Abstract
Background: Cardiogenic shock (CS) remains the leading cause of ST elevation myocardial infarction (STEMI)-related mortality. Contemporary studies have shown no sex-related differences in mortality. Methods: STEMI-CS patients undergoing primary percutaneous coronary intervention (PPCI) were included based on a dedicated prospective STEMI database. We compared sex-specific differences in CS characteristics at baseline, during hospitalization, and in subsequent clinical outcomes. Endpoints included all-cause mortality and major adverse cardiac events (MACE). Results: Of 3202 consecutive STEMI patients, 210 (6.5%) had CS, of which 63 (30.0%) were women. Women were older than men (73.2 vs. 65.5% y, p < 0.01), and more had hypertension (68.3 vs. 52.8%, p = 0.019) and diabetes (38.7 vs. 24.8%, p = 0.047). Fewer were smokers (13.3 vs. 41.2%, p < 0.01), had previous PCI (9.1 vs. 22.3% p = 0.016), or required IABP (35.3 vs. 51.1% p = 0.027). Women had higher rates of mortality (53.2 vs. 35.3% in-hospital, p = 0.01; 61.3 vs. 41.9% at 1 month, p = 0.01; and 73.8 vs. 52.6% at 3 years, p = 0.05) and MACE (60.6 vs. 41.6% in-hospital, p = 0.032; 66.1 vs. 45.6% at 1 month, p = 0.007; and 62.9 vs. 80.3% at 3 years, p = 0.015). After multivariate adjustment, female sex remained an independent factor for death (HR-2.42 [95% CI 1.014–5.033], p = 0.042) and MACE (HR-1.91 [95% CI 1.217–3.031], p = 0.01). Conclusions: CS complicating STEMI is associated with greater short- and long-term mortality and MACE in women. Sex-focused measures to improve diagnosis and treatment are mandatory for CS patients.
1. Introduction
Cardiogenic shock (CS) complicates 5% to 10% of cases of patients hospitalized with acute myocardial infarction (AMI) [1], and is the leading cause of death in this population [2,3,4]. Current guidelines suggest that primary percutaneous coronary intervention (PPCI) reduces mortality in these patients [5]. Thus, an early revascularization strategy has been adopted. However, despite current advances in reperfusion methods and the adoption of more aggressive strategies, CS continues to lead to significantly high and consistent mortality rates [6]. Early mortality rates have seen a decrease, but the 1-year survival rate has not changed [7].
In the setting of acute myocardial infarction, it has been repeatedly shown that differences in treatment remain between sexes [8,9,10], and that women presenting with AMI have higher adjusted early mortality rates [11]. Recent trends have shown increases in rates of hospitalization for women less than 65 years of age experiencing acute coronary syndrome (ACS) [12] and, when compared with men, women receive less medical and invasive therapy when suffering from AMI [10,13], including lower in-hospital use of coronary angiography [14] and PCI [15]. It has been suggested that the disparity may be due to higher baseline risk, atypical presentations, and underestimation of patient risk [14]. Although previous trials, including the SHOCK and SHOCK IABP-II trials, which evaluated the etiology, management, and outcomes of patients who developed CS following ST-elevation AMI (STEMI) have shown that mortality rates are high, no significant sex differences in mortality have been seen [16,17,18]. Identification of sex-related differences in outcomes for patients within this already very high-risk population can allow for changes in practice, such as earlier recognition and intervention, which may help mitigate these differences. The goal of this study was to assess any sex differences in clinical outcomes, including mortality, in patients presenting with acute STEMI complicated by CS and undergoing PPCI.
2. Materials and Methods
2.1. Patients and Setting
The clinical data of consecutive patients presenting with STEMI and treated by PPCI at the Department of Cardiology, Rabin Medical Center, Israel, between January 2009 and December 2018 were prospectively entered into a registry for purposes of monitoring patient-related variables and clinical events. Patients who were designated as Killip score 4 and with a primary clinical diagnosis of cardiogenic shock defined as having clinical and biochemical signs of hypoperfusion such as systolic blood pressure (SBP) <90 mmHg for ≥30 min, or required mechanical or medical support to maintain SBP ≥90 mmHg, end-organ hypoperfusion defined by urine output <30 mL/h, or cool extremities were identified for inclusion in the present post hoc study. Those who developed CS were compared between sexes. Exclusion criteria were treatment by thrombolysis and ineligibility for PPCI or a year-long dual antiplatelet regimen. The study was approved by the institutional review boards of the Rabin Medical Center.
