Multi-Omic Candidate Screening for Markers of Severe Clinical Courses of COVID-19
Abstract
:1. Introduction
2. Research Design and Methods
2.1. Patient Inclusion and Clinical Measurements
2.2. Plasma Measurements
2.3. Ethics
2.4. Statistics
2.4.1. Preprocessing
2.4.2. Examining the Significance of Estimated Parameters
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
4. Discussion
4.1. Interleukin-6 and Hyperinflammation
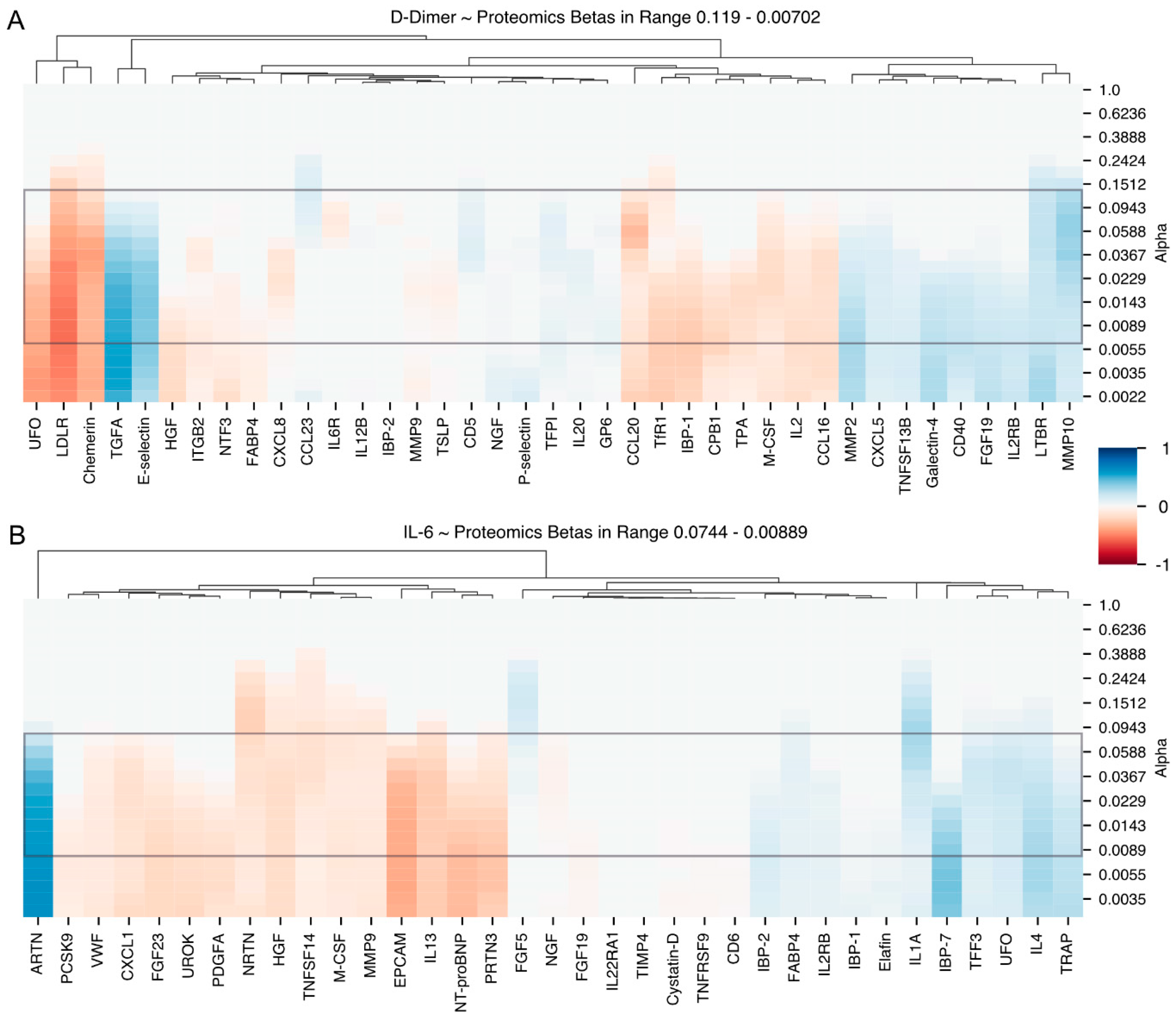
Candidates Associated with Changes in IL-6
4.2. D-Dimers and Hypercoagulopathy
Candidates Associated with Changes in D-dimers
4.3. Strengths and Limitations
5. Clinical Perspectives—Translational Outlook
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shanka-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernandez, J.; Prati, D.; Baselli, G.; Asselta, R.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [PubMed]
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 16, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Sanche, S.; Cassidy, T.; Chu, P.; Perelson, A.S.; Riberiro, R.M.; Ke, R. A simple model of COVID-19 explains disease severity and the effect of treatments. Sci. Rep. 2022, 12, 14210. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chi, Y.; Ge, Y.; Wu, B.; Zhan, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, R.; Kollimuttathuillam, S.; Gowda, A.M.; Singh, B.; Mehta, D.; Maroules, M. The looming storm: Blood and cytokines in COVID-19. Blood Rev. 2021, 46, 100743. [Google Scholar] [CrossRef]
- Gao, C.; Cai, Y.; Zhang, K.; Zhou, L.; Zhang, Y.; Zhang, X.; Li, Q.; Li, W.; Yang, S.; Zhao, X.; et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: A retrospective observational study. Eur. Heart J. 2020, 41, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Lehmann, I.; Eils, R. Hyperinflammation as underlying mechanism predisposing patients with cardiovascular diseases for severe COVID-19. Eur. Heart J. 2021, 42, 1720–1721. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, E.; Perricone, C.; Cafaro, G.; Gerli, R. Hypertension and SARS-CoV-2 infection: Is inflammation the missing link? Cardiovasc. Res. 2020, 116, e193–e194. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Colling, M.E.; Kanthi, Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc. Med. 2020, 25, 471–478. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Lederose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Cavalier, E.; Guiot, J.; Lechner, K.; Dutsch, A.; Eccleston, M.; Herzog, M.; Bygott, T.; Schomburg, A.; Kelly, T.; Holdenrieder, S. Circulating Nucleosomes as Potential Markers to Monitor COVID-19 Disease Progression. Front. Mol. Biosci. 2021, 8, 600881. [Google Scholar] [CrossRef]
- Lionte, C.; Sorodoc, V.; Haliga, R.E.; Bologa, C.; Ceasovschih, A.; Petris, O.R.; Coman, A.E.; Stoica, A.; Sirbu, O.; Puha, G.; et al. Inflammatory and Cardiac Biomarkers in Relation with Post-Acute COVID-19 and Mortality: What We Know after Successive Pandemic Waves. Diagnostics 2022, 12, 1373. [Google Scholar] [CrossRef]
- Nicogossian, A.; Kloiber, O.; Stabile, B. The Revised World Medical Association’s Declaration of Helsinki 2013: Enhancing the Protection of Human Research Subjects and Empowering Ethics Review Committees. World Med. Health Policy 2014, 6, 1–3. [Google Scholar] [CrossRef]
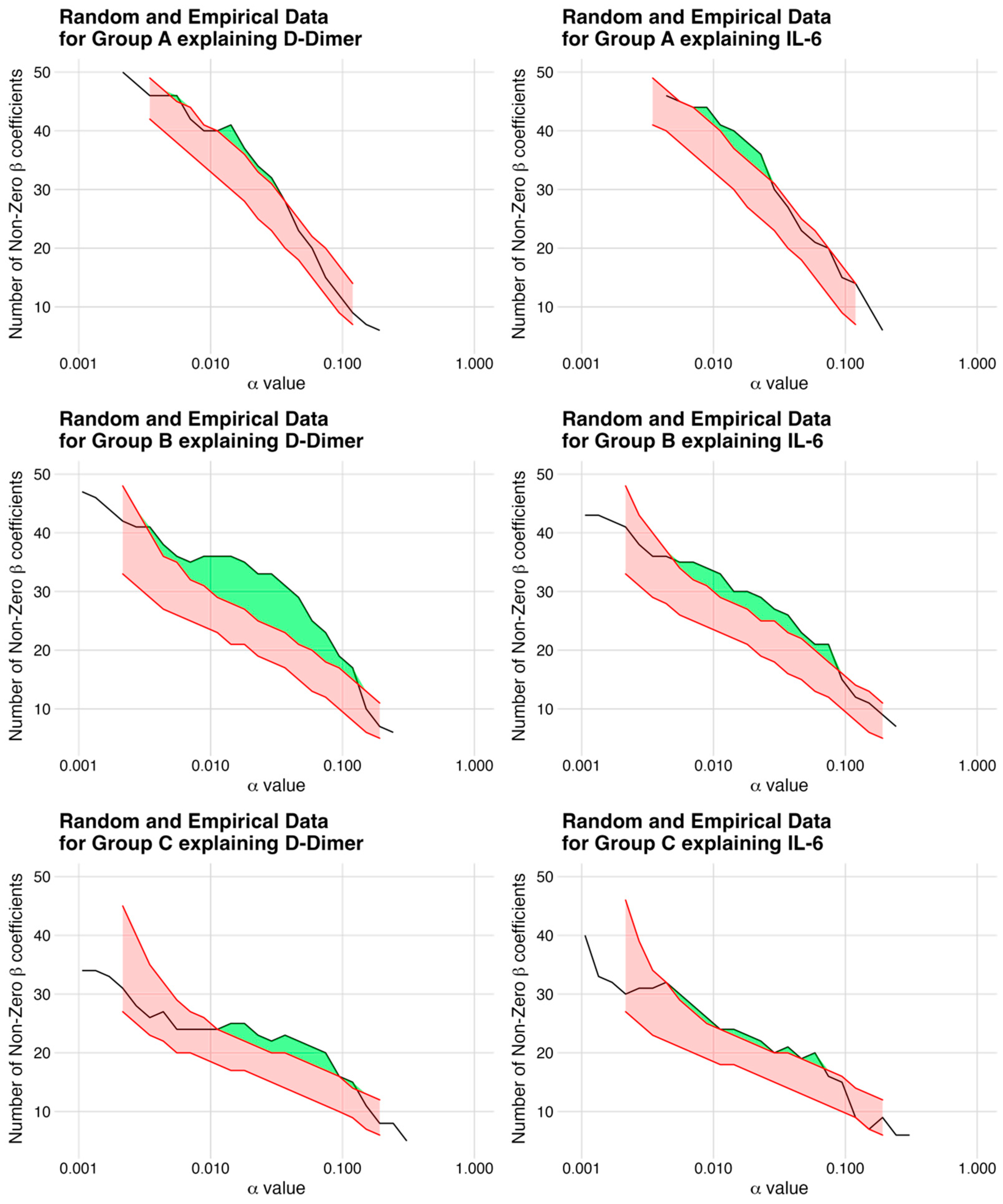
- Carsten Uhlig, S.U. LASSO extension: Using the number of non-zero coefficients to test the global model hypothesis. arXiv 2023, arXiv:2307.16374v1. [Google Scholar]
- uniprot.org. Available online: https://www.uniprot.org (accessed on 8 September 2022).
