Autoimmune Pancreatitis Type 1 with Biliary, Nasal, Testicular, and Pulmonary Involvement: A Case Report and a Systematic Review
Abstract
1. Introduction
- Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is an immune-mediated condition associated with fibroinflammatory lesions that can occur at almost any anatomical site. Autoimmune pancreatitis (AIP) type 1 is the most prominent manifestation of IgG4-RD in the digestive tract with common extra-pancreatic inflammation.
- It often presents as a multiorgan disease that may mimic malignancy, infection, or other immune-mediated conditions.
- A case of a patient with AIP type 1 and other organ involvement (bile ducts, testicles, nasal polyps, and lungs) is described.
- Additionally, a systematic review of AIP type 1 with testicular and nasal involvement was conducted.
2. Methods
3. Case Report
4. Literature Review
5. Results
5.1. AIP Type 1 and Testicular Involvement
5.2. AIP Type 1 and Nasal Cavity Involvement
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wallace, Z.S.; Naden, F.R.P.; Chari, S.; Choi, H.; Della-Torre, E.; Dicaire, J.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-Related Disease. Arthritis Rheumatol. 2020, 72, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Eishi, Y.; Koike, M.; Tsuruta, K.; Okamoto, A.; Egawa, N.; Nakajima, H. A new clinicopathological entity of IgG4-related autoimmune disease. J. Gastroenterol. 2003, 38, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Khosroshahi, A.; Deshpande, V.; Chan, J.K.C.; Heathcote, J.G.; Aalberse, R.; Azumi, A.; Bloch, D.B.; Brugge, W.R.; Carruthers, M.N.; et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012, 64, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Sarles, H.; Sarles, J.C.; Muratore, R.; Guien, C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am. J. Dig. Dis. 1961, 6, 688–698. [Google Scholar] [CrossRef]
- Yoshida, K.; Toki, F.; Takeuchi, T.; Watanabe, S.-I.; Shiratori, K.; Hayashi, N. Chronic pancreatitis caused by an autoimmune abnormality. Dig. Dis. Sci. 1995, 40, 1561–1568. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Vujasinovic, M.; Rosendahl, J.; Stone, J.H.; Beuers, U. IgG4-related diseases of the digestive tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.H.; Klöppel, G.; Lerch, M.M.; Löhr, M.; et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef]