2.2. Interventional Procedure
Patients provided explicit written informed consent before undergoing cardiac catheterization. In the event that patients were incapacitated due to mechanical ventilation, consent was obtained via the agreed decision of three physicians. Pretreatment consisted of aspirin and unfractionated heparin (70 U/kg), clopidogrel 300 or 600 mg, prasugrel 60 mg, or ticagrelor 180 mg, which was administered as a loading dose before or immediately after PCI. The utilization of glycoprotein IIb/IIIa inhibitors, and the choice of stent type and mechanical circulatory support devices, were left to the discretion of the primary operator. All stents were implanted with moderate to high deployment pressure (14 to 18 atm). All patients received dual antiplatelet therapy with aspirin 100 mg daily and a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) for at least 12 months after PCI.
2.3. Study Endpoints
Immediate and in-hospital clinical events were prospectively recorded in the institutional database. During follow-up, patients completed standardized questionnaires on clinical events at 6-month intervals, either by telephone or in the outpatient clinic. When indicated, records from peripheral hospitals were acquired to verify the events. All events were further confirmed and adjudicated by the institutional clinical events adjudication committees. Survival status was assessed by the use of municipal civil registries at 1 and 3 years.
Clinical outcomes included all-cause mortality and major adverse cardiac events (MACE), which comprised death, myocardial infarction (MI), target vessel revascularization (TVR), and coronary artery bypass surgery (CABG). Other endpoints were peri-procedural arrhythmias, vascular complications, bleeding according to the thrombolysis in myocardial infarction (TIMI) classification, renal failure (defined as glomerular filtration rate below 50 mL/min/1.73 m2, calculated according to the Modification of Diet in Renal Disease formula), stent thrombosis, and cardiac death. Anemia was defined as hemoglobin levels lower than 13.0 g/dL for men and 12.0 g/dL for women. Findings were compared between male and female CS-STEMI patients.
2.4. Statistical Analysis
Continuous data are summarized as mean and standard deviation (SD) or median and interquartile range (IQR), and categorical data as frequency (%). Student’s t-test or analysis of variance was used to compare continuous variables between groups, and chi-square or Fisher’s exact test was used for categorical variables. The normality of variable distributions was assessed using the Kolmogorov–Smirnov test. Time-to-event curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Cox regression was performed to determine independent predictors of the primary endpoint, accounting for known baseline cardiovascular risk differences. Effect sizes are presented as hazard ratio (HR) and 95% confidence interval (CI). Stepwise variable selection of significant univariate predictors (p < 0.1) was used to identify variables for inclusion in the multivariate model. All statistical analyses were performed with IBM SPSS V.28 (IBM Corp. Armonk, NY, USA). A p value of <0.05 was considered statistically significant.
3. Results
Among the 3202 consecutive STEMI patients included in our analysis, 210 (6.5%) suffered from CS. Of these, 147 (70.0%) were men and 63 (30.0%) were women. Women were older than men (73.2 ± 12.4 vs. 65.5 ± 12.0 years, p < 0.01) and had higher BMIs (36.7 ± 10.9 vs. 31.3 ± 10.3 p = 0.002). Among comorbid conditions, women were less frequently smokers (31.1 vs. 40.4%, p = 0.006), and less likely to have previously undergone PCI (9.1 vs. 22.3% p = 0.016). Women more frequently had hypertension (68.3 vs. 52.8%, p = 0.019) and diabetes mellitus (38.7 vs. 24.8%, p = 0.047). There were no other differences found in baseline characteristics between the two sexes (Table 1). Ischemic time (5.1 vs. 4.1 h, p = 0.528) and door to balloon time (1.7 vs. 1.5 h, p = 0.525) did not differ between the sexes, nor did the use of IV inotropes or the use of a temporary pacemaker. Women were less likely to receive intra-aortic balloon pump (IABP) treatment (35.3 vs. 51.1% p = 0.027) compared with men. Target vessels were smaller in female patients, as represented by the use of an average stent diameter of 3.0 vs. 3.2 (p = 0.028) in male patients. Men were more often treated with multivessel angioplasties as compared with women (2.3 vs. 2.1 vessels, p = 0.077), but their SYNTAX scores did not differ. Adherence to antiplatelet therapy was equal in both sexes (Table 2).

