Abstract
This review assesses how publications interpret factors that influence the serum or plasma albumin (PA) level in prognostic indices, focusing on inflammation and nutrition. On PubMed, a search for “albumin AND prognosis” yielded 23,919 results. From these records, prognostic indices were retrieved, and their names were used as search strings on PubMed. Indices found in 10 or more original research articles were included. The same search strings, restricted to “Review” or “Systematic review”, retrieved yielded on the indices. The data comprised the 10 latest original research articles and up to 10 of the latest reviews. Thirty indices had 294 original research articles (6 covering two indices) and 131 reviews, most of which were from recent years. A total of 106 articles related the PA level to inflammation, and 136 related the PA level to nutrition. For the reviews, the equivalent numbers were 54 and 65. In conclusion, more publications mention the PA level as a marker of nutrition rather than inflammation. This is in contrast to several general reviews on albumin and nutritional guidelines, which state that the PA level is a marker of inflammation but not nutrition. Hypoalbuminemia should prompt clinicians to focus on the inflammatory aspects in their patients.
1. Introduction
Prognostic index models can be used either for prediction or explanation [1]. A prediction model aims to generate a risk score that grades patient prognosis. However, the main limitation of prediction models is that they do not explain the impact or role of the model’s individual co-factors. As an example, the APACHE III prognostic index [2] often yields high performance, but it does not account for the impact or role of each of its numerous co-factors. Such assessments demand explanatory models which aim to advance scientific knowledge by interpreting the role of individual co-factors [1].
Hypoalbuminemia is consistently associated with adverse outcomes, and it is considered a strong prognostic predictor [3,4,5].
In contrast, the possible pathological mechanisms behind hypoalbuminemia are more obscure, and they have been debated for decades [6,7,8,9]. For the vast majority of diseases (such as malignancies, diabetes, or sepsis), reasons for hypoalbuminemia are difficult to assess in observational studies because the level of serum or plasma albumin (PA) depends on both synthesis, breakdown, external loss, and the distribution between the vascular and extra-vascular space [10].
For some diseases related to specific organs (liver or kidneys), hypoalbuminemia can partly be related directly to these disorders (e.g., reduced synthesis in patients with cirrhosis [11]). Among the mechanisms not directly related to specific organs, two main explanations have dominated the reasons for hypoalbuminemia: malnutrition and inflammation [8,10,12]. As malnutrition and inflammation are positively correlated in many diseases, hypoalbuminemia is often statistically associated with both of these.
This literature review provides an overview of all retrieved prognostic indices that incorporate PA. For indices that appear in at least 10 prognostic studies, we will describe how PA is interpreted based on the 10 newest research articles and reviews.
2. Materials and Methods
2.1. Initial Literature Searches
To obtain an overview of publications that included prognostic indices with PA levels, we used the search terms “albumin AND prognosis” in the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) without any restrictions. A search on 22 December 2017 yielded 18,007 results, for which all titles and abstracts were examined to see whether the publications comprised such indices. This search was performed once again on 1 October 2021, yielding 5912 more results. From these initial literature searches, we found the indices shown in Table A1 and Table A2, Appendix A.
2.2. Literature Searches for the Specific Prognostic Indices
Table A1 gives an overview of the search terms used for all the initially retrieved indices as well as the number of hits on the search date (18 February 2022). After automatically retrieving the records, we sorted them chronologically (according to “Most recent”, i.e., not according to “Publication date”) and manually examined the abstracts (newest first) to deduce whether the publications were in agreement with the search terms. In this manual process, we only included English-language publications presenting human studies and excluded casuistic studies. For indices with <10 records, we counted all manually retrieved records, whereas we finished counting at 10 for indices with ≥10 records.
2.3. Final Inclusion of Prognostic Indices and Publications
In the final stage, we only proceeded with prognostic indices that had at least 10 manually retrieved publications (Table A1, right column). For these indices, we assessed the 10 newest original research publications as well as a maximum of the 10 newest reviews regarding the indices. The reviews were retrieved using the same search terms as depicted in Table A1 but with restrictions to “Review” and “Systematic review” in the PubMed database. Reviews discussing specific diseases were retrieved on 18 February 2022, whereas reviews regarding broader patient groups were retrieved in week 10, 2023. We only included reviews in which the index was the main topic, regardless of whether the index was evaluated for one or several diseases or patient groups. Thus, we excluded reviews where the main topic was a disease assessed via several prognostic indices.
2.4. Reporting of Results
For each prognostic index, we looked through the 10 newest original research publications and retrieved reviews (where possible) to deduce whether they interpreted the PA-level results. Concerning the research publications, we focused on the abstract, introduction, and discussion sections as these were most likely to contain information about the role of PA. For reviews, we scrolled through all of the text. For all publications, we finalized each search by retrieving all “alb” (case letter insensitive) in the pdf file to deduce whether we had missed sentences that possibly dealt with albumin. If a publication interpreted the PA-level results, we further evaluated whether they reported these as markers of inflammation, nutrition, or other conditions (e.g., liver disorders or external loss). We differentiated between prognostic indices developed for specific diseases and prognostic indices developed for broader patient groups.
