Efficacy, Safety, and Subject Satisfaction of PrabotulinumtoxinA for Moderate-to-Severe Crow’s Feet: A Phase IV, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients
2.3. Study Medication and Procedures
2.4. Efficacy Outcome Assessments
2.5. Safety Assessments
2.6. Statistical Analysis
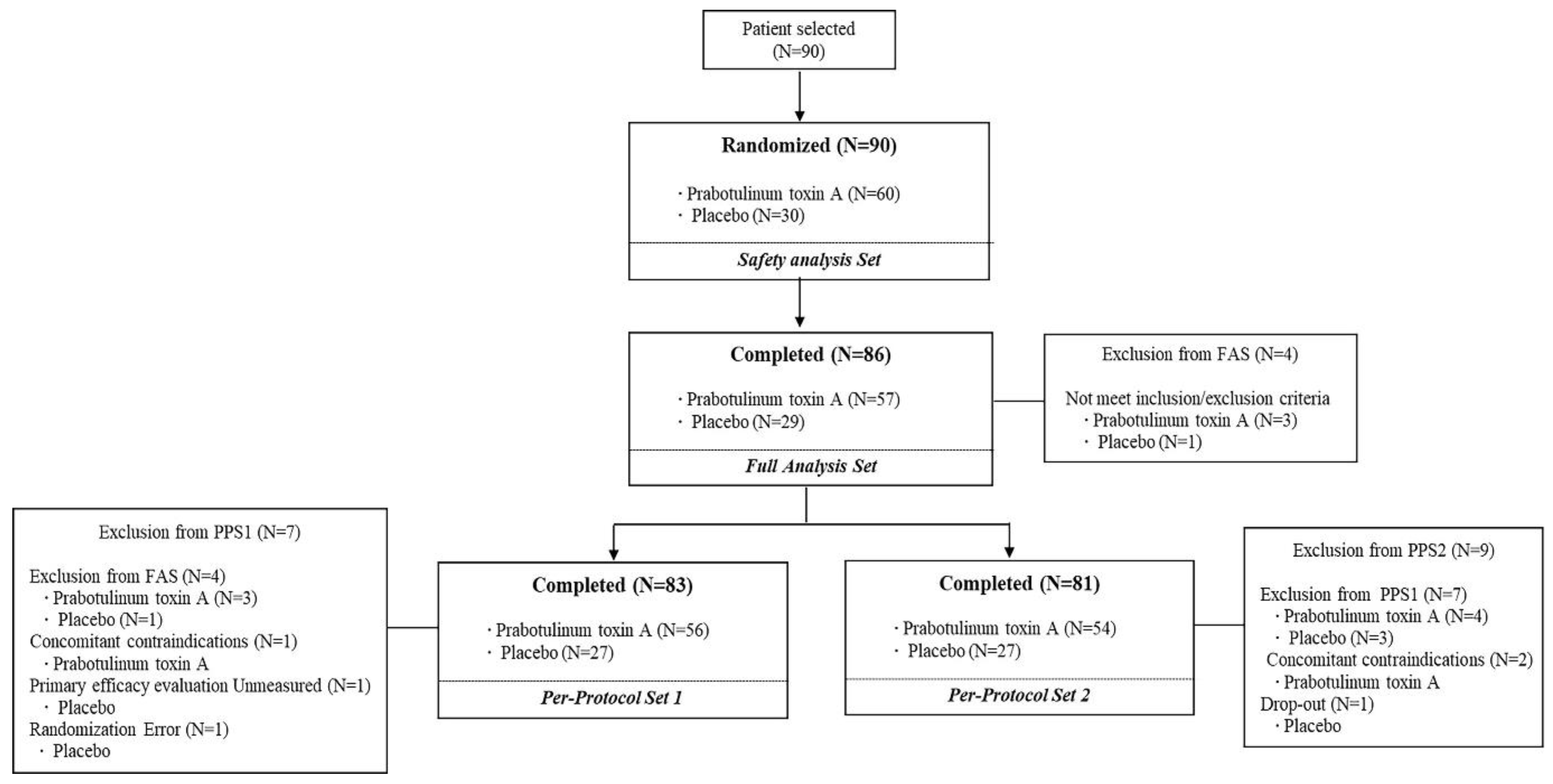
3. Results
3.1. Patients
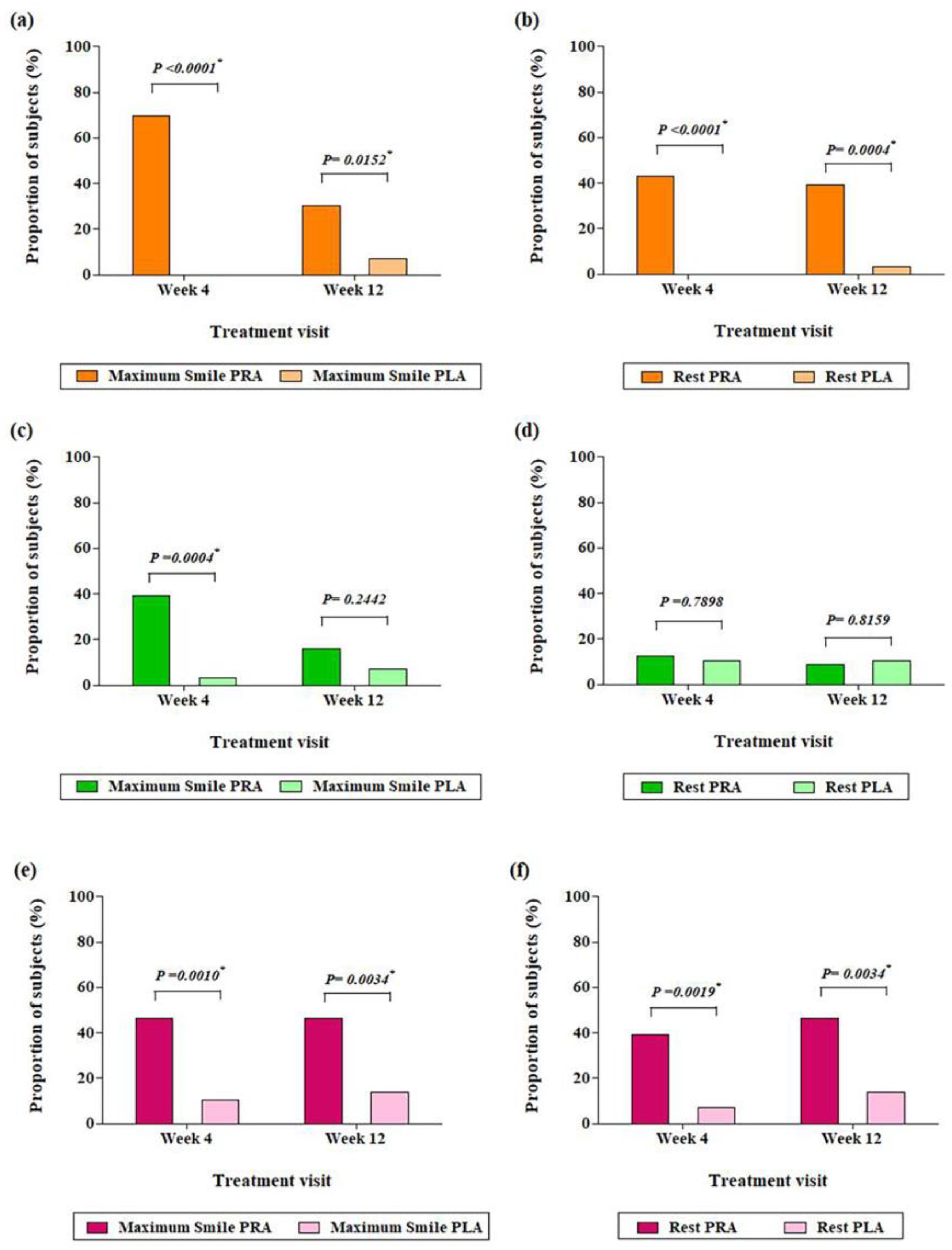
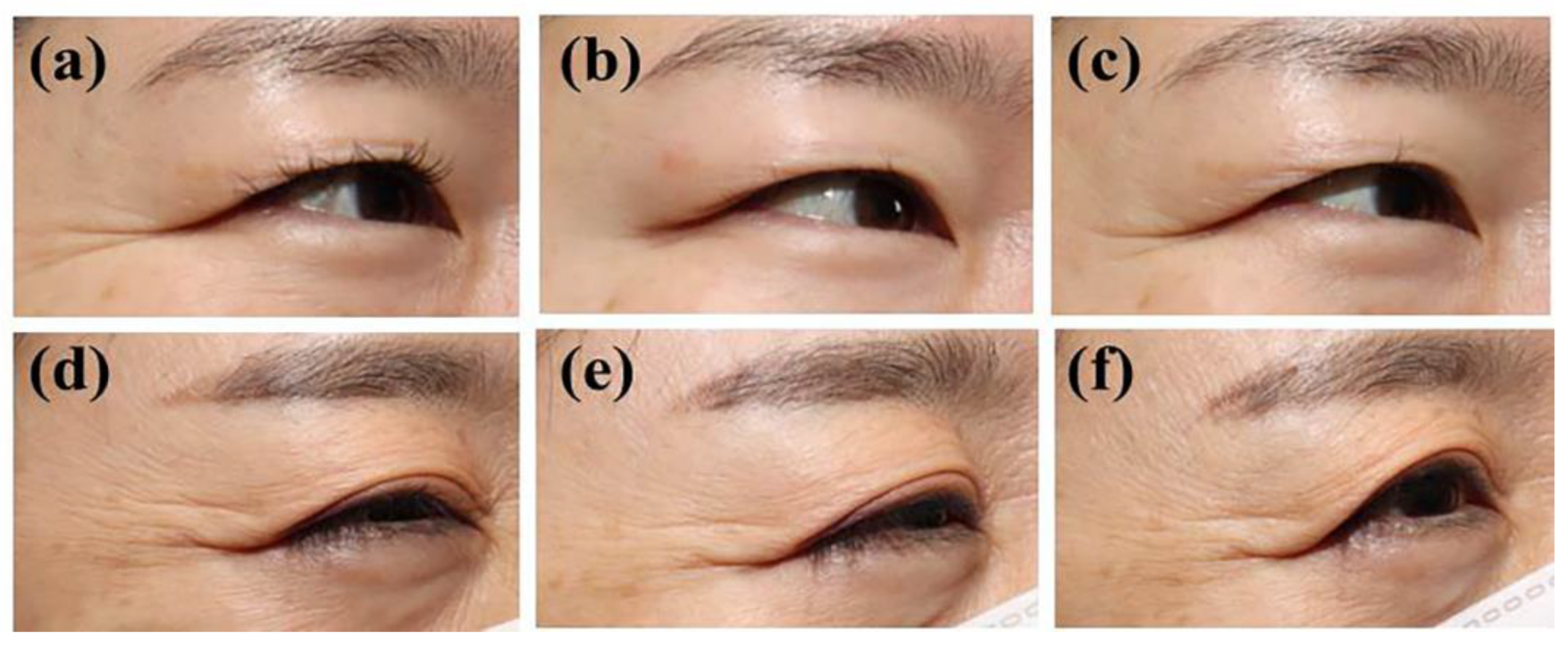
3.2. Efficacy Outcome Assessments
3.2.1. Primary Outcome Assessments
3.2.2. Secondary Outcome Assessments
- Results of the Investigators’ Evaluation
- Results of the IRP’s Evaluation
- Patients’ Self-Assessment Results
3.3. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamura, B.M.; Odo, M.Y. Classification of periorbital wrinkles and treatment with Botulinum Toxin Type A. Surg. Cosmet. Dermatol. 2011, 3, 129–134. [Google Scholar]
- Lowe, N.J.; Ascher, B.; Heckmann, M.; Kumar, C.; Fraczek, S.; Eadie, N.; Botox Facial Aesthetics Study Team. Double-blind, randomized, placebo-controlled, dose-response study of the safety and efficacy of botulinum toxin type A in subjects with crow’s feet. Dermatol. Surg. 2005, 31, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.I.; Jung, N.; Won, C.H.; Kim, B.J.; Lee, Y.W. Efficacy and Safety of Prabotulinumtoxin A and Onabotulinumtoxin A for Crow’s Feet: A Phase 3, Multicenter, Randomized, Double-Blind, Split-Face Study. Dermatol. Surg. 2019, 45, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, Y.H.; Hong, J.P.; Oh, T.S. Safety, efficacy, and onset of a novel botulinum toxin type A (Nabota) for the treatment of glabellar frown lines: A single-arm, prospective, phase 4 clinical study. Arch. Craniofac. Surg. 2018, 19, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. 