Table 1.
Baseline characteristics.

Table 2.
Procedural characteristics.
Patients suffering from CS who underwent PPCI were followed for rates of mortality and MACE. Mortality at separate time points was found to be significantly higher among women during in-hospital (53.2 vs. 35.3%, p = 0.01), 1-month (61.3 vs. 41.9%, p = 0.01), and after 3 years (73.8 vs. 52.6%, p = 0.05) following intervention (Figure 1). A Cox regression analysis was conducted, adjusting for confounding variables, and mortality remained higher for women (HR 2.42 [95% CI 1.014–5.033], p = 0.042). Other factors associated with increased risk of mortality included: diabetes mellitus (HR 2.81 [95% CI 1.055–4.345], p = 0.038), renal failure (HR 3.08 [95% CI 1.201–6.038], p = 0.02), and PVD (HR 4.23 [95% CI 1.640–7.496], p = 0.003). Notably, age, the usage of an IABP, the timing of day versus night, and door-to-balloon time were not found to have an impact on rates of mortality (Table 3). Rates of MACE were higher among women during their admission (60.6 vs. 41.6%, p = 0.032), at 1-month (66.1 vs. 45.6%, p = 0.007), and at 3 years (62.9 vs. 80.3, p = 0.015) (Figure 2). (For the individual components, please see Supplementary Table S1). After multivariate analysis, MACE rates remained higher for women (HR 1.91 [95% CI 1.217–3.031], p = 0.01). Other factors associated with increased risk of MACE included age (HR 1.12 [95% CI 1.126–1.324], p = 0.005), renal failure (HR 1.88 [95% CI 1.189–2.823], p = 0.007), and PVD (HR 1.83 [95% CI 1.072–2.823], p = 0.021) (Table 4). Rates of recurrent myocardial infarction, stent thrombosis, and the need for revascularization did not differ between sexes at all time points.
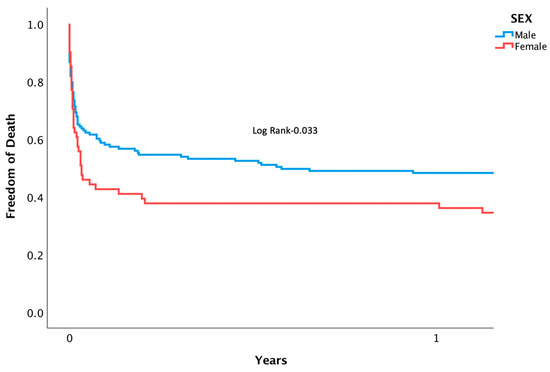
Figure 1.
Kaplan-meier curve displaying length of time patients were free from death following PPCI for AMI complicated by CS.

Table 3.
Cox analysis for mortality.
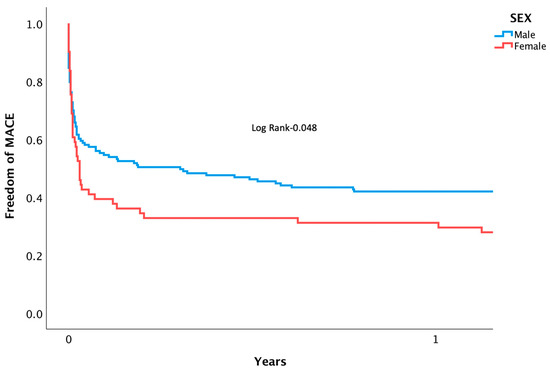
Figure 2.
Kaplan-meier curve displaying length of time patients were free from MACE following PPCI for AMI complicated by CS.

