Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Course and Treatment Outcomes
2.3. MEFV Gene Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
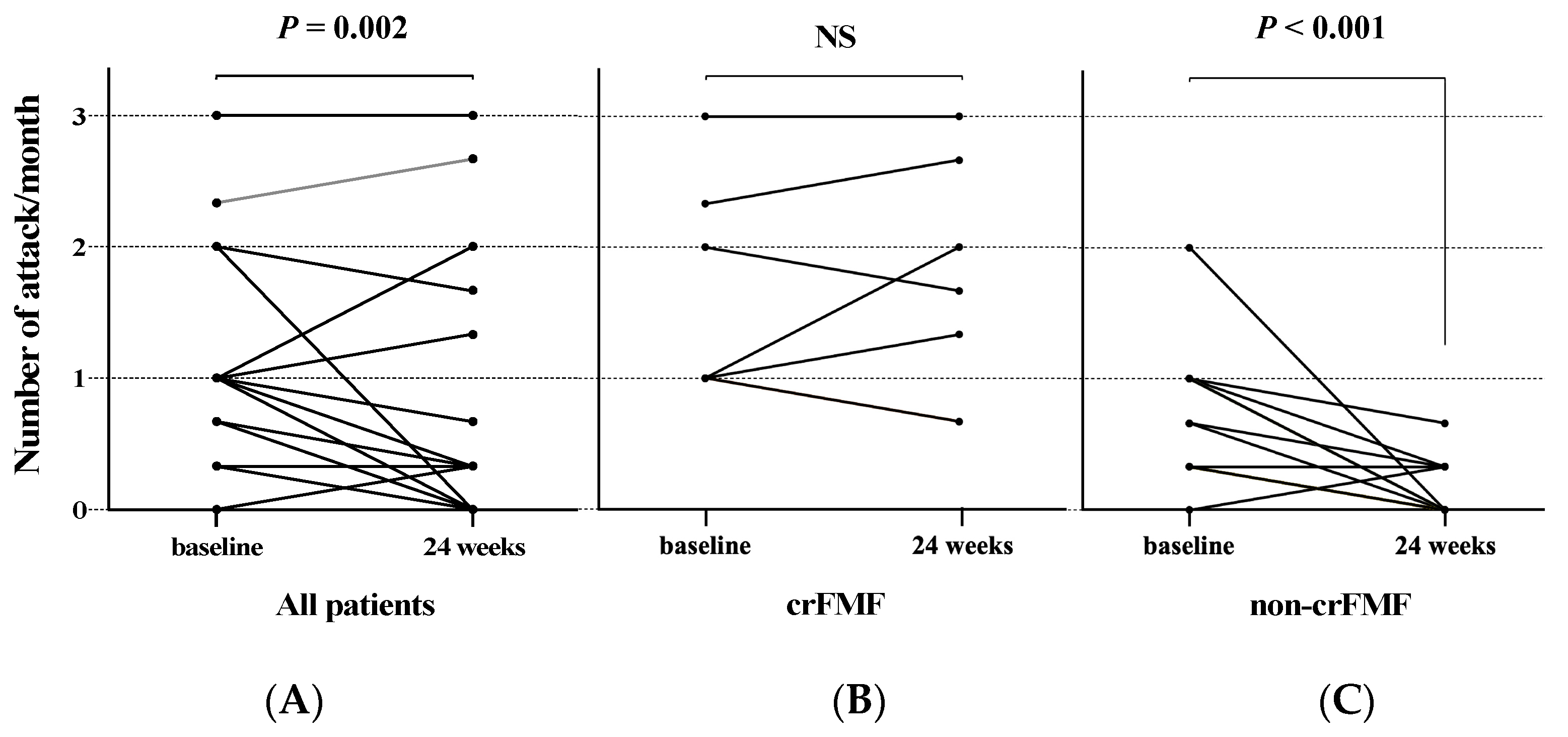
3.2. Comparison of Colchicine Responsiveness between crFMF and Non-crFMF Patients
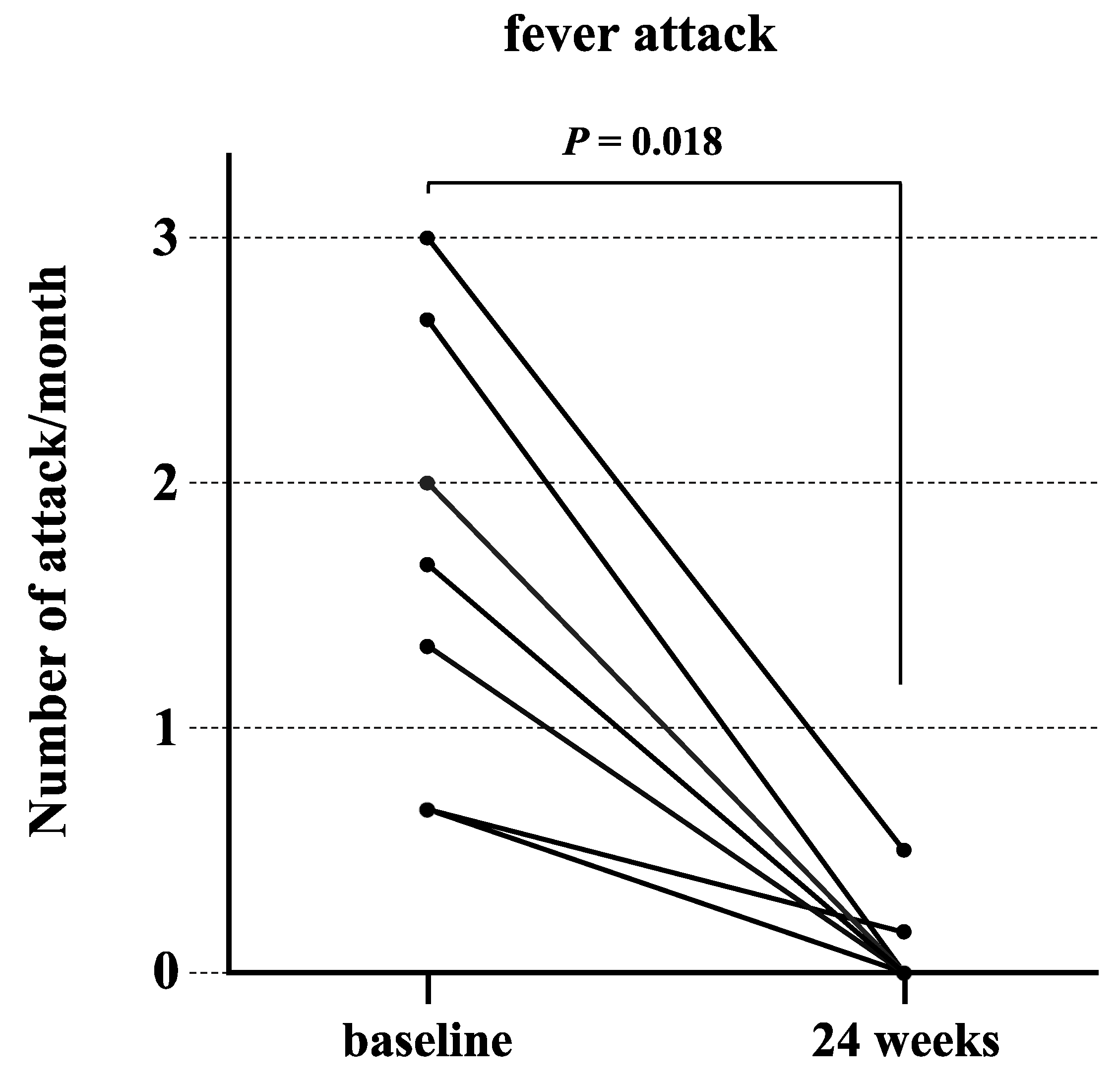
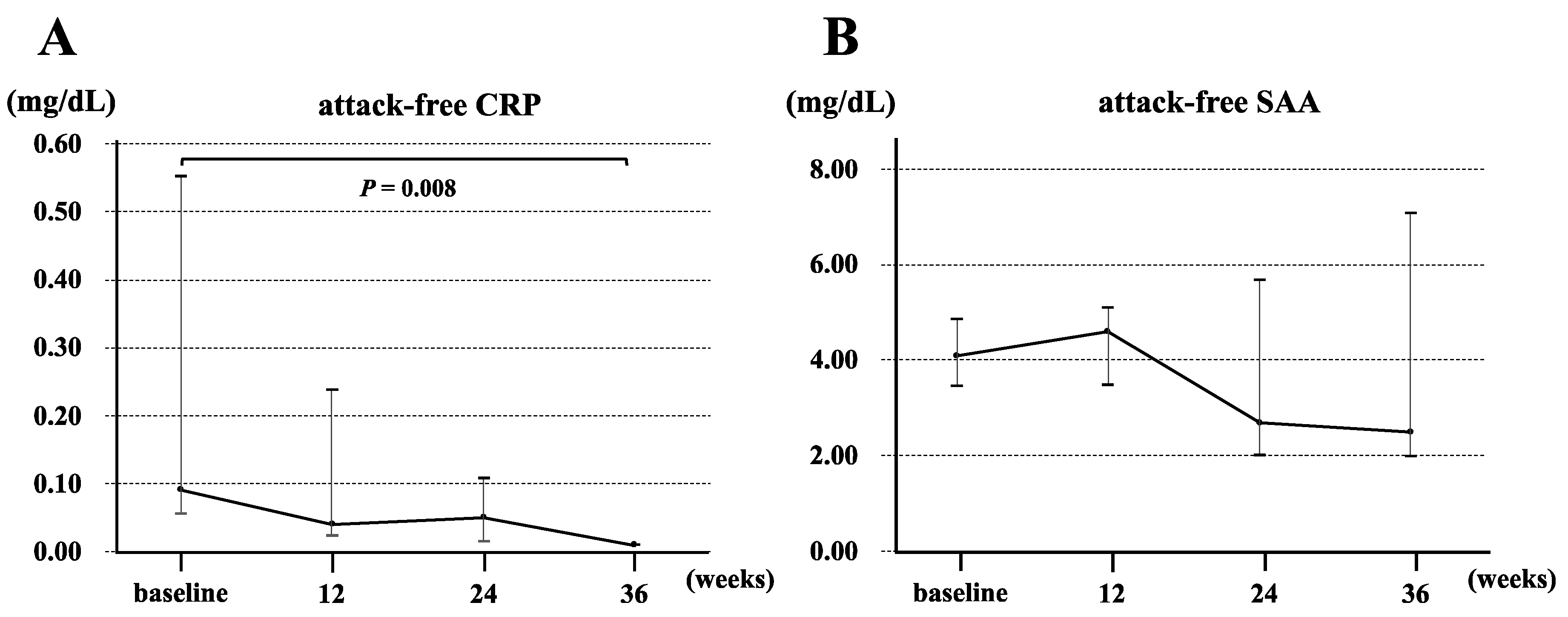
3.3. Clinical Characteristics of Patients with crFMF and Clinical Response to Canakinumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FMF | Familial Mediterranean Fever |
| crFMF | colchicine-resistant Familial Mediterranean Fever |
| AA | amyloid A |
| IL | interleukin |
| CRP | C-reactive protein |
| IQR | interquartile range |
| SAA | serum amyloid A |
| EULAR | European League Against Rheumatism |
| TCZ | tocilizumab |
References
- Ben-Chetrit, E.; Levy, M. Familial Mediterranean fever. Lancet 1998, 351, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.C.; Schwabe, A.D. Prophylactic colchicine therapy in familial Mediterranean fever. A controlled, double-blind study. Ann. Intern. Med. 1974, 81, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Agematsu, K.; Yazaki, M.; Nonaka, F.; Nakamura, A.; Toma, T.; Kishida, D.; Uehara, R.; Nakamura, Y.; Jiuchi, Y.; et al. Familial Mediterranean fever: Genotype-phenotype correlations in Japanese patients. Medicine 2014, 93, 158–164. [Google Scholar] [CrossRef] [PubMed]
- El Roz, A.; Ghssein, G.; Khalaf, B.; Fardoun, T.; Ibrahim, J.N. Spectrum of MEFV Variants and Genotypes among Clinically Diagnosed FMF Patients from Southern Lebanon. Med. Sci. 2020, 8, 35. [Google Scholar] [CrossRef]