- Nikolic, S.; Brehmer, K.; Panic, N.; Valente, R.; Löhr, J.-M.; Vujasinovic, M. Cardiovascular and Lung Involvement in Patients with Autoimmune Pancreatitis. J. Clin. Med. 2020, 9, 409. [Google Scholar] [CrossRef]
- Nikolic, S.; Panic, N.; Hintikka, E.S.; Dani, L.; Rutkowski, W.; Hedström, A.; Steiner, C.; Löhr, J.-M.; Vujasinovic, M. Efficacy and safety of rituximab in autoimmune pancreatitis type 1: Our experiences and systematic review of the literature. Scand. J. Gastroenterol. 2021, 56, 1355–1362. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Mucelli, R.M.P.; Valente, R.; Verbeke, C.S.; Haas, S.L.; Löhr, J.-M. Kidney Involvement in Patients with Type 1 Autoimmune Pancreatitis. J. Clin. Med. 2019, 8, 258. [Google Scholar] [CrossRef]
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Ishizaka, N.; Akamizu, T.; Sato, Y.; Kawano, M.; et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Moyer, A.M.; Yi, E.S.; Hogan, M.C.; Pearson, R.K.; Chari, S.T. IgG4–related paratesticular pseudotumor in a patient with autoimmune pancreatitis and retroperitoneal fibrosis: An extrapancreatic manifestation of IgG4–related disease. Hum. Pathol. 2012, 43, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- De Buy Wenniger, L.M.; Scheltema, J.M.; Verheij, J.; Beuers, U. Testicular Inflammation as a New Manifestation of IgG4-associated Disease. Urology 2013, 82, e15–e16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohno, K.; Matsuda, Y.; Arai, T.; Kimura, Y. Nasal manifestations of IgG4-related disease: A report of two cases. Auris Nasus Larynx 2015, 42, 483–487. [Google Scholar] [CrossRef]
- Cain, R.B.; Colby, T.V.; Balan, V.; Patel, N.P.; Lal, D. Perplexing Lesions of the Sinonasal Cavity and Skull Base: IgG4-related and Similar Inflammatory Diseases. Otolaryngol. Neck Surg. 2014, 151, 496–502. [Google Scholar] [CrossRef]
- Moteki, H.; Yasuo, M.; Hamano, H.; Uehara, T.; Usami, S.-I. IgG4-related chronic rhinosinusitis: A new clinical entity of nasal disease. Acta Otolaryngol. 2011, 131, 518–526. [Google Scholar] [CrossRef]
- Hanaoka, M.; Kammisawa, T.; Koizumi, S.; Kuruma, S.; Chiba, K.; Kikuyama, M.; Shirakura, S.; Sugimoto, T.; Hishima, T. Clinical features of IgG4-related rhinosinusitis. Adv. Med. Sci. 2017, 62, 393–397. [Google Scholar] [CrossRef]