Table 4.
Cox analysis for MACE.
4. Discussion
In this study examining the course of STEMI patients treated with PPCI presenting with CS, we observed that female patients suffer from worse short- and long-term outcomes when compared with men. Information regarding the worse outcomes experienced by women suffering from CAD and ACS requiring PCI is well known [10,11,12,19,20,21]. However, outcomes for women compared with men in CS following STEMI have yet to be clearly defined. In this observational study, we compared mortality rates of women and men within this population with the intention of further clarifying sex differences in clinical outcomes and management. Considering the already poor outcomes for patients suffering from CS following STEMI, recognition of differences between the sexes is necessary.
Previous studies have suggested that sex differences can be attributed to the fact that women present at older age or with an increased burden of comorbidities and that, when these differences in baseline characteristics are controlled for, outcome differences do not exist [22,23]. Other studies looking specifically at CS patients undergoing PPCI following AMI, including the SHOCK registry, also showed no differences in outcomes between the sexes [24,25], or that sex is not independently associated with clinical outcomes in similar populations [26]. However, other studies have found that women are at increased risk for in-hospital mortality [27,28] and that, in general, women are more likely to die in hospital following AMI [29]. Women similarly show higher unadjusted mortality rates 24 h after randomization, as published in the IABP-II sub study trial (18% vs. 9%, p = 0.004) [30]. A decrease in cardiac power, a measure from the SHOCK trial defined as the product of cardiac output and mean arterial blood pressure, was found to be the strongest independent correlate with in-hospital mortality, and was significantly associated with female sex [31]. However, the inconsistency in study results is an example of the discordance in information regarding sex and mortality outcomes within this population.
Our study found higher rates of death in women at three separate time points: during admission, after 1 month, and after 3 years. In our multivariate model, PVD was the strongest predictor of mortality from CS. Increased in-hospital CV morbidity and mortality in patients with PVD and ACS has been reported [32,33]. This has been thought to be related to an increased systemic atherosclerotic burden, impaired arterial remodeling, and increased disease progression [34,35], all of which may contribute to the development of CS and the deterioration of patients in an acute setting. Of important note, this study also assessed other possibly contributing elements, including clinical factors such as previous MI, left ventricular function, and the timing of the procedure, namely door-to-balloon and ischemic time. We also assessed procedural characteristics, including use of a radial versus femoral approach and non-culprit vessel angioplasty. None of these variables showed statistical significance in relation to mortality, contradictory to previous studies [36,37,38,39]. The contrasting results may be due in part to variability in the definitions of shock parameters and measurements across studies [36]. Another explanation may be that the time patients spent in shock was an important risk factor, but this data was not collected.
Female patients were more likely to be older and to be diagnosed with diabetes mellitus and hypertension. However, after multivariate analysis, a significant difference in mortality between the sexes remained. Women tend to delay health care-seeking behavior [40], and biological sex differences in the extent of endothelial dysfunction, an important prognostic factor for patients with AMI [41], exist. These differences have been postulated to be due to higher rates of inflammation, loss of arterial compliance, and increase in remodeling possibly due to loss of sex-protecting hormones [42]. Estrogen has shown a similar role in premenopausal women in reducing inflammation in the setting of systemic inflammatory response syndrome (SIRS) complicated by increases in inflammatory cytokines and mediators from systemic hypoperfusion [43]. Complicating the matter further is the fact that women have a higher prevalence of CS following STEMI, compared with men [27,43,44,45], consistent with our cohort (10.9% vs. 5.7%). This could be partly attributed to the fact that women are at increased risk for more sex-specific triggers of CS, such as peripartum cardiomyopathy, microvascular dysfunction, spontaneous coronary artery dissection (SCAD), and takotsubo cardiomyopathy [46].