- Giaglis, S.; Papadopoulos, V.; Kambas, K.; Doumas, M.; Tsironidou, V.; Rafail, S.; Kartalis, G.; Speletas, M.; Ritis, K. MEFV alterations and population genetics analysis in a large cohort of Greek patients with familial Mediterranean fever. Clin. Genet. 2007, 71, 458–467. [Google Scholar] [CrossRef]
- Mattit, H.; Joma, M.; Al-Cheikh, S.; El-Khateeb, M.; Medlej-Hashim, M.; Salem, N.; Delague, V.; Mégarbané, A. Familial Mediterranean fever in the Syrian population: Gene mutation frequencies, carrier rates and phenotype-genotype correlation. Eur. J. Med. Genet. 2006, 49, 481–486. [Google Scholar] [CrossRef]
- Booty, M.G.; Chae, J.J.; Masters, S.L.; Remmers, E.F.; Barham, B.; Le, J.M.; Barron, K.S.; Holland, S.M.; Kastner, D.L.; Aksentijevich, I. Familial Mediterranean fever with a single MEFV mutation: Where is the second hit? Arthritis Rheum. 2009, 60, 1851–1861. [Google Scholar] [CrossRef]
- Sag, E.; Akal, F.; Atalay, E.; Akca, U.K.; Demir, S.; Demirel, D.; Batu, E.D.; Bilginer, Y.; Ozen, S. Anti-IL1 treatment in colchicine-resistant paediatric FMF patients: Real life data from the HELIOS registry. Rheumatology 2020, 59, 3324–3329. [Google Scholar] [CrossRef]
- Ozen, S.; Ben-Cherit, E.; Foeldvari, I.; Amarilyo, G.; Ozdogan, H.; Vanderschueren, S.; Marzan, K.; Kahlenberg, J.M.; Dekker, E.; De Benedetti, F.; et al. Long-term efficacy and safety of canakinumab in patients with colchicine-resistant familial Mediterranean fever: Results from the randomised phase III CLUSTER trial. Ann. Rheum. Dis. 2020, 79, 1362–1369. [Google Scholar] [CrossRef]
- Kacar, M.; Savic, S.; van der Hilst, J.C.H. The Efficacy, Safety and Tolerability of Canakinumab in the Treatment of Familial Mediterranean Fever: A Systematic Review of the Literature. J. Inflamm. Res. 2020, 13, 141–149. [Google Scholar] [CrossRef]