- Shi, Q.; Ning, X.; Li, H.; Ma, X.; Wang, K.; Bian, W.; Zhang, Y.; Xia, J.; Zheng, X.; Liu, Y.; et al. Characteristics of IgG4-related disease complicated with allergic rhinitis or chronic rhinosinusitis: A large cross-sectional cohort study. Sci. Rep. 2022, 12, 12039. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakamaru, Y.; Akazawa, S.; Mizumachi, T.; Maeda, M.; Takagi, D.; Hatanaka, K.C.; Fukuda, S. Nasal manifestations of immunoglobulin G4-related disease. Laryngoscope 2013, 123, 829–834. [Google Scholar] [CrossRef]
- Chari, S.T.; Smyrk, T.C.; Levy, M.J.; Topazian, M.D.; Takahashi, N.; Zhang, L.; Clain, J.E.; Pearson, R.K.; Petersen, B.T.; Vege, S.S. Diagnosis of Autoimmune Pancreatitis: The Mayo Clinic Experience. Clin. Gastroenterol. Hepatol. 2006, 4, 1010–1016. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Maier, P.; von Beckerath, V.; Haas, S.L.; Arnelo, U.; Del Chiaro, M.; Kartalis, N.; Pozzi-Mucelli, R.M.; Fernandez-Moro, C.; et al. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatology 2018, 18, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Tada, M.; Isayama, H.; Sasahira, N.; Umefune, G.; Akiyama, D.; Watanabe, T.; Saito, T.; Takagi, K.; Takahara, N.; et al. Outcome of Long-term Maintenance Steroid Therapy Cessation in Patients with Autoimmune Pancreatitis: A Prospective Study. J. Clin. Gastroenterol. 2016, 50, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Isogawa, A.; Tada, M.; Isayama, H.; Takahara, N.; Miyabayashi, K.; Mizuno, S.; Mohri, D.; Kawakubo, K.; Sasaki, T.; et al. Long-Term Prognosis of Autoimmune Pancreatitis in Terms of Glucose Tolerance. Pancreas 2012, 41, 691–695. [Google Scholar] [CrossRef]
- Hart, P.A.; Kamisawa, T.; Brugge, W.R.; Chung, J.B.; Culver, E.L.; Czakó, L.; Frulloni, L.; Go, V.L.W.; Gress, T.M.; Kim, M.-H.; et al. Long-term outcomes of autoimmune pancreatitis: A multicentre, international analysis. Gut 2013, 62, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.M.; Beuers, U.; Vujasinovic, M.; Alvaro, D.; Frøkjær, J.B.; Buttgereit, F.; Capurso, G.; Culver, E.L.; De Madaria, E.; Della-Torre, E.; et al. European Guideline on IgG4-related digestive disease—UEG and SGF evidence-based recommendations. United Eur. Gastroenterol. J. 2020, 8, 637–666. [Google Scholar]
- Bösmüller, H.; von Weyhern, C.H.; Adam, P.; Alibegovic, V.; Mikuz, G.; Fend, F. Paratesticular fibrous pseudotumor—An IgG4-related disorder? Virchows Arch. Int. J. Pathol. 2011, 458, 109–113. [Google Scholar] [CrossRef]

