.; Caglar, D. Wound management. Pediatr. Rev. 2015, 36, 207–215, quiz 216. [Google Scholar] [CrossRef]
- Messner, J.; Harwood, P.; Johnson, L.; Itte, V.; Bourke, G.; Foster, P. Lower limb paediatric trauma with bone and soft tissue loss: Ortho-plastic management and outcome in a major trauma centre. Injury 2020, 51, 1576–1583. [Google Scholar] [CrossRef]
- Laine, J.C.; Cherkashin, A.; Samchukov, M.; Birch, J.G.; Rathjen, K.E. The Management of Soft Tissue and Bone Loss in Type IIIB and IIIC Pediatric Open Tibia Fractures. J. Pediatr. Orthop. 2016, 36, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Doak, J.; Ferrick, M. Nonoperative management of pediatric grade 1 open fractures with less than a 24-hour admission. J. Pediatr. Orthop. 2009, 29, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, N.; Khanna, A.; Maffulli, N. Open tibial fractures in the paediatric population: A systematic review of the literature. Br. Med. Bull. 2009, 91, 75–85. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Shi, Y.; Lu, Y.; Yu, B. Children with open tibial fractures show significantly lower infection rates than adults: Clinical comparative study. Int. Orthop. 2019, 43, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Nandra, R.S.; Wu, F.; Gaffey, A.; Bache, C.E. The management of open tibial fractures in children: A retrospective case series of eight years’ experience of 61 cases at a paediatric specialist centre. Bone Jt. J. 2017, 99-B, 544–553. [Google Scholar] [CrossRef]
- Yang, E.C.; Eisler, J. Treatment of isolated type I open fractures: Is emergent operative debridement necessary? Clin. Orthop. 2003, 410, 289–294. [Google Scholar] [CrossRef]
- Iobst, C.A.; Tidwell, M.A.; King, W.F. Nonoperative management of pediatric type I open fractures. J. Pediatr. Orthop. 2005, 25, 513–517. [Google Scholar] [CrossRef]
- Adili, A.; Bhandari, M.; Schemitsch, E.H. The biomechanical effect of high-pressure irrigation on diaphyseal fracture healing in vivo. J. Orthop. Trauma 2002, 16, 413–417. [Google Scholar] [CrossRef]
- Bazzi, A.A.; Brooks, J.T.; Jain, A.; Ain, M.C.; Tis, J.E.; Sponseller, P.D. Is nonoperative treatment of pediatric type I open fractures safe and effective? J. Child. Orthop. 2014, 8, 467–471. [Google Scholar] [CrossRef]
- Padgett, A.M.; Torrez, T.W.; Kothari, E.A.; Conklin, M.J.; Williams, K.A.; Gilbert, S.R.; Ashley, P. Comparison of nonoperative versus operative management in pediatric gustilo-anderson type I open tibia fractures. Injury 2023, 54, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R.J.; Minhas, S.V.; Patrick, B.C.; Janicki, J.A. Current Practice in the Management of Type I Open Fractures in Children: A Survey of POSNA Membership. J. Pediatr. Orthop. 2015, 35, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Rai, S.; Tang, X.; Liu, R.; Li, J. External fixation versus elastic stable intramedullary nailing in the treatment of open tibial shaft fractures in children. J. Orthop. Surg. 2021, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, L.E.; Bhaskar, A.R.; Cole, W.G.; Howard, A.W. Treatment of open femur fractures in children: Comparison between external fixator and intramedullary nailing. J. Pediatr. Orthop. 2007, 27, 748–750. [Google Scholar] [CrossRef]
- Skaggs, D.L.; Friend, L.; Alman, B.; Chambers, H.G.; Schmitz, M.; Leake, B.; Kay, R.M.; Flynn, J.M. The effect of surgical delay on acute infection following 554 open fractures in children. J. Bone Jt. Surg. Am. 2005, 87, 8–12. [Google Scholar] [CrossRef]
- Hubbard, E.W.; Riccio, A.I. Pediatric Orthopedic Trauma: An Evidence-Based Approach. Orthop. Clin. N. Am. 2018, 49, 195–210. [Google Scholar] [CrossRef]