2016, 16, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Bruce, S.; de Coninck, A.; Connolly, S.; Cox, S.E.; Davis, P.G.; Campo, A.; Lei, X.; Somogyi, C.; Lee, E.; et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crows feet lines: A multicenter, randomized, controlled trial. Dermatol. Surg. 2014, 40, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.P.; Xia, J.; Costa, C.S.; Gemperli, R.; Tatini, M.D.; Bulsara, M.K.; Riera, R. Botulinum toxin type A for facial wrinkles. Cochrane Database Syst. Rev. 2021, 7, CD011301. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.D.; Gendler, E.C.; Poff, B.; Fleming, L.; Bachtell, N.; Garcia, E.; Burkholder, D. Development and validation of a 6-point grading scale in patients undergoing correction of nasolabial folds with a collagen implant. Dermatol. Surg. 2010, 36 (Suppl. 3), 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Ascher, B.; Rzany, B.J.; Grover, R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow’s feet: Double-blind, placebo-controlled, dose-ranging study. Dermatol. Surg. 2009, 35, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Lask, G.; Yamauchi, P.; Moore, D. Bilateral, double-blind, randomized comparison of 3 doses of botulinum toxin type A and placebo in patients with crow’s feet. J. Am. Acad. Dermatol. 2002, 47, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B. Patient satisfaction. J. Cutan. Aesthet. Surg. 2010, 3, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zagui, R.M.; Matayoshi, S.; Moura, F.C. Adverse effects associated with facial application of botulinum toxin: A systematic review with meta-analysis. Arq. Bras. Oftalmol. 2008, 71, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lu, H.; Yang, X.; Jin, X.; Wu, R.; Zhao, J.; Chen, L.; Qi, Z. Adverse Events of Botulinum Toxin Type A in Facial Rejuvenation: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2016, 40, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Boodhoo, T.I.; Pogoda, J.M.; James, L.M.; Demos, G.; Terashima, Y.; Gu, J.; Eadie, N.; Bowen, B.L. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: A meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J. Am. Acad. Dermatol. 2009, 61, 961–970 e961–911. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, P.; Jones, D.H.; Avelar, R.L.; Jonker, A.; Monroe, R.; Carruthers, J. PrabotulinumtoxinA for Treatment of Millennials With Moderate to Severe Glabellar Lines: Post Hoc Analyses of the Phase III Clinical Study Data. Dermatol. Surg. 2022, 48, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Solish, N.; Ascher, B.; Avelar, R.L.; Bertucci, V.; Bodokh, I.; Carruthers, J.; Cartier, H.; Delmar, H.; Denfeld, R.; Heckmann, M.; et al. PrabotulinumtoxinA vs OnabotulinumtoxinA for the Treatment of Adult Males With Moderate to Severe Glabellar Lines: Post-hoc Analyses of the Phase III Clinical Study Data. Aesthetic Surg. J. 2022, 42, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