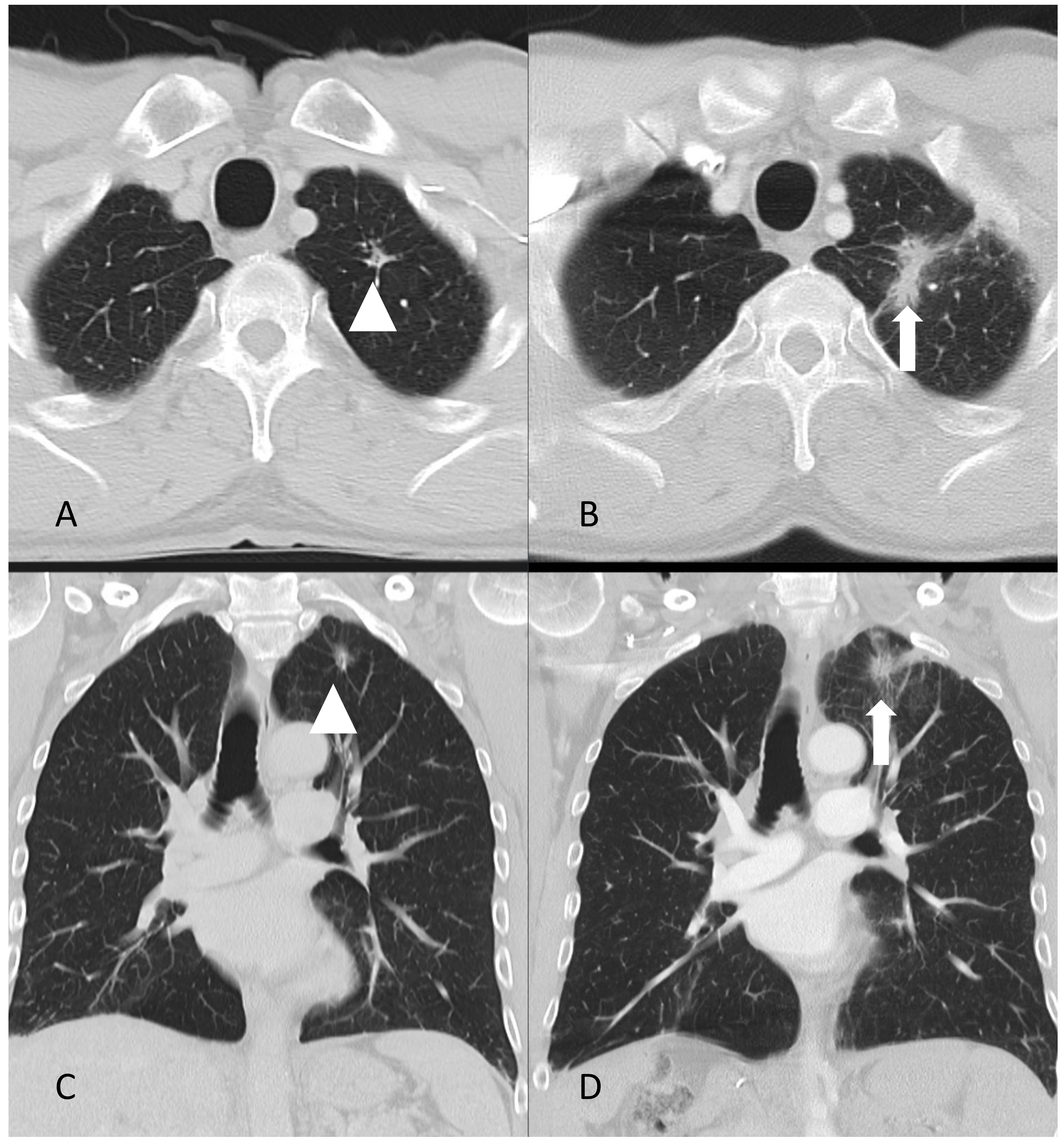


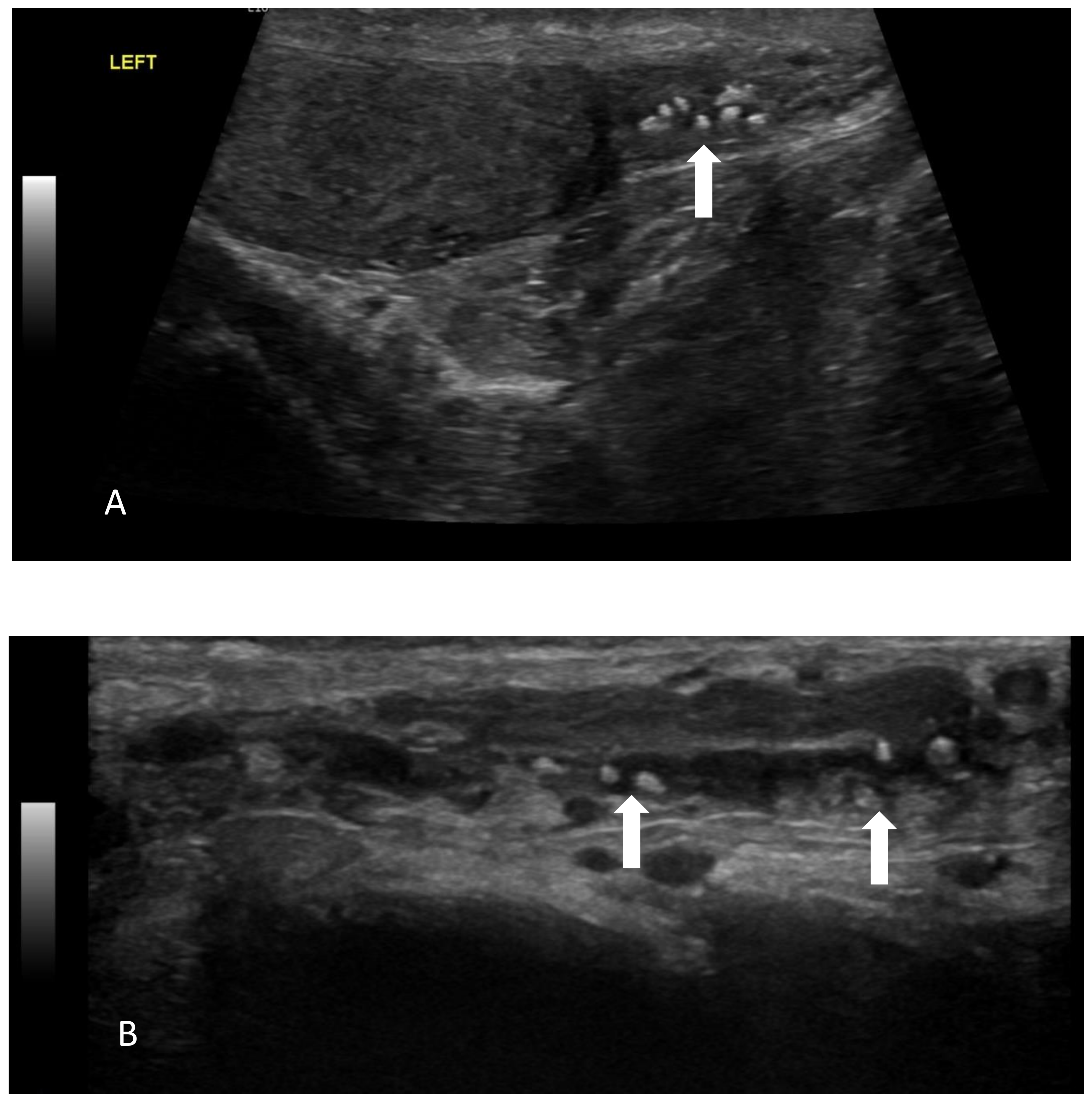
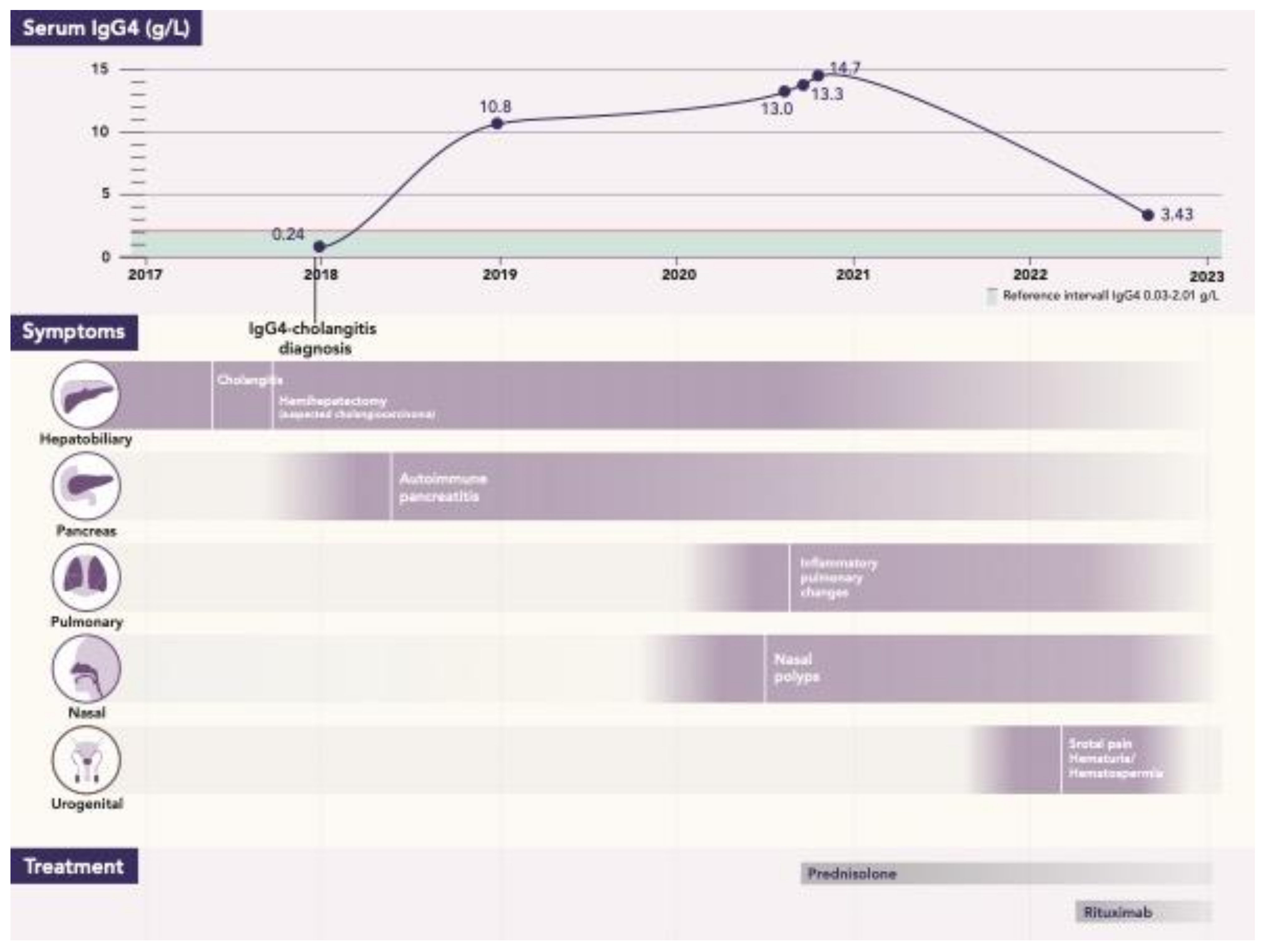


| Author, Year, Country | Age | Medical History and Clinical Presentation | Treatment | Histology | Follow-Up |
|---|---|---|---|---|---|
| Hart et al. [12], 2012, USA | 67 | IgG4-RD in pancreas (histologically confirmed), retroperitoneal fibrosis causing left hydronephrosis and bilateral kidney involvement with renal failure. Patient was successfully treated with 3-month course of corticosteroids and ureteral stenting. At the same time, patient developed DM. Five years after IgG4-RD diagnosis, patient presented with painless right scrotal mass that had been gradually increasing in size for three months. | Due to the potential risk of malignancy, patient underwent right inguinal radical orchiectomy. | Grossly, para-testicular tissue was involved with cystic formation. Microscopy showed lymphoplasmacytic inflammatory infiltrate, storiform fibrosis, obliterative phlebitis, perineural inflammation, and IgG4 positive cells (>50/HPF and IgG4/IgG ratio of 60%). The testicular parenchyma was not involved. | A pancreas relapse was suspected, and corticosteroids were introduced postoperatively. However, magnetic resonance imaging showed no signs of active inflammation in the pancreas, so corticosteroids were tapered off. |
| de Buy Wenniger et al. [13], 2013, the Netherlands | 64 | IgG4-RD in pancreas (histologically confirmed after Whipple operation performed due to suspected pancreatic cancer). Seven years after IgG4-RD diagnosis, patient presented with scrotal pain. | Patient was initially treated with antibiotics for suspected epididymitis. Ultrasound showed inhomogeneous alterations, and the patient underwent left orchiectomy due to suspected malignancy. Right orchiectomy was performed seven months later due to abscess-forming inflammation in the right testis. | IgG4 staining of the tissue of the right testis showed a high number of infiltrating IgG4-positive cells around a seminiferous tubule (focally maximum 50/HPF). The earlier resected left testis showed similar changes (maximum 80 IgG4-positive cells/HPF). | Not reported. |
| Author, Year, Coutry | Age, Sex | Medical History and Clinical Presentation | Histology | Treatment | Follow-Up |