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Baggen, J.; Vanstreels, E.; Jansen, S.; Daelemans, D. Cellular host factors for SARS-CoV-2 infection. Nat. Microbiol. 2021, 6, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.H.; Tansey, M.G.; Lampe, P.A.; Fahrner, T.J.; Enomoto, H.; Simburger, K.S.; Leitner, M.L.; Araki, T.; Johnson, E.M., Jr.; Milbrandt, J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 1998, 21, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, M.J.; Lowery, B.D.; Farber-Eger, E.; Wang, T.J.; Bradham, W.; Ormseth, M.J.; Chung, C.P.; Stein, C.M.; Gupta, D.K. Heart Failure Risk Associated with Rheumatoid Arthritis-Related Chronic Inflammation. J. Am. Heart Assoc. 2020, 9, e014661. [Google Scholar] [CrossRef] [PubMed]
- Minty, A.; Chalon, P.; Derocq, J.M.; Dumon, X.; Guillemot, J.C.; Kaghad, M.; Labit, C.; Leplatois, P.; Liauzun, P.; Miloux, B.; et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 1993, 362, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Frontiers in Pharmacology 2019, 10, 1387. [Google Scholar] [CrossRef]
- Watany, M.M.; Abdou, S.; Elkolaly, R.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. Evaluation of admission levels of P, E and L selectins as predictors for thrombosis in hospitalized COVID-19 patients. Clin Exp Med. 2022, 22, 567–575. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Silva, M.; Videira, P.A.; Sackstein, R. E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front. Immunol. 2018, 8, 1878. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Rando, E.; Al Ismail, D.; De Angelis, M.; Cancelli, F.; Miele, M.C.; Aronica, R.; Mauro, V.; Di Timoteo, F.; Loffredo, L.; et al. Role of Serum E-Selectin as a Biomarker of Infection Severity in Coronavirus Disease 2019. J. Clin. Med. 2021, 10, 4018. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Fogagnolo, A.; Campo, G.; Zucchetti, O.; Verri, M.; Ottaviani, I.; Tunstall, T.; Grasso, S.; Scaramuzzo, V.; Murgolo, F.; et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care 2021, 25, 74. [Google Scholar] [CrossRef]
- Soares-Schanoski, A.; Sauerwald, N.; Goforth, C.W.; Periasamy, S.; Weir, D.L.; Lizewski, S.; Lizewski, R.; Ge, Y.; Kuzmina, N.A.; Nair, V.D.; et al. Asymptomatic SARS-CoV-2 Infection Is Associated With Higher Levels of Serum IL-17C, Matrix Metalloproteinase 10 and Fibroblast Growth Factors Than Mild Symptomatic COVID-19. Front. Immunol. 2022, 13, 821730. [Google Scholar] [CrossRef]
- Simats, A.; García-Berrocoso, T.; Penalba, A.; Giralt, D.; Llovera, G.; Jiang, Y.; Ramiro, L.; Bustamante, A.; Martinez-Saez, E.; Canals, F.; et al. CCL23: A new CC chemokine involved in human brain damage. J. Intern. Med. 2018, 283, 461–475. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of low-density lipoprotein receptors: Implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation 1987, 76, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Fraunberger, P.; Schaefer, S.; Werdan, K.; Walli, A.K.; Seidel, D. Reduction of Circulating Cholesterol and Apolipoprotein Levels during Sepsis. Clin. Chem. Lab. Med. 1999, 37, 357–362. [Google Scholar] [CrossRef]