It has been well-established that attempts at all but timely PPCI in the treatment of CS complicated by STEMI fail to prevent short- and long-term MACE [47,48,49]. As discussed, although rates of in-hospital mortality have decreased over time, CS remains the most common cause of death. Increased use of angiography, the adoption of an earlier revascularization strategy, and the increased rates of administration of novel P2Y12 inhibitors and statins may be contributing to the relative decline in death [50,51,52]. However, even with the increased use of early revascularization, mortality rates remain high, and differences in outcomes exist between men and women [2]. It has been suggested that mortality in STEMI complicated by CS may be reduced by emphasizing rapid LV unloading and immediate coronary revascularization [53], which require even more prompt initiation of treatment, especially in women. Our study’s patient population was similar to the one found in a recent study by Yahn et al., which also analyzed sex-differences in outcomes for CS patients [54]. This larger single center study found no differences in outcomes between sexes; however, it did note a significant disparity in utilization of mechanical circulatory support (MCS). Our study also revealed women to be less likely to be treated with MCS, but showed no difference in use of IV inotropes. This may account for differences in outcomes within our smaller patient cohort; however, no study, as of yet, has been able to show a significant improvement in rates of mortality for patients with CS-AMI who are treated with MCS devices such as IABP or impella, which are used with the aim to aid in rapidly unloading the left ventricle and improving coronary blood flow [17,55]. Reasons for women to be less likely to receive MCS may be related to under-recognition of the severity of CS in this population. Large, randomized control trials investigating the use of newer MCS devices, such as impella, in the setting of improved treatment measures previously mentioned are necessary. Furthermore, implementing and further validating a cardiogenic shock classification system, such as the SCAI classification, may also improve outcomes [56].
5. Study Limitations
This study is an observational post hoc analysis of our cohort, a design that in itself presents limitations. It is also a single-center trial. Although based on an all-comer prospective registry, biases may have influenced the selection of data, the method of therapy, and other factors associated with outcome, although our main discussion topic remained significant after multivariate analysis. The observational nature of the study also prevents us from attributing a causal relationship between female sex and mortality. Finally, as rates of CS are relatively low, our final analysis included a relatively small number of patients. Nevertheless, this is one of the largest real-world studies comparing outcomes in patients suffering from CS complicating STEMI, and it shows a clear independent impact of female sex on worse prognosis.
6. Conclusions
Our study revealed a significant increase in mortality in women compared with men suffering from cardiogenic shock after STEMI and undergoing PPCI. The recognition of the increased risk is necessary to better steer management and improve prognosis for female patients. We anticipate that future studies assessing differences in outcomes will shed more light on this important topic and enable better care of both male and female patients suffering from this ominous medical condition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12196259/s1, Table S1: Outcomes.
Author Contributions
