Abstract
The aim of this study was to explore the impact of dry eye disease (DED) on the uncorrected distance visual acuity (UDVA) and refractive status after small incision lenticule extraction (SMILE). This prospective cohort study enrolled 29 patients (DED group, 11 eyes; non-DED group, 18 eyes) who underwent SMILE in our center from July to September 2022. The examinations on DED, refractive status and UDVA were performed before surgery, and on day 7 and 20 after surgery. The results showed that on day 20 after SMILE, subjects in the non-DED group reported greater changes of ocular surface disease index value increase and tear-film breakup time reduction compared to baseline than those in the DED group (p < 0.001 and p = 0.048, respectively). Compared to preoperative status, DED patients had greater improvements of UDVA and better optometric outcomes on day 20 after surgery than non-DED subjects (p = 0.008 and 0.026, respectively). Multiple linear regression analysis showed age, contact lens daily wearing time, and tear meniscus height before surgery were of the highest value to predict UDVA on day 20 after SMILE in contact lens wearers (p = 0.006, 0.010 and 0.043, respectively). In conclusion, preoperative tear function could affect UDVA after SMILE. The impact of DED on UDVA and refraction should be taken into consideration before surgery.
1. Introduction
Myopia is one of the most common ocular diseases in Eastern Asians, especially adolescents and young people [1,2]. More than 6 million people have been reported to undergo small incision lenticule extraction (SMILE) globally since 2011, among which more than half were performed in China [3]. Several factors have been shown to potentially affect visual function postoperatively, including surgery types [4,5], higher-order aberration [4,6], pupil size [7], and dry eye disease (DED) [8,9].
DED is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles [10]. The main clinical manifestations of DED are ocular discomfort, dryness, burning sensation, grittiness, photophobia, pain, visual disturbance due to partial or total tear film instability, increased tear film osmolarity, and subacute inflammation of the ocular surface [11]. As one of the most common ocular surface diseases impairing vision-related quality of life, its prevalence varies from 4.29% to 50.33% in the Chinese population [12]. Many risk factors have been confirmed to be closely related to the development and deterioration of DED, among which refractive surgery is an important one, especially in Asians [13].
DED and corneal refractive surgery influence each other. On one hand, due to a strong willingness to free themselves from glasses, most patients used to be contact-lens wearers, which is an already-known risk factor of DED [13]. Therefore, a great number of patients have had DED before corneal refractive surgery. On the other hand, DED is one of the most common complications after corneal refractive surgery, which might be attributed to many factors including impaired corneal sensation and decreased nutrition on corneal epithelium due to the damage of the sub-basal and stromal nerve plexus, decreased mucin secretion due to the damage of goblet cells, and abnormal aqueous and lipid tear production due to reduced blinking frequency. All of them affect the quality and quantity of tear films [14,15,16,17]. Compared with SMILE, laser-assisted in situ keratomileusis (LASIK), which is involved with flap creation and photoablation of corneal stroma, induces more keratocyte apoptosis and inflammation, and higher risks of flap-related complications [18,19]. Reinstein DZ et al. confirmed that after the surgery, SMILE could maintain stromal tensile strength better than LASIK [20]. Nevertheless, the reports on DED after SMILE are not rarely seen even though it has a less negative impact on the corneal sub-basal nerve plexus, biomechanical stability and ocular surface microenvironment [17,18,21,22,23,24,25,26]. Moreover, the impact of preoperative DED on the prediction of uncorrected distance visual acuity (UDVA) and refractive status after SMILE has not been fully addressed up till now.
Therefore, we performed this prospective study in order to investigate the impact of pre-operative DED on postoperative UDVA and refractive status, and explore the predictive potential of DED parameters on UDVA.