- Ugurlu, S.; Ergezen, B.; Egeli, B.H.; Selvi, O.; Ozdogan, H. Anakinra treatment in patients with familial Mediterranean fever: A single-centre experience. Rheumatology 2021, 60, 2327–2332. [Google Scholar] [CrossRef]
- Hashkes, P.J.; Spalding, S.J.; Giannini, E.H.; Huang, B.; Johnson, A.; Park, G.; Barron, K.S.; Weisman, M.H.; Pashinian, N.; Reiff, A.O.; et al. Rilonacept for colchicine-resistant or -intolerant familial Mediterranean fever: A randomized trial. Ann. Intern. Med. 2012, 157, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Livneh, A.; Langevitz, P.; Zemer, D.; Zaks, N.; Kees, S.; Lidar, T.; Migdal, A.; Padeh, S.; Pras, M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997, 40, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Demirkaya, E.; Erer, B.; Livneh, A.; Ben-Chetrit, E.; Giancane, G.; Ozdogan, H.; Abu, I.; Gattorno, M.; Hawkins, P.N.; et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 644–651. [Google Scholar] [CrossRef]
- Nakayama, M.; Oda, H.; Nakagawa, K.; Yasumi, T.; Kawai, T.; Izawa, K.; Nishikomori, R.; Heike, T.; Ohara, O. Accurate clinical genetic testing for autoinflammatory diseases using the next-generation sequencing platform MiSeq. Biochem. Biophys. Rep. 2017, 9, 146–152. [Google Scholar] [CrossRef]
- Fujimoto, K.; Hidaka, Y.; Koga, T.; Kaieda, S.; Yamasaki, S.; Nakashima, M.; Hoshino, T.; Ida, H. Clinical and Genetic Analysis of 22 Japanese Patients with Familial Mediterranean Fever: An Examination of MEFV and 10 Other Genes Related to Autoinflammatory Syndromes. Intern. Med. 2020, 59, 1373–1378. [Google Scholar] [CrossRef]