- Cure, E.; Cumhur Cure, M. Strong relationship between cholesterol, low-density lipoprotein receptor, Na(+)/H(+) exchanger, and SARS-CoV-2: This association may be the cause of death in the patient with COVID-19. Lipids Health Dis. 2021, 20, 179. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Karathanasis, S.K.; Yang, Z.H.; Freeman, L.; Kotani, K.; Remaley, A.T. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9843–9853. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Hopps, E.; Lo Presti, R.; Caimi, G. Matrix Metalloproteases in Arterial Hypertension and their Trend after Antihypertensive Treatment. Kidney Blood Press. Res. 2017, 42, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Dentali, F.; Nicolini, E.; Maresca, A.M.; Tayebjee, M.H.; Franz, M.; Guasti, L.; Venco, A.; Schiffrin, E.L.; Lip, G.Y.; et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: A systematic review and meta-analysis. J. Hypertens. 2012, 30, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef]
- Carolina, D.A.-M.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar]
- Perreau, M.; Suffiotti, M.; Marques-Vidal, P.; Wiedemann, A.; Levy, Y.; Laouénan, C.; Ghosn, J.; Fenwick, C.; Comte, D.; Roger, T.; et al. The cytokines HGF and CXCL13 predict the severity and the mortality in COVID-19 patients. Nat. Commun. 2021, 12, 4888. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 7) | |
|---|---|
| Sex | |
| Male, n | 5 |
| Female, n | 2 |
| Age, mean (SD) | 65 (8) |
| Days of hospitalization, mean (SD) | 15 (10) |
| Death (if yes), n | 5 |
| Diabetes, n | 3 |
| Hypertension, n | 5 |
| Hypercholesterinemia, n | 3 |
| Smoking, n | 0 |
| Former Smoking, n | 1 |
| Coronary artery disease, n | 2 |
| Previous myocardial infarction, n | 1 |
| Previous CABG, n | 0 |
| Renal disease, n | 2 |
| Pulmonary disease, n | 3 |
| PCR positive, n | 6 |
| Intubated on admission, n | 7 |
| Days intubated before admission, mean (SD) | 5 (4) |
| Intubated total (days), mean (SD) | 17 (8) |
| Renal replacement therapy, n | 3 |
| Need for renal replacement therapy (days), mean (SD) | 8 (11) |
| ECMO, n | 3 |
| Need for ECMO (days), mean (SD) | 5 |
| Catecholamines, n | 6 |
| Need for Catecholamines (days), mean (SD) | 3 (3) |
| Antibiotics, n | 7 |
| Need for antibiotics (days), mean (SD) | 9 (7) |
| Thrombotic Event, n | 2 |
| Haemorrhagic Event, n | 3 |
| Arrhythmia during hospitalization, n | 5 |
| Malignant Arrhythmic Event, n | 3 |
| Pulmonary Infiltrate, n | 6 |
| Medication on admission | |
| ASS, n | 3 |
| ACE-inhibitor, n | 2 |
| AT1-antagonist, n | 2 |
| Betablocker, n | 4 |
| Diuretics, n | 2 |
| Antidiabetics, n | 3 |
| Statin, n | 3 |
| Laboratory at admission | |
| Leukocytes (103 cells/L), mean (SD) | 13 (4) |
| Hemoglobin (g/dL), mean (SD) | 10 (2) |
| Creatinine (mg/dL), mean (SD) | 3 (2) |
| GFR (mL/min/1.73), mean (SD) | 36 (26) |
| D-dimer (mg/L FEU), mean (SD) | 7 (10) |
| Troponin high-sensitive (ng/L), mean (SD) | 57 (95) |
| CK (U/L), mean (SD) | 953 (795) |
| CK-MB (U/L), mean (SD) | 48 (60) |
| CRP (mg/L), mean (SD) | 285 (127) |
| PCT (ng/L), mean (SD) | 2 (3) |
| IL-6 (ng/L), mean (SD) | 367 (231) |
| NT-proBNP (ng/L), mean (SD) | 2616 (3465) |
| Dataset | Total Samples | Total Biomarkers | Selected Samples | Selected Biomarkers |
|---|---|---|---|---|
| Group A | 93 | 84 | 79 | 48 |
| Routine Markers | ||||
| Group B | 80 | 185 | 36 | 184 |
| Proteomic Markers | ||||
| Group C | 80 | 862 | 26 | 662 |
| Metabolomic Markers |
| Group | Candidates |
|---|---|
| D-Dimer | |