2. Materials and Methods
2.1. Study Population
This prospective cohort study was approved by the Institutional Review Board of Eye, Ear, Nose & Throat Hospital of Fudan University and conformed to the tenets of the Helsinki Declaration. A total of 29 subjects who underwent SMILE in Eye, Ear, Nose & Throat Hospital of Fudan University from July to September 2022 were enrolled. Written informed consents were obtained from all participants. Eleven patients met the following criteria of DED as previously described [27]: (1) a frequent or sustained occurrence of at least two dry eye symptoms (ocular discomfort/foreign body sensation, photophobia, grittiness, pain, dryness, blurred and fluctuating vision); (2) the presence of any two of the following three signs: (i) tear film breakup time <10 s, (ii) Schirmer I test (SIT) <10 mm/5 min, and (iii) corneal fluorescein staining (CFS) score ≥ 1 [28,29]. They were assigned in a preoperative DED group (n = 11), and the other 18 patients were in a non-DED group (n = 18).
The exclusion criteria included: (1) <18-year-old; (2) pregnancy or in lactation; (3) history of autoimmune diseases or ocular trauma; (4) active ocular or periocular infection or inflammation; (5) concomitant lid margin abnormality; (6) history of ocular or periocular surgery within 6 months before enrollment; (7) history of artificial tear usage within 2 weeks before enrollment; (8) history of lacrimal punctal occlusion; (9) patients who could not cooperate with the examinations and surgery.
2.2. Ocular Examinations
2.2.1. Order of Ocular Examinations
Eligible subjects were required to fulfil a self-reported questionnaire, Ocular Surface Disease Index (OSDI), before ocular examination to avoid the influence of clinical examinations on their responses. Sociodemographic data were also obtained prior to ocular examination, which included age, gender, occupation and history of contact lens wear. Then, all participants underwent a thorough ocular examination including best corrected visual acuity (BCVA), slit-lamp biomicroscopy, direct ophthalmoscopy, fundus photography, tonometry, optometric examination, and Oculus Keratograph 5M (Wetzlar, Germany). Fluorescence tear film breakup time (FBUT) and CFS were performed during slit-lamp biomicroscopy examination. SIT without anesthesia was finally performed when all the other examinations were finished so as to avoid the impact on corneal epithelium and fluorescence staining. These examinations were performed before surgery, and repeated on day 7 and 20 after surgery. Moreover, UDVA and BCVA were obtained after the surgery, both of which were evaluated with Standard for logarithmic visual acuity charts (GB/T 11533-2011, China) [30]. The eye with DED was included in patients with monocular DED, while the more severe eye was enrolled if the patients were bilaterally affected. The primary endpoints were UDVA and refractive status on Day 20 after SMILE. DED parameters on Day 7 and Day 20 postoperatively were also evaluated.
2.2.2. OSDI
OSDI questionnaire consists of three sections (a total of 12 questions), and evaluates the severity of ocular discomfort symptoms, visual functions related life quality and environmental triggers in the recent week. OSDI scores = (sum of scores for questions answered × 25)/(number of answered questions) [31].
2.2.3. Oculus Keratograph 5M
Oculus K5M was used to obtain non-invasive breakup time (NIBUT), tear meniscus height (TMH), lipid layer color (LLC), lipid layer uniformity (LLU), and meibomian gland (MG) loss. After blinking twice in a dark room, the patients were required to keep their eyes open as long as they could until the next blink took place. Then, the upper and lower eyelids were everted by the same operator to capture the meibography with infrared system. The examinations were repeated three times.
2.2.4. Slit-Lamp Biomicroscopy
All subjects underwent a slit-lamp examination. Those having any other ocular abnormalities that might potentially interfere with the tear film were excluded. FBUT and CFS were assessed under cobalt blue light during slit-lamp examination as previously reported [27].
2.2.5. SIT
Without topical anesthesia, Schirmer paper strips (5 × 40 mm, Jingming, Tianjin, China) were folded at the notch and placed in the 1/3 of the external lower conjunctival sac cautiously. Then, the patients were asked to close their eyes gently for 5 min. The length of wetting by tears from the notch was measured and recorded.
2.3. Image Analysis and Measurement
2.3.1. Assessment of DED Parameters Obtained by K5M
DED parameters were measured based on the placido rings projected on the tear film. The changes of tear film during two blinks were presented as color-coded tear maps, in which the color closest to red indicated the location with the most instable tear film. The duration between the first blink and tear film break-up at this location was recorded as the first NIBUT. The mean value of the first NIBUT in different zones of the cornea was calculated as the average NIBUT. TMH was measured from the lower lid margin with the application of a caliper tool in the customized software, just as previously described [32]. The measurement of NIBUT and TMH was performed on three scans and the average values were calculated.