- Koga, T.; Migita, K.; Kawakami, A. Biologic therapy in familial Mediterranean fever. Mod. Rheumatol. 2016, 26, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S. Update in familial Mediterranean fever. Curr. Opin. Rheumatol. 2021, 33, 398–402. [Google Scholar] [CrossRef]
- Ozturk, C.; Halicioglu, O.; Coker, I.; Gulez, N.; Sutçuoglu, S.; Karaca, N.; Aksu, G.; Kutukculer, N. Association of clinical and genetical features in FMF with focus on MEFV strip assay sensitivity in 452 children from western Anatolia, Turkey. Clin. Rheumatol. 2012, 31, 493–501. [Google Scholar] [CrossRef]
- Tomokawa, T.; Koga, T.; Endo, Y.; Michitsuji, T.; Kawakami, A. Efficacy and safety of canakinumab for colchicine-resistant or colchicine-intolerant familial Mediterranean fever: A single-centre observational study. Mod. Rheumatol. 2022, 32, 797–802. [Google Scholar] [CrossRef]
- Seshadri, S.; Duncan, M.D.; Hart, J.M.; Gavrilin, M.A.; Wewers, M.D. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J. Immunol. 2007, 179, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Wu, J.; Zhang, Z.; Datta, P.; Ibrahimi, I.; Taniguchi, S.; Sagara, J.; Fernandes-Alnemri, T.; Alnemri, E.S. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006, 13, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Bilginer, Y.; Akpolat, T.; Ozen, S. Renal amyloidosis in children. Pediatr. Nephrol. 2011, 26, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
| Variables | n = 27 |
|---|---|
| Typical FMF, n (%) | 17 (63.0) |
| Male, n (%) | 11 (40.7) |
| Age at FMF onset (years), median (IQR) | 30 (19.5–40.5) |
| Age at colchicine introduction (years), median (IQR) | 39 (29–47) |
| Duration from FMF onset to diagnosis (years), median (IQR) | 4.0 (1.5–9.0) |
| Use of colchicine, n (%) | 27 (100) |