J.H.A., L.P. and H.V.A. all conceived the original study design. L.P. was responsible for data extraction, analysis and writing of the manuscript and J.H.A. was responsible for searching for studies and writing the manuscript. All authors made a substantial contribution to interpreting the data and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institute of Health under award number HL155729.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review boards of the Rabin Medical Center for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. In the event that patients were incapacitated due to mechanical ventilation, consent was obtained via the agreed decision of three physicians.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reyentovich, A.; Barghash, M.H.; Hochman, J.S. Management of refractory cardiogenic shock. Nat. Rev. Cardiol. 2016, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Samad, N.A.; Yarzebski, J.; Gurwitz, J.; Bigelow, C.; Gore, J.M. Temporal Trends in Cardiogenic Shock Complicating Acute Myocardial Infarction. N. Engl. J. Med. 1999, 340, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Gore, J.M.; Thompson, C.A.; Gurwitz, J.H. Recent magnitude of and temporal trends (1994–1997) in the incidence and hospital death rates of cardiogenic shock complicating acute myocardial infarction: The second National Registry of Myocardial Infarction. Am. Heart J. 2001, 141, 65–72. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization In Acute Myocardial Infarction Complicated By Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Dhakam, S.; Khalid, L. A Review of Cardiogenic Shock in Acute Myocardial Infarction. Curr. Cardiol. Rev. 2008, 4, 34–40. [Google Scholar] [CrossRef]
- Aissaoui, N.; Puymirat, E.; Tabone, X.; Charbonnier, B.; Schiele, F.; Lefevre, T.; Durand, E.; Blanchard, D.; Simon, T.; Cambou, J.P.; et al. Improved outcome of cardiogenig shock at the acute stage of myocardial infarction: A report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur. Heart J. 2012, 33, 2535–2543. [Google Scholar] [CrossRef]
- Ayanian, J.Z.; Epstein, A.M. Differences in the Use of Procedures between Women and Men Hospitalized for Coronary Heart Disease. N. Engl. J. Med. 1991, 325, 221–225. [Google Scholar] [CrossRef]
- Steingart, R.M.; Packer, M.; Hamm, P.; Coglianese, M.E.; Gersh, B.; Geltman, E.M.; Sollano, J.; Katz, S.; Moyé, L.; Basta, L.L.; et al. Sex Differences in the Management of Coronary Artery Disease. N. Engl. J. Med. 1991, 325, 226–230. [Google Scholar] [CrossRef]
- Liakos, M.; Parikh, P.B. Gender Disparities in Presentation, Management, and Outcomes of Acute Myocardial Infarction. Curr. Cardiol. Rep. 2018, 20, 64. [Google Scholar] [CrossRef]
- Jneid, H.; Fonarow, G.C.; Cannon, C.P.; Hernandez, A.F.; Palacios, I.F.; Maree, A.O.; Wells, Q.; Bozkurt, B.; LaBresh, K.A.; Liang, L.; et al. Sex Differences in Medical Care and Early Death After Acute Myocardial Infarction. Circulation 2008, 118, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Gabet, A.; Danchin, N.; Juillière, Y.; Olié, V. Acute coronary syndrome in women: Rising hospitalizations in middle-aged French women, 2004–2014. Eur. Heart J. 2017, 38, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Rathore, S.S.; Wenger, N.K.; Frederick, P.D.; Abramson, J.L.; Barron, H.V.; Manhapra, A.; Mallik, S.; Krumholz, H.M. Sex and Racial Differences in the Management of Acute Myocardial Infarction, 1994 through 2002. N. Engl. J. Med. 2005, 353, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.; Goodman, S.G.; Yan, R.T.; Bugiardini, R.; Bierman, A.S.; Eagle, K.A.; Johnston, N.; Huynh, T.; Grondin, F.R.; Schenck-Gustafsson, K.; et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am. Heart J. 2012, 163, 66–73. [Google Scholar] [CrossRef]
- Radovanovic, D.; Erne, P.; Urban, P.; Bertel, O.; Rickli, H.; Gaspoz, J.-M.; on behalf of the AMIS Plus Investigators. Gender differences in management and outcomes in patients with acute coronary syndromes: Results on 20 290 patients from the AMIS Plus Registry. Heart 2007, 93, 1369–1375. [Google Scholar] [CrossRef]
- Hochman, J.S.; Buller, C.E.; Sleeper, L.A.; Boland, J.; Dzavik, V.; Sanborn, T.A.; Godfrey, E.; White, H.D.; Lim, J.; LeJemtel, T.; et al. Cardiogenic Shock Complicating Acute Myocardial Infarction—Etiologies, Management and Outcome: A Report from the SHOCK Trial Registry. J. Am. Coll Cardiol. 2000, 36 (Suppl. 3), 1063–1070. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Perl, L.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Lev, E.; Kornowski, R.; Porter, A. Impact of female sex on long-term acute coronary syndrome outcomes. Coron. Artery Dis. 2015, 26, 11–16. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Kornowski, R.; Sagie, A.; Vaknin-Assa, H.; Perl, L.; Porter, A.; Lev, E.; Assali, A. Gender Differences in Left Ventricular Function Following Percutaneous Coronary Intervention for First Anterior Wall ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2014, 114, 1473–1478. [Google Scholar] [CrossRef]