| PrabotulinumtoxinA Group (N = 57) | Placebo Group (N = 29) | |
|---|---|---|
| Mean age (±SD), years | 50.51 (±8.33) | 48.66 (±6.80) |
| Sex, N (%) | ||
| Male | 5 (8.77) | 4 (13.79) |
| Female | 52 (91.23) | 25 (86.21) |
| Investigator’s crow’s feetseverity rating, N (%) | ||
| Moderate | 22 (38.60) | 9 (31.03) |
| Severe | 35 (61.40) | 20 (68.97) |
| Subjects’ appearance satisfaction rating, n (%) | ||
| Grade 1 | 8 (14.04) | 3 (10.34) |
| Grade 2 | 14 (24.56) | 7 (21.14) |
| Grade 3 | 14 (24.56) | 10 (34.38) |
| Grade 4 | 16 (28.07) | 7 (24.14) |
| Grade 5 | 1 (1.75) | 2 (6.90) |
| Grade 6 | 4 (7.02) | 0 (0.00) |
| Grade 7 | 0 (0.00) | 0 (0.00) |
| Week 4 | Week 12 | |||
|---|---|---|---|---|
| PrabotulinumtoxinA Group (N = 57) | Placebo Group (N = 29) | PrabotulinumtoxinA Group (N = 57) | Placebo Group (N = 29) | |
| Proportion of subjects satisfied with crow’s feet treatment | ||||
| Number of subjects | 56 | 29 | 56 | 29 |
| Satisfied ‡, N (%) | 26 (46.43) | 2 (6.90) | 21 (37.50) | 2 (6.90) |
| Treatment difference | 39.53 | 30.17 | ||
| [95% Confidence interval] †, p-value | [22.91, 56.14], 0.0003 * | [14.25, 46.09], 0.0031 * | ||
| Proportion of subjects satisfied with appearance | ||||
| Number of subjects | 56 | 29 | 56 | 29 |
| Satisfied ‡, N (%) | 18 (32.14) | 3 (10.34) | 16 (28.57) | 2 (6.90) |
| Treatment difference | 21.94 | 21.13 | ||
| [95% Confidence interval] †, p-value | [5.07, 38.80], 0.0289 * | [6.39, 35.87], 0.0227 * | ||
| Proportion of subjects convinced that they looked younger | ||||
| Number of subjects | 56 | 29 | 56 | 29 |