| Dose of colchine (mg/day), average (SD) | 0.98 (0.44) |
| Colchicine resistance, n (%) | 7 (25.9) |
| Attack frequency (number of attacks per month), median (IQR) | 1 (0.33–1) |
| Attack-free CRP (mg/dL), median (IQR) | 0.11 (0.04–0.22) |
| Attack-free SAA (μg/dL), median (IQR) | 3.6 (2.5–6.8) |
| MEFV variants, n (%) | |
| E148Q/normal | 5 (18.5) |
| E148Q/L110P | 3 (11.1) |
| P369S/R408Q | 3 (11.1) |
| M694I/E148Q | 2 (7.4) |
| M694I/normal | 1 (3.7) |
| M694I/E148Q/L110P | 1 (3.7) |
| E148Q/G304R | 1 (3.7) |
| E148Q/P369S | 1 (3.7) |
| E148Q/R408Q/P158S | 1 (3.7) |
| E148Q/L110P/P369S/R408Q | 1 (3.7) |
| R354Q/normal | 1 (3.7) |
| S503C/normal | 1 (3.7) |
| R503C/normal | 1 (3.7) |
| G304R/normal | 1 (3.7) |
| R202Q/normal | 1 (3.7) |
| No variant | 3 (11.1) |
| Follow-up period (months), median (IQR) | 36.4 (11.7–71.1) |
| Autoimmune comorbidities, n (%) | 5 (18.5) |
| Characteristics | All Patients | ||
|---|---|---|---|
| crFMF (n = 7) | Non-crFMF (n = 20) | p Value | |
| Typical FMF, n (%) | 5 (71.4) | 12 (60.0) | 0.678 |
| Male, n (%) | 4 (57.1) | 7 (35.0) | 0.633 |
| Age at colchicine introduction, † years | 43 (42–46) | 36.5 (28–47.3) | 0.431 |
| Duration from FMF onset to diagnosis, † years | 5.0 (3.5–11) | 3.5 (1.0–9.0) | 0.431 |
| Dose of colchine (mg/day), average (SD) | 1.14 (0.38) | 0.93 (0.46) | 0.174 |
| Attack frequency before initiation of colchicine, † number of attacks per month | 1.0 (1.0–2.17) | 0.66 (0.33–1.0) | 0.004 * |
| Attack frequency 6 months after initiation of colchicine, † number of attacks per month | 1.67 (1.0–2.33) | 0.0 (0.0–0.1) | <0.001 * |
| Attack-free CRP, † mg/dL | 0.09 (0.06–0.55) | 0.11 (0.03–0.21) | 0.882 |