|---|---|---|---|---|---|
| Moteki et al. [16], 2011, Japan | Mean age 57.6 ± 6.5 years (range of 50–68) 6 males and 1 female | A total of 7 patients (out of 31 presented) had both autoimmune pancreatitis and nasal involvement. Patients had nasal problems including nasal obstruction, rhinorrhea, postnasal drip, and anosmia, and were diagnosed as having allergic rhinitis and/or chronic rhinosinusitis. All 7 patients had elevated IgG4 in serum: 2970, 387, 773, 1610, 1910, 209, 1500 (cut-off: 135 mg/dL) | Histology was performed in 5 patients. IgG4-positive plasma cell infiltration in nasal mucosa was present in all patients: severe in 4 (>30/HPF) and moderate in 1 (10–30/HPF). | In total, 70% of patients who had chronic rhinosinusitis were treated with oral prednisolone (40 mg per day for 4 weeks, tapered 5 mg per week). Other patients were not treated because of mild symptoms, unstable diabetes mellitus, or other comorbidities. | After approximately 3 months of prednisolone administration, at the point when it was possible to reduce the dosage to 5–15 mg/day, the characteristic findings of autoimmune pancreatitis had improved in six patients. Serum IgG and IgG4 levels were markedly decreased. Nose, nasal discharge, and obstruction symptoms were also improved. |
| Suzuki et al. [19], 2013, Japan | Mean age 67.3 ±8.9 years (range 55–79) 6 males and 1 female | A total of 7 patients (out of 23 presented) had both autoimmune pancreatitis and nasal involvement. Nasal symptoms included nasal obstruction, nasal crusting, and rhinorrhea. | High infiltration of IgG4 plasma cells (>50/HPF) in nasal mucosa in all 7 patients: 220.8 ± 106.5 (range of 101–391) | Oral corticosteroids and intranasal corticosteroids were used (no details reported) | NA |
| Cain et al. [15], 2014, USA | 60, male | Nasal congestion, cervical lymphadenopathy, retro-orbital pain, and vision changes. MRI revealed a unilateral cavernous sinus-enhancing soft tissue mass. Serum IgG4 was elevated (195 mg/dL) Six months previously he had undergone a partial pancreatectomy for a pancreatic head mass, which was found to be autoimmune pancreatitis. | The pancreatectomy specimen showed focal lymphoplasmacytic infiltrate and marked sclerosis, consistent with IgG4-associated autoimmune pancreatitis. | Prednisone 40 mg daily, with