We further categorized author affiliations as either two or more countries or a single country (regardless of patient populations). For the latter, we recorded the authors’ continent and country affiliation.
3. Results
3.1. General Overview
From the initially retrieved 23,919 records, going back to 1957, we found the prognostic indices shown in Table A1. Using the search strings shown in Table A1, we found 38 indices with 10 or more publications in PubMed. In the manual assessments of the PubMed-derived records, we found that 30 of these indices had 10 or more original research studies in English that were not casuistic. For the remaining eight indices, alternative search strings (not shown) were used, but these resulted in either a very low (0–2) or very high number of records. As an example, the search string “serum creatinine to albumin ratio” yielded 3554 records, none of which had the search string per se (PubMed: Quoted phrase not found: “serum creatinine to albumin ratio”), and our manual assessment did not yield relevant studies, as many assessed creatinine and albumin in urine but not in serum or plasma.
Table A2 gives an overview of the formulas used for computing all of the prognostic indices retrieved from our initial assessment of the initially retrieved 23,919 records. These formulas are mainly relevant in the context of inflammation vs. nutrition. C-reactive protein or Body Mass Index (BMI) are examples of other index parameters that are markers of inflammation and nutrition, respectively.
3.2. Prognostic Indices That Were Further Assessed
Table 1 gives an overview of the 30 relevant prognostic indices and the diseases or patient groups for which they have mainly been used.

Table 1.
Prognostic indices with at least 10 original research articles and the diseases or patient groups noted in published studies and reviews.
We characterized 12 of these indices as covering specific disorders and 18 as covering broader patient groups.
3.3. Prognostic Indices Mainly Related to Specific Disorders
These 12 indices could further be grouped into the following: lung cancer (ALI), liver disorders (ALBI-T Score, ALBI, Child–Pugh Score, NAFLD Fibrosis Score [NFS]), kidney disorders (Protein Energy Wasting [PEW] Index, Malnutrition–Inflammation Score), specific hematological malignancies (International Prognostic Score for Advanced Hodgkin’s Disease, International Staging System [ISS] for Multiple Myeloma), gastrointestinal bleeding (AIMS65), pneumonia (SMART-COP), or spinal metastases (New England Spinal Metastasis Score) (Table 1).
Most of the 177 retrieved references were recent; 161 (91.0%) were from 2017 or later, and the oldest reference was from 2009 (Table 2).

Table 2.
Prognostic indices for specific patient groups, along with interpretations of the role of plasma albumin.
3.4. Prognostic Indices Mainly Related to Specific Disorders: Original Research Studies
The 12 indices were covered by 119 studies, as one study dealt with both the Child–Pugh Score and ALBI [55] (Table 2). A total of 83 studies (69.7%) did not mention anything about the role of PA. Eight of the nine studies that interpreted PA levels according to “miscellaneous” (i.e., neither due to inflammation nor nutritional status) dealt with prognostic indices specifically generated for liver patients (Table 2) and all related hypoalbuminemia to liver disorders. Among the studies that interpreted PA levels in relation to inflammation and/or nutritional status, 3 studies interpreted PA levels according to inflammation only, 19 studies interpreted PA levels according to nutrition only, and 10 interpreted PA levels according to both inflammation and nutrition.
3.5. Prognostic Indices Mainly Related to Specific Disorders: Reviews
For 2 indices, we did not find any reviews, whereas we found from 1 to 10 reviews for the other 10 prognostic indices, meaning that we retrieved 58 reviews altogether (Table 2). Forty-three reviews (74.1%) did not deal with the role of PA in the indices. Among the remaining 15 reviews, 8 related PA to inflammation, 5 related PA to nutritional status, and 10 related PA to miscellaneous factors. Liver disorders were listed as reasons for hypoalbuminemia dominating among the miscellaneous factors [33,62,64,65,105,141,163,164], with two liver disorder indices, ALBI and NFS, showing the greatest level of prevalence. The reasons for hypoalbuminemia among the miscellaneous factors (other than liver disorders) were acidosis [105], PA loss in dialysis [136], and nephrosis [141].
3.6. Prognostic Indices Mainly Related to Broader Patient Groups: Original Research Studies
Our sample of original research studies included 175 studies with 18 indices related to broader patient groups (Table 1). Five studies discussed 2 of the 18 indices [190,191,192,193,194] (Table 3).

Table 3.
Prognostic indices for broader patient groups, along with interpretations of the role of plasma albumin.
Nearly all studies were from 2022 or 2021, and the three oldest were from 2016 [373,374,376].
Thirty-six studies (20.6%) did not mention any factors that may have an impact on the PA level, including all 10 studies with the APACHE III Score, 7 with the Gustave Roussy Immune (GRIM) Score, 6 with the Platelet–Albumin–Bilirubin (PALBI) Grade, and 4 with the Glasgow Prognostic Score (Table 3). Moreover, 13 studies (including all 10 studies dealing with the Ischemia-modified Albumin/Albumin ratio) only mentioned factors that were neither related to inflammation nor nutrition (e.g., liver disorders). Among the remaining 126 studies, 24 (19.1%) associated the PA level with inflammation only, 37 (29.4%) associated the PA level with nutrition only, and 65 (51.6%) associated the PA level with both inflammation and nutrition.