| Attack-free SAA, † μg/day | 4.1 (3.48–4.88) | 3.3 (2.5–6.85) | 0.779 |
| MEFV Exson 10 variants, n (%) | 0 (0) | 4 (20.0) | 0.545 |
| MEFV Exson 2 variants, n (%) | 2 (28.6) | 17 (85.0) | 0.011 * |
| MEFV Exson 3 variants, n (%) | 2 (28.6) | 4 (20.0) | 0.633 |
| MEFV Exson 5 variants, n (%) | 0 (0) | 2 (10.0) | 1.00 |
| No variant | 3 (42.9) | 0 (0) | 0.012 * |
| Follow-up period, † months | 51.8 (31.8–86.5) | 34.6 (10.7–64.3) | 0.370 |
| Autoimmune comorbidities, n (%) | 2 (28.6) | 3 (15.0) | 0.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Sumichika, Y.; Saito, K.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; Asano, T.; Sato, S.; Migita, K. Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study. J. Clin. Med. 2023, 12, 6272. https://doi.org/10.3390/jcm12196272
Yoshida S, Sumichika Y, Saito K, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Asano T, Sato S, Migita K. Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study. Journal of Clinical Medicine. 2023; 12(19):6272. https://doi.org/10.3390/jcm12196272
Chicago/Turabian StyleYoshida, Shuhei, Yuya Sumichika, Kenji Saito, Haruki Matsumoto, Jumpei Temmoku, Yuya Fujita, Naoki Matsuoka, Tomoyuki Asano, Shuzo Sato, and Kiyoshi Migita. 2023. "Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study" Journal of Clinical Medicine 12, no. 19: 6272. https://doi.org/10.3390/jcm12196272
APA StyleYoshida, S., Sumichika, Y., Saito, K., Matsumoto, H., Temmoku, J., Fujita, Y., Matsuoka, N., Asano, T., Sato, S., & Migita, K. (2023). Effectiveness of Colchicine or Canakinumab in Japanese Patients with Familial Mediterranean Fever: A Single-Center Study. Journal of Clinical Medicine, 12(19), 6272. https://doi.org/10.3390/jcm12196272