Based on the image captured immediately after blinking, LLC and LLU were evaluated. LLC was classified into five colors: multicolor, red-green, both of which were considered as normal, and blue-grey, hoary, and achromatic, which were abnormal. LLU was divided into even distribution and uneven distribution, which were assigned the scores 0 and 1, respectively. The grades of MG loss were scored according to the proportion of MG dropout area over the entire gland area. Score 0, 1, 2, and 3 represented no dropout, dropout area ≤ 1/3, 1/3 < dropout area ≤ 2/3, and dropout area > 2/3, respectively [33].
2.3.2. Measurement of FBUT
The participants were asked to naturally blink several times until the cornea was fully covered with fluorescein sodium solution. Then, they were told to keep eyes open as long as possible under the cobalt blue light. The duration from the last blink to the first black spot on the corneal surface was measured and recorded as FBUT. The outcome of FBUT was the average of three repeated examinations for each eye.
2.3.3. Evaluation of CFS
CFS was assessed within 1–3 min after fluorescein instillation [34]. Based on the National Eye Institute grid, the cornea was divided into 5 quadrants (central, superior, inferior, nasal and temporal) and corneal epithelial staining in each quadrant was evaluated and scored 0–3 according to the following criteria: 0, no staining; 1, <15 dots; 2, 16–30 dots; 3, >30 dots or strip/bulk staining or corneal filaments [28]. The total score ranged from 0–15, and the score ≥ 1 was considered as positive.
2.4. Surgical Procedure and Postoperative Treatment
The procedure of SMILE was performed as previously described [35,36]. In brief, 500-kHz femtosecond laser system (Visumax; Carl Zeiss, Jena, Germany) was set to 110–160 μm as the intended cap thickness and 7.6 mm as the intended cap diameter. The lenticule optical zone diameter was set between 6.1 and 6.8 mm based on the diameter of scotopic pupil. With a 2 mm wide small incision, the vertical side cut was created at 90- or 120-degree superior cornea. The refractive stromal lenticule was dissected after laser cutting and extracted through the incision. After the surgery, all patients were administered 0.5% levofloxacin eye drops 4 times/d for 7 days, and 0.1% fluorometholone eye drops tapered every 3 days from 8 times/d to once a day within 3 weeks.
2.5. Statistical Analysis
Stata 16.0 software (Stata Corp., College Station, TX, USA) and SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. The Shapiro–Wilk test was used to test the normality of data. The data with normal distribution were presented as means ± standard deviation, otherwise as median (range). Student’s t or t’ test, Wilcoxon rank sum test, and Fisher’s exact method were used to analyze normally distributed data, non-normally distributed data and enumeration data, respectively. As for the comparison on data in one group among different time points, two-way analysis of variance, Friedman test and Cochran’s Q test were performed. Bonferroni correction was used in subgroup pairwise comparisons. Regression analysis was performed in contact lens wearers. A total of 14 variables were included in the univariate linear regression analysis, including age, wearing duration, wearing frequency, daily wearing time, UDVA, spherical equivalent (SE), OSDI value, SIT, FBUT, CFS score, first NIBUT, average NIBUT, TMH, and grade score of MG loss. Variables with p values less than 0.35 in univariate linear regression analysis were selected for multiple linear regression analysis to explore the predictive parameters for UDVA. A p value < 0.05 was considered statistically significant.
3. Results
A total of 29 patients (12 males and 17 females, 29 eyes) with a median age of 24 (range: 17–39) years were enrolled. Among them, 15 eyes had the history of wearing contact lens, and the majority was soft contact lens (14/15, 93.33%). Preoperative LogMAR UDVA of all subjects was 1.06 ± 0.31, and SE was −5.08 ± 1.64 diopters (D). The demographic data and clinical characteristics of the DED group and non-DED group were summarized in Table 1. Before the surgery, subjects in the DED group reported a worse UDVA, a higher OSDI score, a lower SIT value and a higher proportion of abnormal LLC than those in the non-DED group (p = 0.036, <0.001, 0.041 and 0.008, respectively).