rapid resolution of his symptoms. Transitioned from prednisone to azathioprine and continued to do well without relapse. | Repeat MRI after 1 month of prednisone showed a marked decrease in size of the previously noted cavernous sinus mass with minimal residual enhancement. This mass was therefore determined to be an inflammatory pseudotumor secondary to IgG4-RD. Surveillance MRI 2 years later showed complete resolution of the cavernous sinus mass. |
| Ohno et al. [14], 2015, Japan | 55, male | Swelling of the bilateral submandibular glands (4 cm in diameter) and elevated serum IgG4 (514 mg/dL). One month later, hyposmia and diffuse, erosive, crusty lesions on nasal mucosa were found. Two years after admittance, the patient was diagnosed with autoimmune pancreatitis. | Biopsy from the submandibular gland and the inferior turbinate mucosa showed dense infiltration of IgG4-positive plasma cells and a high IgG4/IgG ratio (submandibular gland 220/HPF, 70%; nasal mucosa 100/HPF, 50%) with fibrosis. | Initially, patient was treated with oral prednisolone (start 0.6 mg/kg) with tapering (suspended after one year). Two years later, the patient was diagnosed with autoimmune pancreatitis and has been followed regularly without general steroid administration. | After prednisolone treatment, the nasal mucosa normalized and the hyposmia improved. Control biopsy of nasal mucosa improved (IgG4+ lowered to 3/HPF and IgG4/IgG ratio to 5%). Serum IgG4 persisted at a high level, independently of the dose of oral prednisolone. |
| Hanaoka et al. [17], 2017, Japan | Mean age 59.8 ± 9.3 years (range 48–69) 3 males and 2 females | A total of 5 patients (out of 108) had both autoimmune pancreatitis and nasal involvement. The chief nasal complaints were hyposmia and nasal obstruction. | Abundant infiltration of IgG4-positive plasma cells (>10/HPF) was found in 3 of 4 patients in whom histology was performed (45, 20, 190/HPF). In one patient, IgG4 was 8/HPF. Neither storiform fibrosis nor obstructive phlebitis was observed in any patient. | All patients were treated with steroid therapy. Endoscopic sinus surgery was performed in 4 of 5 patients. | No recurrence was noted. |