Many indices were primarily used for disease entities with a common pathophysiology (Table 4).

Table 4.
Prognostic indices for broader patient groups (distributed according to main disease entity).
Among the 175 publications, 91 (52%) discussed malignancies, 19 (10.9%) discussed infections, and the remaining 65 (37.1%) included discussions of various other disease entities and patient groups. All 10 studies with the Gustave Roussy Immune (GRIM) Score and the Systemic Inflammation Score dealt with malignancies, which was also the case for 9 of the 10 studies regarding the Albumin/Alkaline Phosphate Ratio; the Glasgow Prognostic Score; the Hemoglobin, Albumin, Lymphocyte, Platelet Score; and the Naples Prognostic Score.
3.7. Prognostic Indices Mainly Related to Broader Patient Groups: Reviews
The 73 retrieved reviews featured 13 of the 18 indices, of which 5 (C-reactive protein/Albumin ratio, COntrolling NUTritional status (CONUT) Score, Geriatric Nutritional Risk Index, Glasgow Prognostic Score, and Prognostic Nutritional Index) had at least 10 reviews (Table 3). Sixty-eight reviews (93.2%) were from 2019 or later. The four oldest reviews (from 1991, 2000, 2008, and 2014) all dealt with the APACHE III Score.
Six reviews (8.2%) stated that inflammation only had an impact on the PA level, 20 (27.4%) only mentioned nutrition, and 40 (54.8%) mentioned both inflammation and nutrition (Table 3). Factors other than inflammation or nutrition (e.g., liver disorders) were mentioned in 13 reviews (17.8%), whereas 7 reviews (all the 5 on APACHE III and 2 on Glasgow Prognostic Score) did not mention any factors that could have an impact on the PA level.
Fifty-six reviews (76.7%) dealt with malignancies, five (6.8%) dealt with infections, and the remaining twelve (16.4%) dealt with other diseases or patient groups (Table 4).
3.8. Authors’ Continent and Country Affiliations
Three countries (the USA, China, and Japan) accounted for 46.2% of the 119 original research studies mainly related to specific disorders, and 15 studies (12.5%) had authors from two or more countries (Table 5).

Table 5.
Publications on indices according to authors’ continent and country.
Among the 58 reviews, 14 (24.1%) had authors from two or more countries, 12 (20.7%) were from the USA, 7 (12.1%) were from China, and 6 (10.3%) were from the UK; the remaining 19 reviews had authors from 13 different countries.
Among the original research studies mainly related to broader patient groups, all except eight had all of their authors affiliated with one country only (Table 5). Among these 167 studies, 148 (88.6%) had author affiliations in Asian countries, of which China, Japan, and Turkey dominated with 72, 40, and 27 studies, respectively. A total of 52 reviews (71.2%) were from authors with affiliations with institutions in China; Taiwan had six reviews (8.2%). Four reviews were from authors from more than one country, and the remaining eleven reviews were from eight different countries.
4. Discussion
Among the 425 original research articles and reviews that included discussions on indices and PA levels, 156 (36.7%) related PA levels to inflammation, and 196 (46.1%) related them to nutrition. For the 248 articles/reviews that discussed broader patient groups, the equivalent metrics were 135 (54.4%) for inflammation and 162 (65.3%) for nutrition. Some manuscripts discussed indices mainly related to specific diseases (e.g., the Child–Pugh Score specifically designated for liver disorders), thus placing less emphasis on inflammation and nutrition.
Many patient groups are characterized by both inflammation and malnutrition, e.g., in patients with malignancies [437]. Because hypoalbuminemia also often characterizes such patient groups [4], it is statistically correlated with both inflammation and malnutrition. Two models, one in which PA is mainly a marker of nutrition and one in which it is mainly an inflammatory marker, are depicted in Figure 1.
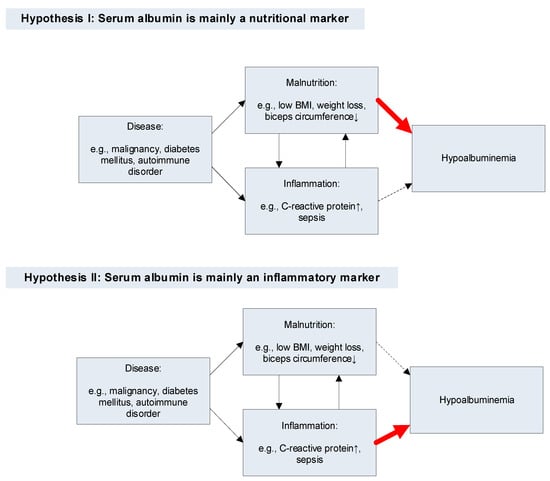
Figure 1.
Pathways for hypoalbuminemia according to two hypotheses. Thin black arrow: statistical association. Thick red arrow: main cause of hypoalbuminemia. Thin dashed arrow: weak causal association between the factor (inflammation [Hypothesis I] or malnutrition [Hypothesis II]) and hypoalbuminemia.