Table 1.
Preoperative demographic data and clinical characteristics of DED and non-DED group.
3.1. Postoperative UDVA and Refractive Status
On day 20 after surgery, the LogMAR UDVA and SE of all subjects were −0.03 ± 0.08 and (−0.22 ± 0.39) D, both of which were significantly improved compared to the baseline values (both p < 0.001). However, the comparisons on UDVA between the DED group and non-DED group did not have significant differences (p = 0.248). It was unexpected that, compared to preoperative status, DED patients had greater improvements of UDVA and better optometric outcomes on day 20 after SMILE than non-DED subjects (p = 0.008 and 0.026, respectively) (Table 2).

Table 2.
Comparison of UDVA, SE and DED parameters between DED and Non-DED group on day 20 after surgery.
3.2. Postoperative DED Parameters
Compared to pre-operative values, a dramatically higher increment of OSDI score (median: 9.09, range: 0.00–66.67, p = 0.005) was reported by non-DED subjects on day 7 after SMILE (Figure 1). Non-DED patients also showed a significant FBUT reduction (p = 0.048), an increasing proportion of abnormal LLC and uneven LLU on day 20 after surgery. However, such changes were not found in the DED group. The changes of MG loss after surgery were not analyzed because no evidence supported any detectable changes of MG morphology within a short duration in eyes undergoing normal SMILE surgery and routine medical treatments [25].
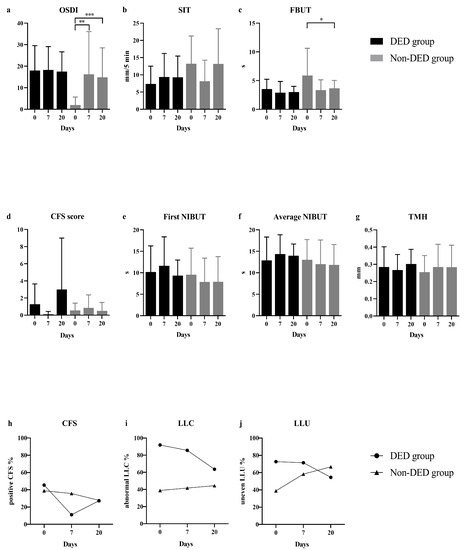
Figure 1.
Comparisons of DED parameters before SMILE and on day 7 and day 20 after surgery. OSDI scores (a), SIT (b) and FBUT (c) values in DED patients did not show any significant changing trends before and after surgery. However, an increased OSDI score and significant FBUT reduction were found in non-DED subjects. SIT values in non-DED patients decreased on day 7, and recovered on day 20 without significant difference. The alterations of CFS score (d) in both groups did not have any statistically significant differences. The proportion of eyes with positive CFS (h) gradually decreased in non-DED group after surgery, while decreased on day 7 and increased on day 20 in DED group. Comparisons on the first NIBUT (e), the average NIBUT (f) and TMH (g) before and after surgery did not find any significant differences in both two groups. Non-DED eyes showed an increasing proportion of abnormal LLC (i) and uneven LLU (j) after SMILE, which were decreased in DED eyes. * p < 0.05, ** p < 0.01 and *** p < 0.001. DED, dry eye disease; OSDI, ocular surface disease index; SIT, Schirmer I test; FBUT, fluorescein tear film breakup time; CFS, corneal fluorescein staining; NIBUT, non-invasive breakup time; TMH, tear meniscus height; LLC, lipid layer color; LLU, lipid layer uniformity (LLU).