| Shi et al. [18], 2022, China | Mean age at disease onset was 56 years (range 46–62) Male:female = 1.34:1 | 122 patients (out of 408) had pancreas involvement. The main clinical nasal manifestations were sneezing, nasal obstruction, nasal itching, and rhinorrhea. Median serum IgG4 was 650 (312–1520) mg/dL. | A total of 46 patients underwent nasal tissue biopsies, and among them, 28 patients had dense IgG4-positive plasma cells (>10/HPF) and a ratio of IgG4/IgG of > 40%. The other 4 patients had storiform fibrosis and/or obliterative phlebitis. | Patients with allergic rhinitis and chronic rhinosinusitis were sensitive to glucocorticoid therapy, and their nasal symptoms alleviated or disappeared after treatment, while chronic rhinosinusitis alone is insensitive to glucocorticoid therapy (information on treatment is available only for the whole cohort of patients in this study). | Follow up period was 51.5 (33–72) months. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourie, M.; Bogdanovic, D.; Mahmutyazicioglu, K.; Ghazi, S.; Panic, N.; Fjellgren, E.; Hellkvist, L.; Thiel, T.; Kjellman, A.; Kartalis, N.; et al. Autoimmune Pancreatitis Type 1 with Biliary, Nasal, Testicular, and Pulmonary Involvement: A Case Report and a Systematic Review. J. Clin. Med. 2023, 12, 6340. https://doi.org/10.3390/jcm12196340
Kourie M, Bogdanovic D, Mahmutyazicioglu K, Ghazi S, Panic N, Fjellgren E, Hellkvist L, Thiel T, Kjellman A, Kartalis N, et al. Autoimmune Pancreatitis Type 1 with Biliary, Nasal, Testicular, and Pulmonary Involvement: A Case Report and a Systematic Review. Journal of Clinical Medicine. 2023; 12(19):6340. https://doi.org/10.3390/jcm12196340
Chicago/Turabian StyleKourie, Mourad, Darko Bogdanovic, Kamran Mahmutyazicioglu, Sam Ghazi, Nikola Panic, Eva Fjellgren, Laila Hellkvist, Tomas Thiel, Anders Kjellman, Nikolaos Kartalis, and et al. 2023. "Autoimmune Pancreatitis Type 1 with Biliary, Nasal, Testicular, and Pulmonary Involvement: A Case Report and a Systematic Review" Journal of Clinical Medicine 12, no. 19: 6340. https://doi.org/10.3390/jcm12196340
APA StyleKourie, M., Bogdanovic, D., Mahmutyazicioglu, K., Ghazi, S., Panic, N., Fjellgren, E., Hellkvist, L., Thiel, T., Kjellman, A., Kartalis, N., Danielsson, O., Dani, L., Löhr, J.-M., & Vujasinovic, M. (2023). Autoimmune Pancreatitis Type 1 with Biliary, Nasal, Testicular, and Pulmonary Involvement: A Case Report and a Systematic Review. Journal of Clinical Medicine, 12(19), 6340. https://doi.org/10.3390/jcm12196340