However, PA as a nutritional marker was described as a “myth” already more than three decades ago [438,439]. Since then, many reviews have emphasized PA as being a negative acute-phase protein and, as a result, a marker of inflammation [5,7,8,9,10,440,441,442,443]. Moreover, nutrition guidelines from the USA and Europe have stated that the PA level is not a valid marker of nutritional status [444,445,446]. Perhaps the most convincing studies are those of PA levels in undernourished patients who have no concomitant inflammation. A review comprising 63 studies of such patients, most with anorexia nervosa, found that their PA levels were normal [447]. In one study, 16 anorectic patients had a mean PA level of 40.5 g/L, and 16 healthy controls had 41.1 g/L, both of which were within normal limits (35–50 g/L) [448]. Curiously, another five anorectic patients who were dying had much lower mean PA levels (33.5 g/L); however, these were not ascribed to their nutritional status but to organ failure with concomitant extravasation of the PA. Mechanisms related to factors that determine the PA level are beyond the scope of this review, but we would like to refer interested readers to two excellent reviews that give useful details [6,7].
The compelling amount of evidence against the notion of the PA level being a marker of nutrition in conjunction with the influx of recent articles that still describe the PA level as a marker of nutrition prompted us to investigate this systematically. To delineate the topic, indices with PA reported in at least 10 original publications were selected.
There were 289 studies and reviews (68.0%) from Asia, most of which were from China (160/289 [55.4%]), followed by Japan (59/289 [20.4%]) and Turkey (33/289 [11.4%]). There were no significant differences between continents or countries vs. factors related to the PA level, although the numbers for most of the continents or countries shown in Table 5 are too small to generate useful statistics. The reasons for why so many studies and reviews dealing with the reported indices emanate from Asia are unknown. The authors of all of the general reviews on albumin mentioned above were either from the USA [5,9,440,442,443], Europe [8,10,441], or both [7]. Still, we cannot conclude why so many studies and reviews mentioned hypoalbuminemia as a marker of nutrition.
The PA level is known to be a strong prognostic predictor regardless of patient population or disease entity [3,4,5], and this is probably well-known by most clinicians. However, according to the many studies and reviews reported here, of which most are very recent, it seems that fewer clinicians are aware of the fact that hypoalbuminemia is not very valid as a marker of nutrition. Specifically, we suspect that many protein drinks, which are not cheap, are given to patients solely due to hypoalbuminemia. Whether this is beneficial for individual patients should be assessed clinically by using factors other than the PA level. The reviews we studied generally related the beneficial aspects of albumin supplementation to factors other than nutrition, such as immunomodulatory, anti-inflammatory, or antioxidant properties [449]; however, whether albumin supplementation is beneficial at all (e.g., for sepsis patients) is also hotly debated [450]. Regardless of this, it is important to focus on inflammatory aspects in patients with hypoalbuminemia. Even in patients with specific organ dysfunctions, such as in the kidney [105,451] or liver [452], inflammation is often an important aspect of pathogenesis and the PA level.
This review has some limitations that need to be addressed. Firstly, this review was written by only one author, who is biased due to his studies that also indicate an important role for inflammation in relation to the PA level [453,454,455,456,457,458,459,460], of which only one study includes nutritional data (the Body Mass Index) [459]. Moreover, this also implies that there was no crosschecking of the scrutinized publications. However, if factors were overlooked or misinterpreted in a few of the references, this probably did not change the overall results. Secondly, the distinction between indices used for specific vs. broader patient groups is partly arbitrary. This distinction was chosen because indices covering broader patient groups are probably more prone to various interpretations than indices that specifically monitor target organs such as the liver. However, indices for specific diseases have also been used for other patient groups, e.g., the Advanced Lung Cancer Inflammation Index (ALI) where seven of the 10 original articles in this review dealt with other malignancies or coronary diseases. Likewise, many of the general indices have mainly assessed certain diseases, e.g., the Naples Prognostic Score where nine of the 10 original studies comprised malignancies. Regardless of these uncertainties, a much higher rate of studies of specific disorders did not discuss reasons for the PA levels (68.9% vs. 20.6% in studies of broader patient groups). Finally, it took some time to look through the PubMed data, which were initially analyzed by skimming 23,919 titles and abstracts to find relevant indices with PA, after which the 425 selected publications were scrutinized. During this process, more publications with the actual indices appeared daily in PubMed. As an example, the search string “advanced lung cancer inflammation index” yielded 54 results on 18 February 2022 (Table A1), but at the time of writing (9 June 2023), the same search string yields 90 results. However, except for reviews of the APACHE III Score, the vast majority of this review’s publications were not more than four years old (Table 1 and Table 2). The studies and reviews published after the ones reported here would probably have little impact on our results, as there is no reason to believe a recent revolutionary change has occurred for a concept discussed for more than three decades.