| D-Dimer + | TGFA, CD5, MMP2, MMP10, IL2RB, P-selectin, E-selectin, TNR5, TNR3, CXCL5, CCL23, Galectin, GPVI, TN13B |
| D-Dimer − | IL1α, IBP1, FABP4, UFO, CCL16, TPA, LDLR, TFR1, IL6RA, IL8, CBPB1, NTF3, IL2, CCL20, Chemerin, M-CSF, HGF, MMP9 |
| IL-6 | |
| IL-6 + | IL4, FGF5, PPA5, TFF3, IBP7, ARTN, IL1α, IBP1, FABP4, UFO |
| Il-6 − | TNF14, UROK, PDGFA, VWF, CXCL1, EPCAM, PRTN3, IL13, NRTN, FGF23, M-CSF, HGF, MMP9 |
| D-dimers | IL-6 | ||||||
|---|---|---|---|---|---|---|---|
| Marker | t-Value | β | Adj. p-Value | Marker | t-Value | β | Adj. p-Value |
| Group A | |||||||
| C-reactive protein | 2.365 | 0.260 | 1.000 | Uric Acid | 3.004 | 0.324 | 0.284 |
| Mean corpuscular volume | −2.197 | −0.243 | 1.000 | Creatinine | 2.596 | 0.284 | 0.892 |
| High-sensitivity troponin T | −2.205 | −0.244 | 1.000 | Gamma-glutamyl Transferase | −2.144 | −0.237 | 1.000 |
| IPC | −2.794 | −0.303 | 0.519 | ||||
| IPFAB | −2.794 | −0.303 | 0.519 | ||||
| Group B | |||||||
| Retinoic Acid Receptor Responder 2 | −2.014 | −0.326 | 1.000 | Interleukin-1 alpha | 2.799 | 0.433 | 0.302 |
| Fibroblast growth factor 5 | 2.636 | 0.412 | 0.452 | ||||
| Trefoil factor 3 | 2.579 | 0.404 | 0.519 | ||||
| Neurturin | −2.506 | −0.395 | 0.617 | ||||
| Tumor necrosis factor ligand super-family member 14 | −2.962 | −0.453 | 0.199 | ||||
| Group C | |||||||
| Triacylglycerols (17:2_34:2) | −2.799 | −0.496 | 0.259 | Triacylglycerols (22:6_32:1) | 3.855 | 0.618 | 0.020 |
| Triacylglycerols (16:0_37:3) | 2.694 | 0.482 | 0.330 | ||||
| Medium-Chain Acyl-Coenzyme A Dehydrogenase | −2.778 | −0.493 | 0.271 | ||||
| 3-Hydroxy-3-Methylglutaryl-Coenzyme A Lyase | −3.241 | −0.552 | 0.090 | ||||
| Multiple Carboxylase | −3.265 | −0.555 | 0.085 | ||||
| D-dimers | IL-6 | ||||||
|---|---|---|---|---|---|---|---|
| Relevance | Absolute | Alpha | Candidates | Relevance | Absolute | Alpha | Candidates |
| 7% | 3 | 0.0143 | 41 | 8% | 3 | 0.0229 | 36 |
| 28% | 8 | 0.0464 | 29 | 14% | 3 | 0.0744 | 21 |
| 15% | 3 | 0.0744 | 20 | 10% | 2 | 0.0588 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutsch, A.; Uhlig, C.; Bock, M.; Graesser, C.; Schuchardt, S.; Uhlig, S.; Schunkert, H.; Joner, M.; Holdenrieder, S.; Lechner, K. Multi-Omic Candidate Screening for Markers of Severe Clinical Courses of COVID-19. J. Clin. Med. 2023, 12, 6225. https://doi.org/10.3390/jcm12196225
Dutsch A, Uhlig C, Bock M, Graesser C, Schuchardt S, Uhlig S, Schunkert H, Joner M, Holdenrieder S, Lechner K. Multi-Omic Candidate Screening for Markers of Severe Clinical Courses of COVID-19. Journal of Clinical Medicine. 2023; 12(19):6225. https://doi.org/10.3390/jcm12196225
Chicago/Turabian StyleDutsch, Alexander, Carsten Uhlig, Matthias Bock, Christian Graesser, Sven Schuchardt, Steffen Uhlig, Heribert Schunkert, Michael Joner, Stefan Holdenrieder, and Katharina Lechner. 2023. "Multi-Omic Candidate Screening for Markers of Severe Clinical Courses of COVID-19" Journal of Clinical Medicine 12, no. 19: 6225. https://doi.org/10.3390/jcm12196225
APA StyleDutsch, A., Uhlig, C., Bock, M., Graesser, C., Schuchardt, S., Uhlig, S., Schunkert, H., Joner, M., Holdenrieder, S., & Lechner, K. (2023). Multi-Omic Candidate Screening for Markers of Severe Clinical Courses of COVID-19. Journal of Clinical Medicine, 12(19), 6225. https://doi.org/10.3390/jcm12196225