3.3. Regression Analysis
Regression analysis was performed in contact lens wearers because they were more likely to have visual fluctuation due to DED [37]. A total of 14 preoperative parameters were included in the univariate linear regression analysis. It revealed that age and wearing frequency had the highest significance to predict UDVA on 20 days after SMILE (p = 0.004 and 0.034, respectively). In addition, wearing duration, daily wearing time, baseline UDVA, CFS score, TMH, and grade score of MG loss potentially influenced UDVA on day 20 after surgery as shown by univariate linear regression analysis (all p < 0.35), which were also included in multiple linear regression analysis. It turned out that age, daily wearing time, and preoperative TMH were independent risk factors associated with UDVA in contact lens wearers on day 20 after SMILE (Coef = 0.021, 0.018, and −0.286; p = 0.006, 0.010, and 0.043, respectively) (Table 3).

Table 3.
Linear regression to determine preoperative predictors of UDVA on day 20 after SMILE in contact lens wearers.
4. Discussion
Most patients undergoing refractive surgery used to be contact lens wearers because they had a strong willingness to free themselves from glasses. Moreover, the majority of them had long-term usage of video terminals simultaneously. Nevertheless, both contact lens wearing and overuse of video terminals were the already-known risk factors of DED. It has been confirmed that DED potentially influences the examinations before refractive surgeries and possibly causes measurement biases [9]. Therefore, this study is performed to evaluate the potential of pre-operative DED parameters to predict UDVA and refractive status after SMILE.
Unexpectedly, subjects in DED group were found to have an optometric status closer to emmetropia and greater improvement of UDVA than those in non-DED group on day 20 after SMILE. Although baseline UDVA was worse in DED group, preoperative DED probably affected the outcome in three aspects. First, it is already known that epithelial thinning in DED eyes interferes an accurate preoperative measurement of corneal topography [38]. Moreover, the unstable pre-corneal tear film impairs the optical regularity of corneal epithelium and causes increased ocular forward and corneal backward light scattering [39]. All these factors may affect the accuracy of preoperative optometric examination in the DED group and lead to the potential risk of more cutting thickness than the theoretical value, which causes a refractive status closer to emmetropia on day 20 postoperatively when corneal edema has not completely diminished. Second, DED patients required shorter time to obtain the ocular surface microenvironment recovery to a preoperative level than non-DED patients. Therefore, on day 20 after SMILE, DED eyes were more likely to have a refractive status closer to emmetropia and better UDVA. However, such disparity was temporary and might disappear when the ocular surface is completely recovered in non-DED patients. Third, it should be also noted that patients with preoperative DED usually had better compliance regarding the usage of artificial tears after surgery, which also contribute to a more regular corneal surface and better refractive outcome [40].
Previous studies have revealed that DED is a major ocular surface adverse event leading to dissatisfactory visual recovery after SMILE [24,41,42]. Nevertheless, our study found that patients in the non-DED group had more severely deteriorated DED symptoms (OSDI score) and signs (FBUT) of dry eye after SMILE, which was partly consistent with previous research [43]. Considering the fact that patients with preoperative DED usually have corneal nerve injury due to a worse ocular surface microenvironment and a reduced corneal sensation [10], it was reasonable to deduce that non-DED patients whose corneal innervation and sensation were intact before surgery might have more severe subjective ocular discomfort symptoms, more apparent changes of objective DED signs and larger measurement bias due to newly developed DED after SMILE than those who already had DED before surgery.
Our study revealed that older age, longer contact lens daily wearing time and lower TMH were the risk factors associated with worse prediction of UDVA after SMILE in contact lens wearers. Since the incidence of DED increases with age [12,13], it is reasonable to suppose that older subjects have a worse ocular surface microenvironment, and need a longer time to have physiological homeostasis of tear film restored. It has been proven that the component of tear film (including lipid layer, aqueous layer and mucin layer) as well as tear dynamics were both affected in long-term contact lens wearers [44,45,46,47,48] and its degree positively correlated with the length of contact lens wearing time [49]. Containing 75%~90% of total tear volume [50,51], TMH is considered as a sensitive indicator of tear fluid volume and used in the diagnosis of aqueous deficient DED [52,53]. Our previous study showed that wearing contact lenses caused a reduction of TMH and decreased area of lower tear meniscus [54], and lower TMH correlated with shorter NIBUT [55]. Therefore, lower TMH potentially affects the accuracy of preoperative measurement and had a negative impact on visual recovery after SMILE [56,57].