5. Conclusions
Although numerous reviews and nutritional guidelines have advocated against using the PA level as a marker of nutrition, this is still how it is interpreted in many studies and reviews of indices commonly used with albumin. Many of these publications also interpreted the PA level as a marker of inflammation, whereas very few reported it as a marker of inflammation but not nutrition. This may have an impact on the prognosis of patients with hypoalbuminemia if clinicians focus more on nutritional than inflammatory aspects, both of which are important to assess in frail patients. Regarding nutrition, many measures have been proposed, such as body mass index, waist/hip ratio, or body fat content. There is no universally accepted gold standard for assessing a patient’s nutritional status, but the PA level should not be used, solely or partly, to fill this void. In contrast, the PA level is important in assessing the inflammatory status of a patient.
Funding
This research study received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Appendix A

Table A1.
Indices (sorted alphabetically), search strings, and numbers of hits (retrieved from PubMed, 18 February 2022).
Table A1.
Indices (sorted alphabetically), search strings, and numbers of hits (retrieved from PubMed, 18 February 2022).
| Index Name | Search String | No. of Hits | |
|---|---|---|---|
| PubMed 1 | Manual 2 | ||
| Advanced Lung Cancer Inflammation Index (ALI) | “advanced lung cancer inflammation index” | 54 | ≥10 |
| Age, Comorbidities, and albumin (ACA) index | “ACA index” | 4 | 2 |
| AGR-PLR Score (APS) | “AGR-PLR index” | 2 | 2 |
| AIMS65 | “AIMS65” | 105 | ≥10 |
| ALBI-TNM = ALBI-T Score | “ALBI-T” OR “ALBI-T score” | 23 | ≥10 |
| Albumin–Bilirubin (ALBI) Score | “albumin-bilirubin score” OR “albumin-bilirubin grade” OR “albumin-bilirubin index” OR “ALBI” | 1282 | ≥10 |
| Albumin–Globulin score (AGS) | “albumin-globulin score” | 7 | 7 |
| Albumin–Indocyanine Green Evaluation (ALICE) Grade | “albumin-indocyanine green evaluation grade” OR “ALICE grade” | 7 | 6 |
| Albumin/Alkaline Phosphate Ratio (AAPR) | “albumin to alkaline phosphate ratio” OR “AAPR” | 181 | ≥10 |
| Albumin/Fibrinogen Ratio (AFR) | “albumin to fibrinogen ratio” | 34 | ≥10 |
| Albumin/Gamma-Glutamyltransferase (AGR) Ratio | “albumin to gamma-glutamyltransferase ratio” OR “AGR ratio” | 4 | 2 |
| Albumin/globulin ratio | “albumin to globulin ratio” | 208 | ≥10 |
| Acute Physiology, Age, Chronic Health Evaluation (APACHE) III Score | “APACHE III” OR “APACHE 3” | 667 | ≥10 |
| BALAD Score | “BALAD score” | 5 | 5 |
| BALAD-2 Score | “BALAD-2” | 9 | 9 |
| Blood Urea Nitrogen/Albumin Ratio | “blood urea nitrogen to albumin ratio” OR “BUN to albumin ratio” | 298 | ≥10 |
| C-reactive protein/albumin ratio | “C-reactive protein to albumin ratio” OR “CRP to albumin ratio” | 240 | ≥10 |
| Child–Pugh Score | “child-pugh score” | 2214 | ≥10 |
| Cirrhosis acute gastrointestinal bleeding (CAGIB) score | “CAGIB score” | 2 | 2 |
| COntrolling NUTritional status (CONUT) Score | “controlling nutritional status score” OR “CONUT score” | 363 | ≥10 |
| Creatinine/albumin ratio | “serum creatinine” AND “albumin” AND “ratio” | 1365 | 1 |
| DCTA scoring system | “DCTA scoring system” | 4 | 1 |
| Expanded A-drop score | “expanded A-drop score” | 11 | 1 |
| EZ-ALBI score | “EZ-ALBI score” | 2 | 2 |
| Fibrinogen/albumin ratio (FAR) | “fibrinogen to albumin ratio” | 63 | ≥10 |
| Gamma-Glutamyltransferase/albumin ratio | “gamma-glutamyltransferase to albumin ratio” | 1 | 1 |
| Geriatric Nutritional Risk Index | “geriatric nutritional risk index” | 481 | ≥10 |
| Glasgow multifactor prognostic scoring systems | “glasgow multifactor prognostic scoring system” | 2 | 2 |
| Glasgow Prognostic Score | “glasgow prognostic score” | 932 | ≥10 |
| Gustave Roussy Immune (GRIM) Score | “gustave roussy immune score” OR “GRIM score” | 12 | ≥10 |