| Younger §, N (%) | 20 (35.71) | 9 (31.03) | 18 (32.14) | 6 (20.69) |
| Treatment difference | 4.41 | 11.58 | ||
| [95% Confidence interval] †, p-value | [–17.35, 26.16], 0.6886 | [–7.77, 30.93], 0.2691 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-K.; Kim, M.S.; Kwon, S.-H.; Chung, B.Y.; Han, S.H.; Kim, H.J. Efficacy, Safety, and Subject Satisfaction of PrabotulinumtoxinA for Moderate-to-Severe Crow’s Feet: A Phase IV, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Med. 2023, 12, 6326. https://doi.org/10.3390/jcm12196326
Lee S-K, Kim MS, Kwon S-H, Chung BY, Han SH, Kim HJ. Efficacy, Safety, and Subject Satisfaction of PrabotulinumtoxinA for Moderate-to-Severe Crow’s Feet: A Phase IV, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Journal of Clinical Medicine. 2023; 12(19):6326. https://doi.org/10.3390/jcm12196326
Chicago/Turabian StyleLee, Soo-Kyung, Myoung Shin Kim, Soon-Hyo Kwon, Bo Young Chung, Se Hee Han, and Hyoung Jun Kim. 2023. "Efficacy, Safety, and Subject Satisfaction of PrabotulinumtoxinA for Moderate-to-Severe Crow’s Feet: A Phase IV, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial" Journal of Clinical Medicine 12, no. 19: 6326. https://doi.org/10.3390/jcm12196326
APA StyleLee, S.-K., Kim, M. S., Kwon, S.-H., Chung, B. Y., Han, S. H., & Kim, H. J. (2023). Efficacy, Safety, and Subject Satisfaction of PrabotulinumtoxinA for Moderate-to-Severe Crow’s Feet: A Phase IV, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Journal of Clinical Medicine, 12(19), 6326. https://doi.org/10.3390/jcm12196326