- Caniano, D.A.; Ruth, B.; Teich, S. Wound management with vacuum-assisted closure: Experience in 51 pediatric patients. J. Pediatr. Surg. 2005, 40, 128–132. [Google Scholar] [CrossRef]
- Halvorson, J.; Jinnah, R.; Kulp, B.; Frino, J. Use of vacuum-assisted closure in pediatric open fractures with a focus on the rate of infection. Orthopedics 2011, 34, e256–e260. [Google Scholar] [CrossRef]
- Hoffmann, H.; Kettelhack, C. Fast-track surgery--conditions and challenges in postsurgical treatment: A review of elements of translational research in enhanced recovery after surgery. Eur. Surg. Res. 2012, 49, 24–34. [Google Scholar] [CrossRef]
| Modified Gustilo and Anderson Classification |
|---|
| Type I A: wound requiring no skin graft or flap, level of contamination is minimal, the periosteum is intact with minimal to moderate muscle damage and no bone comminution |
| Type I B: wound requiring skin graft or flap, level of contamination is moderate to severe, the periosteum is intact with minimal to moderate muscle damage and no bone comminution |
| Type II A: wound requiring no skin graft or flap, level of contamination is minimal to moderate, the periosteum is stripped with minimal to moderate muscle damage and no bone comminution or minimal comminution |
| Type II B: wound requiring skin graft or flap, level of contamination is moderate to severe, the periosteum is stripped with minimal to moderate muscle damage, and bone comminution is minimal to severe or segmental fracture |
| Type III: wound requiring skin graft or flap, level of contamination is severe, the periosteum is stripped with muscular and neurovascular injury and unstable, comminute or segmental bone fractures with or without bone loss |
| Clean Wound with a Vaccine Dose within Ten Years | Not Reaccommodated |
|---|---|
| Wound > 1 cm deep, incurred > 6 h earlier, devitalized tissue, grossly contaminated → vaccine dose within 5 years | Not recommended |
| Wound > 1 cm deep, incurred > 6 h earlier, devitalized tissue, grossly contaminated → not received a tetanus immunization within the past 5 years or if their status is unknown | Recommended |
| Tetanus Immunization |
|---|
| Patient < 7 years old: DTaP (diphtheria, tetanus, and pertussis). |
| Patient between 7 and 10 years old: give Td (tetanus and diphtheria). For older children, give Tdap (tetanus, diphtheria, and pertussis). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulisa, A.G.; Marsiolo, M.; Basiglini, L.; Aletto, C.; Giordano, M.; Falciglia, F. Management of Open Pediatric Fractures: Proposal of a New Multidisciplinary Algorithm. J. Clin. Med. 2023, 12, 6378. https://doi.org/10.3390/jcm12196378
Aulisa AG, Marsiolo M, Basiglini L, Aletto C, Giordano M, Falciglia F. Management of Open Pediatric Fractures: Proposal of a New Multidisciplinary Algorithm. Journal of Clinical Medicine. 2023; 12(19):6378. https://doi.org/10.3390/jcm12196378
Chicago/Turabian StyleAulisa, Angelo Gabriele, Martina Marsiolo, Luca Basiglini, Cristian Aletto, Marco Giordano, and Francesco Falciglia. 2023. "Management of Open Pediatric Fractures: Proposal of a New Multidisciplinary Algorithm" Journal of Clinical Medicine 12, no. 19: 6378. https://doi.org/10.3390/jcm12196378
APA StyleAulisa, A. G., Marsiolo, M., Basiglini, L., Aletto, C., Giordano, M., & Falciglia, F. (2023). Management of Open Pediatric Fractures: Proposal of a New Multidisciplinary Algorithm. Journal of Clinical Medicine, 12(19), 6378. https://doi.org/10.3390/jcm12196378