Several limitations should be acknowledged. First, the sample size of our study is not large enough, which likely contributes to the lack of statistical significance for some parameters. Second, the follow-up is not long enough to evaluate the long-term impact of pre-operative DED on UDVA and visual recovery after SMILE. Further multicenter studies with a larger sample size and longer follow-up are needed.
5. Conclusions
In conclusion, age, contact lens daily wearing time, and preoperative TMH are the three dependent factors to predict LogMAR UDVA on day 20 after SMILE in contact lens wearers. Pre-operative existing DED and SMILE affect each other. The current study provides supporting evidence that the impact of DED on the prediction of UDVA and refraction should be taken into consideration by refractive surgeons before SMILE so as to make individualized therapies for patients with pre-operative DED.
Author Contributions
Conceptualization, Q.L., J.H.; methodology, Q.L., Y.S., J.H.; data curation and collection, Y.S., J.W., X.Z., Z.Y.; data analysis, Y.S., J.W., Z.Y.; writing-original draft preparation, Y.S.; writing-review and editing, Q.L., Y.S.; critical revision, X.Z.; supervision, Q.L.; funding acquisition, Q.L., J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 81970767, 81970766 and 82171102.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Eye, Ear, Nose & Throat Hospital of Fudan University (protocol code EENTIRB-20190301).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors thank Jifang Wang, Pei Yang, Hong Zhang, Xichen Wan and Shuyun Zhou for their support in data curation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baird, P.N.; Saw, S.M.; Lanca, C.; Guggenheim, J.A.; Smith Iii, E.L.; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Chen, Z.; Miao, H.; Wei, R.; Li, M.; Zhao, J.; Zhou, X. Ten-year outcomes following small incision lenticule extraction for up to -10Dioptres myopia. Clin. Exp. Optom. 2023, 16, 1–6. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Y.; Wu, X.; Li, Y.; Xiang, A.; Lu, Y.; Fu, Q.; Hu, T.; Du, K.; Wen, D. Clinical outcomes after small-incision lenticule extraction versus femtosecond laser-assisted LASIK for high myopia: A meta-analysis. PLoS ONE 2021, 16, e0242059. [Google Scholar] [CrossRef]
- Lau, Y.T.; Shih, K.C.; Tse, R.H.; Chan, T.C.; Jhanji, V. Comparison of Visual, Refractive and Ocular Surface Outcomes between Small Incision Lenticule Extraction and Laser-Assisted In Situ Keratomileusis for Myopia and Myopic Astigmatism. Ophthalmol. Ther. 2019, 8, 373–386. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Z. Comparison of early visual quality in patients with moderate myopia using different optical zones in small incision lenticule extraction (SMILE). BMC Ophthalmol. 2021, 21, 46. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Y.; Xu, L.; Zuo, T.; Li, H.; Dou, R.; Zhang, J. Comparison of the Optical Quality between Small Incision Lenticule Extraction and Femtosecond Laser LASIK. J. Ophthalmol. 2016, 2016, 2507973. [Google Scholar] [CrossRef]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Benitez-Del-Castillo, J.; Labetoulle, M.; Baudouin, C.; Rolando, M.; Akova, Y.A.; Aragona, P.; Geerling, G.; Merayo-Lloves, J.; Messmer, E.M.; Boboridis, K. Visual acuity and quality of life in dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2017, 15, 169–178. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Tamimi, A.; Sheikhzadeh, F.; Ezabadi, S.G.; Islampanah, M.; Parhiz, P.; Fathabadi, A.; Poudineh, M.; Khanjani, Z.; Pourmontaseri, H.; Orandi, S.; et al. Post-LASIK dry eye disease: A comprehensive review of management and current treatment options. Front. Med. 2023, 10, 1057685. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xia, W.; Wang, M.; Chang, X.; Wang, J.; Jin, S.; Wang, J.; Wei, W.; Rudan, I. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 020503. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Alio Del Barrio, J.L.; Wilkins, M.; Cochener, B.; Ang, M. Refractive surgery. Lancet 2019, 393, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Toda, I. Dry Eye After LASIK. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES109–DES115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yam, G.H.; Lin, M.T.; Teo, E.; Koh, S.K.; Deng, L.; Zhou, L.; Tong, L.; Mehta, J.S. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J. Adv. Res. 2021, 29, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, H.; Kamiya, K.; Shimizu, K. Dry Eye After Small Incision Lenticule Extraction and Femtosecond Laser-Assisted LASIK: Meta-Analysis. Cornea 2017, 36, 85–91. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, X.; Wu, J.; Zhang, Z.; Li, T.; Zhou, Z.; Zhang, S.; Li, G. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: Comparison of corneal wound healing and inflammation. Br. J. Ophthalmol. 2014, 98, 263–269. [Google Scholar] [CrossRef]
- Moshirfar, M.; Somani, S.N.; Patel, B.C. Small Incision Lenticule Extraction. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Reinstein, D.Z.; Archer, T.J.; Randleman, J.B. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J. Refract. Surg. 2013, 29, 454–460. [Google Scholar] [CrossRef]
- Ishii, R.; Shimizu, K.; Igarashi, A.; Kobashi, H.; Kamiya, K. Influence of femtosecond lenticule extraction and small incision lenticule extraction on corneal nerve density and ocular surface: A 1-year prospective, confocal, microscopic study. J. Refract. Surg. 2015, 31, 10–15. [Google Scholar] [CrossRef]
- Krueger, R.R.; Meister, C.S. A review of small incision lenticule extraction complications. Curr. Opin. Ophthalmol. 2018, 29, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Teo, E.P.; Lwin, N.C.; Yam, G.H.; Mehta, J.S. Early Corneal Wound Healing and Inflammatory Responses after SMILE: Comparison of the Effects of Different Refractive Corrections and Surgical Experiences. J. Refract. Surg. 2016, 32, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Q.; Jia, Y.; Zhou, D.; Zhou, J. Clinical Outcomes of SMILE and FS-LASIK Used to Treat Myopia: A Meta-analysis. J. Refract. Surg. 2016, 32, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Choi, M.; Lee, K.Y.; Kim, E.K.; Seo, K.Y.; Jun, I.; Kim, K.Y.; Kim, T.I. Comparing Dry Eye Disease after Small Incision Lenticule Extraction and Laser Subepithelial Keratomileusis. Cornea 2020, 39, 501–507. [Google Scholar] [CrossRef]
- Moshirfar, M.; McCaughey, M.V.; Reinstein, D.Z.; Shah, R.; Santiago-Caban, L.; Fenzl, C.R. Small-incision lenticule extraction. J. Cataract. Refract. Surg. 2015, 41, 652–665. [Google Scholar] [CrossRef]
- Le, Q.; Ge, L.; Li, M.; Wu, L.; Xu, J.; Hong, J.; Gong, L. Comparison on the vision-related quality of life between outpatients and general population with dry eye syndrome. Acta Ophthalmol. 2014, 92, e124–e132. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Toker, E.; Asfuroglu, E. Corneal and conjunctival sensitivity in patients with dry eye: The effect of topical cyclosporine therapy. Cornea 2010, 29, 133–140. [Google Scholar] [CrossRef]
- GB/T 11533-2011; Standard for Logarithmic Visual Acuity Charts. People’s Republic of China Ministry of Health: Beijing, China, 2011.