| Hemoglobin albumin Lymphocytes Neutrophils (HLAN) Score | “hemoglobin albumin lymphocytes neutrophils score” OR “HLAN score” | 66 | 1 |
| Hemoglobin, albumin, Lymphocyte, Platelet (HALP) Score | “hemoglobin albumin lymphocyte platelet score” OR “HALP score” | 61 | ≥10 |
| Inflammatory Prognostic Index (IPI) | “inflammatory prognostic index” | 5 | 4 |
| The Instrumental Activities of Daily Living (IADL) Scale and the ACA Index | “instrumental activities of daily living scale and the ACA index” OR (“IADL” AND “ACA index”) | 1 | 1 |
| International Normalized Ratio/albumin Ratio (PTAR) | “international normalized ratio to albumin Ratio” OR “PTAR” | 36 | 4 |
| International Prognostic score for advanced Hodgkin’s disease | “international prognostic score” AND “Hodgkin” | 163 | ≥10 |
| The International Society of Renal Nutrition and Metabolism (ISRNM) protein energy wasting (PEW) index | “international society of renal nutrition and metabolism protein energy wasting index” OR (“ISRNM” AND “PEW”) | 32 | ≥10 |
| International Staging System (ISS) for multiple myeloma | “international staging system” AND “myeloma” | 621 | ≥10 |
| Ischemia-modified albumin/albumin ratio | “ischemia modified albumin to albumin ratio” | 103 | ≥10 |
| Lactate dehydrogenase/albumin ratio | “lactate dehydrogenase to albumin ratio” OR “LDH to albumin ratio” | 9 | 9 |
| Lactate/albumin ratio | “lactate to albumin ratio” | 3 | 3 |
| LANR | “LANR” | 24 | 1 |
| Malnutrition–Inflammation Score = Kalantar Score | “malnutrition-inflammation score” OR “kalantar score” | 235 | ≥10 |
| Memorial Sloan Kettering Prognostic Score (MPS) | “memorial sloan kettering prognostic score” | 2 | 2 |
| Nonalcoholic fatty liver disease (NAFLD) Fibrosis Score | “NAFLD fibrosis score” | 440 | ≥10 |
| Naples Prognostic Score | “naples prognostic score” | 21 | ≥10 |
| Neutrophil Percentage/albumin Ratio (NPAR) 3 | “neutrophil percentage to albumin ratio” | 12 | ≥10 |
| Neutrophil/albumin ratio 3 | “neutrophil to albumin ratio” | 12 | ≥10 |
| New England Spinal Metastasis Score | “new england spinal metastasis score” | 14 | ≥10 |
| NOBLADS | “NOBLADS” | 8 | 8 |
| Nutritional and inflammatory status (NIS) ratio | “NIS ratio” | 3 | 1 |
| Platelet–albumin–Bilirubin (PALBI) grade | ”platelet albumin bilirubin grade” OR ”PALBI grade” | 21 | ≥10 |
| Prognostic Inflammation Score (PIS) | “prognostic inflammation score” | 3 | 2 |
| Prognostic Inflammatory Nutritional Index (PINI) | “prognostic inflammatory nutritional index” | 2 | 1 |
| Prognostic Nutritional Index = Onodera’s Prognostic Nutritional Index | “prognostic nutritional index” | 1141 | ≥10 |
| Red cell distribution width/albumin ratio | “red cell distribution width albumin ratio” OR “RDW albumin ratio” | 242 | 8 |
| SMART-COP | “SMART-COP” | 48 | ≥10 |
| Systemic Inflammation Score (SIS) | “systemic inflammation score” | 52 | ≥10 |
| SWOG Stage | “SWOG” AND albumin | 12 | 4 |
1 Number of hits retrieved in PubMed (https://pubmed.ncbi.nlm.nih.gov/?otool=idkvouhlib, accessed on 18 February 2022). 2 Number of English-language publications (excluding casuistic studies) (0–9, ≥10) found relevant for the index by manually looking through the results retrieved via PubMed (sorted by and retrieved from the newest publications). 3 Through analyzing the studies and reviews, it was revealed that the terms “Neutrophil Percentage to albumin Ratio” and “Neutrophil to albumin Ratio” were used interchangeably; therefore, the two terms have been merged in this article.

Table A2.
Indices that incorporate serum or plasma albumin and their computation (index name sorted alphabetically). All biochemistry parameters refer to levels in blood, serum, or plasma.
Table A2.
Indices that incorporate serum or plasma albumin and their computation (index name sorted alphabetically). All biochemistry parameters refer to levels in blood, serum, or plasma.