- Wada, H.; Miyauchi, K.; Daida, H. Gender differences in the clinical features and outcomes of patients with coronary artery disease. Expert Rev. Cardiovasc. Ther. 2018, 17, 127–133. [Google Scholar] [CrossRef]
- Perl, L.; Peiffer, V.; Fuhrer, A.E.; D’ascenzo, F.; Pietzsch, J.B. Sex differences in discharge destination following acute myocardial infarction. Coron. Artery Dis. 2018, 29, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.; Harpaz, D.; Shotan, A.; Boyko, V.; Leor, J.; Cohen, M.; Mandelzweig, L.; Mazouz, B.; Stern, S.; Behar, S.; et al. Sex differences in management and outcome after acute myocardial infarction in the 1990s: A prospective observational community-based study. Circulation 2000, 102, 2484–2490. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Bonds, D.E.; Lovato, J.; Goff, D.C.; Brancati, F.L. Sex disparities in procedure use for acute myocardial infarction in the United States, 1995 to 2001. Am. Heart J. 2004, 147, 1054–1060. [Google Scholar] [CrossRef]
- Wong, S.; Sleeper, L.A.; Monrad, E.; Menegus, M.A.; Palazzo, A.; Dzavik, V.; Jacobs, A.; Jiang, X.; Hochman, J.S. Absence of gender differences in clinical outcomes in patients with cardiogenic shock complicating acute myocardial infarction: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2001, 38, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Qiu, W.; Bawamia, B.; Veerasamy, M.; Jamieson, S.; Zaman, A. Gender Comparisons in Cardiogenic Shock During ST Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2013, 112, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Antoniucci, D.; Migliorini, A.; Moschi, G.; Valenti, R.; Trapani, M.; Parodi, G.; Bolognese, L.; Santoro, G.M. Does gender affect the clinical outcome of patients with acute myocardial infarction complicated by cardiogenic shock who undergo percutaneous coronary intervention? Catheter. Cardiovasc. Interv. 2003, 59, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Edep, M.E.; Brown, D.L. Effect of early revascularization on mortality from cardiogenic shock complicating acute myocardial infarction in california. Am. J. Cardiol. 2000, 85, 1185–1188. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Wegermann, Z.K.; Li, S.; Mahtta, D.; Grau-Sepulveda, M.; Smilowitz, N.R.; Gulati, M.; Garratt, K.N.; Wang, T.Y.; Jneid, H. Sex Differences in Management and Outcomes of Acute Myocardial Infarction Patients Presenting with Cardiogenic Shock. JACC Cardiovasc. Interv. 2022, 15, 642–652. [Google Scholar] [CrossRef]
- Maynard, C.; Every, N.R.; Martin, J.S.; Kudenchuk, P.J.; Weaver, W.D. Association of gender and survival in patients with acute myocardial infarction. Arch. Intern. Med. 1997, 157, 1379–1384. [Google Scholar] [CrossRef]
- Fengler, K.; Fuernau, G.; Desch, S.; Eitel, I.; Neumann, F.-J.; Olbrich, H.-G.; de Waha, A.; de Waha, S.; Richardt, G.; Hennersdorf, M.; et al. Gender differences in patients with cardiogenic shock complicating myocardial infarction: A substudy of the IABP-SHOCK II-trial. Clin. Res. Cardiol. 2014, 104, 71–78. [Google Scholar] [CrossRef]
- Fincke, R.; Hochman, J.S.; Lowe, A.M.; Menon, V.; Slater, J.N.; Webb, J.G.; LeJemtel, T.H.; Cotter, G.; SHOCK Investigators. Cardiac power is sthe strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. J. Am. Coll Cardiol. 2004, 44, 340–348. [Google Scholar] [CrossRef]
- Roffi, M.; Radovanovic, D.; Iglesias, J.F.; Eberli, F.R.; Urban, P.; Pedrazzini, G.B.; Erne, P.; Rickli, H. Multisite vascular disease in acute coronary syndromes: Increased in-hospital mortality and no improvement over time. Eur. Heart J. Acute Cardiovasc. Care 2018, 9, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Perl, L.; Bental, T.; Vaknin-Assa, H.; Assali, A.; Codner, P.; Talmor-Barkan, Y.; Greenberg, G.; Samara, A.; Witberg, G.; Orvin, K.; et al. Independent Impact of Peripheral Artery Disease on Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2020, 9, e017655. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Eagle, K.A.; Ohman, E.M.; Hirsch, A.T.; Goto, S.; Mahoney, E.M.; Wilson, P.W.F.; Alberts, M.J.; D’agostino, R.; Liau, C.-S.; et al. Comparative Determinants of 4-Year Cardiovascular Event Rates in Stable Outpatients at Risk of or With Atherothrombosis. JAMA 2010, 304, 1350–1357. [Google Scholar] [CrossRef]