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Wei, A.; Le, Q.; Hong, J.; Wang, W.; Wang, F.; Xu, J. Assessment of Lower Tear Meniscus. Optom. Vis. Sci. 2016, 93, 1420–1425. [Google Scholar] [CrossRef]
- Shimazaki, J.; Sakata, M.; Tsubota, K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.C.; Wolffsohn, J.S.; Fowler, C.W. Optimization of anterior eye fluorescein viewing. Am. J. Ophthalmol. 2006, 142, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Sekundo, W.; Kunert, K.S.; Blum, M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: Results of a 6 month prospective study. Br. J. Ophthalmol. 2011, 95, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Tan, D.; Mehta, J.S. Small incision lenticule extraction (SMILE) versus laser in-situ keratomileusis (LASIK): Study protocol for a randomized, non-inferiority trial. Trials 2012, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Bakkar, M.; Carnt, N.; Chalmers, R.; Vijay, A.K.; Marasini, S.; Ng, A.; Tan, J.; Wagner, H.; Woods, C.; et al. CLEAR—Contact lens complications. Cont. Lens Anterior Eye 2021, 44, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Edorh, N.A.; El Maftouhi, A.; Djerada, Z.; Arndt, C.; Denoyer, A. New model to better diagnose dry eye disease integrating OCT corneal epithelial mapping. Br. J. Ophthalmol. 2022, 106, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Maeda, N.; Ikeda, C.; Asonuma, S.; Mitamura, H.; Oie, Y.; Soma, T.; Tsujikawa, M.; Kawasaki, S.; Nishida, K. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6601–6606. [Google Scholar] [CrossRef]
- Rieger, G. The importance of the precorneal tear film for the quality of optical imaging. Br. J. Ophthalmol. 1992, 76, 157–158. [Google Scholar] [CrossRef]
- Shah, R.; Shah, S.; Sengupta, S. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J. Cataract. Refract. Surg. 2011, 37, 127–137. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y. Dry eye after small incision lenticule extraction and LASIK for myopia. J. Refract. Surg. 2014, 30, 186–190. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Shen, Y.; Li, T.; He, L.; Xu, H.; Yu, Y.; Zhou, X. Comparison of dry eye and corneal sensitivity between small incision lenticule extraction and femtosecond LASIK for myopia. PLoS ONE 2013, 8, e77797. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Morgan, P.; Cai, Z.Q.; Straughan, R.A. Prevalence of and risk factors for symptomatic dry eye disease in Singapore. Clin. Exp. Optom. 2015, 98, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Kozareva, D.; Hysi, P.G.; Hammond, C.J. Prevalence and risk factors of dry eye disease in a British female cohort. Br. J. Ophthalmol. 2014, 98, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Kuchiba, A.; Yamaguchi, T.; Amano, S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009, 116, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Lee, J.S.; Kang, S.Y.; Kim, H.M.; Song, J.S. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am. J. Ophthalmol. 2013, 155, 1104–1110.e2. [Google Scholar] [CrossRef]
- Dogan, A.S.; Gurdal, C.; Arslan, N. Corneal confocal microscopy and dry eye findings in contact lens discomfort patients. Cont. Lens Anterior Eye 2018, 41, 101–104. [Google Scholar] [CrossRef]
- Holly, F.J. Physical chemistry of the normal and disordered tear film. Trans Ophthalmol. Soc. UK (1962) 1985, 104 Pt 4, 374–380. [Google Scholar]
- Savini, G.; Barboni, P.; Zanini, M. Tear meniscus evaluation by optical coherence tomography. Ophthalmic Surg. Lasers Imaging 2006, 37, 112–118. [Google Scholar] [CrossRef]
- Mainstone, J.C.; Bruce, A.S.; Golding, T.R. Tear meniscus measurement in the diagnosis of dry eye. Curr. Eye Res. 1996, 15, 653–661. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Jiang, C.; Jiang, A.C.; Xu, J. The analysis of tear meniscus in soft contact lens wearers by spectral optical coherence tomography. Cornea 2009, 28, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Golding, T.R.; Bruce, A.S.; Mainstone, J.C. Relationship between tear-meniscus parameters and tear-film breakup. Cornea 1997, 16, 649–661. [Google Scholar] [CrossRef]
- Benito, A.; Perez, G.M.; Mirabet, S.; Vilaseca, M.; Pujol, J.; Marin, J.M.; Artal, P. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. J. Cataract. Refract. Surg. 2011, 37, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, H.; Kamiya, K.; Yanome, K.; Igarashi, A.; Shimizu, K. Longitudinal assessment of optical quality and intraocular scattering using the double-pass instrument in normal eyes and eyes with short tear breakup time. PLoS ONE 2013, 8, e82427. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).