| Index Name | Computation | References |
|---|---|---|
| Advanced Lung Cancer Inflammation Index (ALI) | [BMI (kg/m2) × ALB (g/dL)]/NLR | [461] |
| Age, Comorbidities, and ALB (ACA) index | 1 point for each of: Age > 75 years; ALB < 3.7 g/dL; Charlson Comorbidity Index Score ≥ 3 | [462,463] |
| AGR-PLR Score (APS) | AGR = ALB (g/L)/globulins (g/L) PLR = Platelet/Lymphocyte Ratio APS score 0: PLR ≤ 132 and AGR > 1.75; score 1: PLR > 132 or AGR ≤ 1.75; score 2: PLR > 132 and AGR ≤ 1.75 | [464] |
| AIMS65 | 1 point for each of: ALB < 3.0 mg/dL; INR > 1.5; Altered mental status; Systolic blood pressure < 90 mm Hg; Age > 65 years | [465] |
| ALBI-TNM = ALBI-T Score | ALBI grade + TNM stage of LCSGJ – 2 ALBI grade: See “ALB-Bilirubin (ALBI) Score” TNM stage of Liver Cancer Study Group of Japan (LCSGJ): Stage I: T1N0M0; Stage II: T2N0M0; Stage III: T3N0M0; Stage IVa: T4N0M0, or any TN1M0; Stage IVb: Any TN0-N1M1 | [50] |
| ALB-Bilirubin (ALBI) Score | (log10 [bilirubin (μmol/L)] × 0.66) + (ALB (g/L) × [−0.085]) | [466] |
| ALB-Globulin score (AGS) 1 | Score 0: ALB > 35 g/L and globulin ≤ 35 g/L; Score 1: ALB ≤ 35 g/L or globulin > 35 g/L; Score 2: ALB ≤ 35 g/L and globulin > 35 g/L | [467] |
| ALB-Indocyanine Green Evaluation (ALICE) Grade | 0.663 × log10 [ICG R15 (%)] − 0.0718 × ALB (g/L) ICG R15 (%) = retention rate of Indocyanine Green, 15 min after its infusion | [468,469] |
| ALB/Alkaline Phosphate Ratio (AAPR) | ALB (g/L)/Alkaline phosphatase (IU/L) | [201] |
| ALB/Fibrinogen Ratio (AFR) | ALB (g/L)/Fibrinogen (g/L) | [470] |
| ALB/Gamma-Glutamyltransferase (AGR) Ratio | ALB (g/L)/Gamma-Glutamyltransferase (IU/L) | [471] |
| ALB/globulin ratio | ALB (g/dL)/[Total protein (g/dL) − ALB (g/dL)] | [472] |
| Acute Physiology, Age, Chronic Health Evaluation (APACHE) III Score | ALB: 11 points: ≤19 g/L; 6 points: 20–24 g/L; 0 points: 25–44 g/L; 4 points: ≥45 g/L + numerous other parameters (APACHE III Score, range 0–299) | [2] |
| BALAD Score | Bilirubin (mg/dL)/ALB (g/dL) score: 0 points: <1.0/>3.5; 1 point: 1.0–2.0/2.8–3.5; 2 points: >2.0/<2.8 Scores for elevated tumor markers, 1 point for each of the: α-Fetoprotein > 400 ng/mL; α-Fetoprotein-L3 > 15% (of α-Fetoprotein); Des-ϒ-Carboxyprothrombin > 100 mAU/mL BALAD Score: Bilirubin/ALB score + scores for elevated tumor markers | [473] |
| BALAD-2 Score | BALAD-2 Score = 0.02 × (α-Fetoprotein − 2.57) + 0.012 × (α-Fetoprotein-L3 − 14.19) + 0.19 × (ln [Des-ϒ-Carboxyprothrombin] − 1.93) + 0.17 × (Bilirubin1/2 − 4.50) − 0.09 × (ALB − 35.11) Units: α-Fetoprotein (1000× ng/mL); α-Fetoprotein-L3 (% of α–Fetoprotein); Des-ϒ-Carboxyprothrombin (1000× ng/mL); Bilirubin (μmol/L); ALB (g/L) | [474,475] |
| Blood Urea Nitrogen/ALB Ratio | Blood urea nitrogen (mg/dL)/ALB (g/dL) | [251] |
| C-reactive protein/ALB ratio | C-reactive protein (mg/L)/ALB (g/L) | [476] |
| Child–Pugh Score | 1, 2, 3 points, respectively, for each of: Total bilirubin: <2, 2–3, >3 mg/dL; ALB: >3.5, 2.8–3.5, <2.8 g/dL; INR: <1.7, 1.7–2.2, >2.2; Ascites: Absent, Slight, Moderate; Encephalopathy: None, Grade 1–2, Grade 3–4 | [477] |
| Cirrhosis acute gastrointestinal bleeding (CAGIB) score | Diabetes (yes = 1, no = 0) × 1.040 + Hepatocellular carcinoma (yes = 1, no = 0) × 0.974 + Bilirubin (μmol/L) × 0.005 − ALB (g/L) × 0.091 + Alanine aminotransferase (IU/L) × 0.001 + Serum creatinine (μmol/L) × 0.012 − 3.964 | [478] |
| COntrolling NUTritional status (CONUT) Score | ALB, g/dL (points): 3.5–4.5 (0), 3.0–3.49 (2), 2.5–2.9 (4), <2.5 (6) Lymphocytes/mL (points): >1600 (0), 1200–1599 (1), 800–1199 (2), <800 (3) Cholesterol, mg/dL (points): >180 (0), 140–180 (1), 100–139 (2), <100 (3) | [479] |
| Creatinine/ALB ratio | Creatinine (μmol/L)/ALB (g/L) | [480] |
| DCTA scoring system | 1 point for each of: D-dimer > 0.63 mg/L; Cholesterol ≤ 3.68 mmol/L; ALB ≤ 34 g/L; High sensitivity cardiac troponin T > 24.06 pg/mL | [481] |