- Hussein, A.A.; Uno, K.; Wolski, K.; Kapadia, S.; Schoenhagen, P.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Peripheral Arterial Disease and Progression of Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2011, 57, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.G.C.; Finn, P.; Hall, J.A.; Harcombe, A.A.; Wright, R.A.; de Belder, M.A. Predictors of outcome after percutaneous treatment for cardiogenic shock. Heart 2005, 91, 339–344. [Google Scholar] [CrossRef]
- Acharya, D., II. Predictors of Outcomes in Myocardial Infarction and Cardiogenic Shock. Cardiol. Rev. 2018, 26, 255–266. [Google Scholar] [CrossRef]
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.N.; Mauri, L.; et al. Faculty Opinions recommendation of Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: A report from the cathpci registry. Cardiovasc. Interv. 2017, 9, 341–351. [Google Scholar]
- Awad, H.H.; Anderson, F.A.; Gore, J.M.; Goodman, S.G.; Goldberg, R.J. Cardiogenic shock complicating acute coronary syndromes: Insights from the Global Registry of Acute Coronary Events. Am. Heart J. 2012, 163, 963–971. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll Cardiol. 2006, 47 (Suppl. 3), S21–S29. [Google Scholar]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef]
- Ostadal, B.; Netuka, I.; Maly, J.; Besik, J.; Ostadalova, I. Gender Differences in Cardiac Ischemic Injury and Protection—Experimental Aspects. Exp. Biol. Med. 2009, 234, 1011–1019. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Milford-Beland, S.; Roe, M.T.; Piana, R.N.; Kao, J.; Shroff, A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am. Heart J. 2009, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Isorni, M.-A.; Aissaoui, N.; Angoulvant, D.; Bonello, L.; Lemesle, G.; Delmas, C.; Henry, P.; Schiele, F.; Ferrières, J.; Simon, T.; et al. Temporal trends in clinical characteristics and management according to sex in patients with cardiogenic shock after acute myocardial infarction: The FAST-MI programme. Arch. Cardiovasc. Dis. 2018, 111, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Coats, L.; Kini, A.S.; Mehran, R. Cardiogenic Shock in Women. Interv. Cardiol. Clin. 2012, 1, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Spencer, F.A.; Gore, J.M.; Lessard, D.; Yarzebski, J. Thirty-year trends (1975 to 2005) in the magnitude, management of, and hospital death rates associated with acute myocardial infarction: A population-based perspective. Circulation 2009, 119, 1211–1219. [Google Scholar] [CrossRef]
- Bangalore, S.; Gupta, N.; Guo, Y.; Lala, A.; Balsam, L.; Roswell, R.O.; Reyentovich, A.; Hochman, J.S. Outcomes with Invasive vs Conservative Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. Am. J. Med. 2015, 128, 601–608. [Google Scholar] [CrossRef]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, E232–E268. [Google Scholar] [CrossRef]
- Wong, S.; Sanborn, T.; Sleeper, L.A.; Webb, J.G.; Pilchik, R.; Hart, D.; Mejnartowicz, S.; Antonelli, T.A.; Lange, R.; French, J.K.; et al. Angiographic findings and clinical correlates in patients with cardiogenic shock complicating acute myocardial infarction: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2000, 36, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.S.; Jeong, M.H.; Cho, K.H.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Hong, T.J.; Seong, I.W.; Chae, J.K.; Kim, C.J.; et al. Effect of Early Statin Treatment in Patients with Cardiogenic Shock Complicating Acute Myocardial Infarction. Korean Circ. J. 2013, 43, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef] [PubMed]
- Yan, I.; Schrage, B.; Weimann, J.; Dabboura, S.; Hilal, R.; Beer, B.N.; Becher, P.M.; Seiffert, M.; Magnussen, C.; Schnabel, R.B.; et al. Sex differences in patients with cardiogenic shock. ESC Heart Fail. 2021, 8, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).