| Expanded A-drop score | 1 point for each of: Age ≥ 70 years (male) or ≥75 years (female); Blood urea nitrogen ≥ 21 mg/dL or dehydration; Oxygen tension ≤ 90% (PaO2 ≤ 60 mmHg); Confusion; Systolic blood pressure ≤ 90 mmHg; Malignancy; Heart rate ≥ 100/min; ALB ≤ 3.09 g/dL; Lactate > 1.7 mmol/L; N-terminal pro-brain natriuretic peptide > 500 pg/mL | [482] |
| EZ-ALBI score | Total bilirubin (mg/dL) − 9 × ALB (g/dL) | [483] |
| Fibrinogen/ALB ratio (FAR) | Fibrinogen (mg/dL)/ALB (g/dL) | [484] |
| Gamma-Glutamyltransferase/ALB ratio | Gamma-Glutamyltransferase (IU/L)/ALB (g/L) | [485] |
| Geriatric Nutritional Risk Index | (1.489 × ALB [g/dL]) + (41.7 × [body weight/ideal body weight]) Lorentz-formula, for calculating the ideal body-weight (w) of a subject: For men: w = (height [cm] − 100) − ((height − 150)/4) For women: w = (height − 100) − ((height − 150)/2.5) | [486] |
| Glasgow multifactor prognostic scoring systems | In all three systems [487,488,489], 1 point for each of: Neutrophils (>15 × 109/L); Blood glucose (>10 mmol/L); Serum urea (>16 mmol/L); Arterial oxygen saturation (<8 kPa); Serum calcium (<2 mmol/L); ALB (<32 g/L); Serum lactate dehydrogenase (>600 units/L) In [487,489]: 1 point for age > 55 years In [487] and [488]: 1 point for serum transaminase >100 and >200 units/L, respectively | [487,488,489,490] |
| Glasgow Prognostic Score 2 | 0 points: C-Reactive protein (CRP) ≥ 10 mg/L and ALB ≥ 35g/L 1 point: CRP > 10 mg/L or ALB < 35 g/L 2 points: CRP > 10 mg/L and ALB < 35 g/L | [491,492] |
| Gustave Roussy Immune (GRIM) Score | 1 point for each of: Lactate dehydrogenase > upper limit normal; ALB < 35 g/L; NLR > 6 | [354] |
| Hemoglobin ALB Lymphocytes Neutrophils (HLAN) Score | (Hemoglobin [g/L] × Lymphocytes [/L] × ALB [g/L])/Neutrophils (/L)/100 | [493] |
| Hemoglobin, ALB, Lymphocyte, Platelet (HALP) Score | (Hemoglobin [g/L] × ALB [g/L] × Lymphocyte [/L])/Thrombocytes (/L). | [364] |
| Inflammatory Prognostic Index (IPI) | (C-reactive protein (mg/L) × NLR)/ALB (g/L) | [494] |
| The Instrumental Activities of Daily Living (IADL) Scale and the ACA Index | IADL scores of 6 to 7 and ACA score 1: 1 point IADL scores ≤ 5 and ACA scores 2 or 3: 2 points IADL ACA (IACA) Index: Score 0 = low risk; Score 1–2 = intermediate risk; Score 3–4 = high risk ACA Score: See “Age, Comorbidities, and ALB (ACA) index” in this table | [495,496] |
| International Normalized Ratio/ALB Ratio (PTAR) | INR/ALB (g/dL) | [497] |
| International Prognostic score for advanced Hodgkin’s disease | 1 point for each of: ALB < 4 g/dL; Hemoglobin < 10.5 g/dL; Male sex; Stage IV disease; Age ≥ 45 years; Neutrophils ≥ 15,000/mm3; Lymphocytes < 600/mm3, or <8% of neutrophil count | [498] |
| The International Society of Renal Nutrition and Metabolism (ISRNM) protein energy wasting (PEW) index | 1 point for each of: ALB < 38 g/L; BMI ≤ 23 kg/m2; Serum creatinine ≤ 818 μmol/L; Protein intake assessed by the normalized protein catabolic rate ≤ 0.8 g/kg/day | [499] |
| International Staging System (ISS) for multiple myeloma | Stage I: Serum beta2-microglobulin < 3.5 mg/L and ALB ≥ 3.5 g/dL; Stage II: neither stage I nor III; Stage III: Serum beta2-microglobulin ≥ 5.5 mg/L. Two categories for stage II: Serum beta2-microglobulin < 3.5 mg/L/ALB < 3.5 g/dL; Serum beta2-microglobulin 3.5 to <5.5 mg/L, irrespective of the ALB level | [500] |
| Ischemia-modified ALB/ ALB ratio | Ischemia-modified ALB (Absorbance units [ABSU])/ALB (g/dL) | [376] |
| Lactate dehydrogenase/ALB ratio | Lactate dehydrogenase (IU/L)/ALB (g/dL) | [501] |
| Lactate/ALB ratio | Lactate (mmol/L)/ALB (g/dL) | [502] |
| LANR | [Lymphocytes (109/L) × ALB (g/L)]/neutrophils (109/L) | [503] |
| Malnutrition–Inflammation Score = Kalantar Score | Three factors were amended to the Subjective Global Assessment (SGA), with its seven factors (each of 0–3 points), thus rendering 0–30 points for the Malnutrition–Inflammation Score: BMI (kg/m2): 0 points: ≥20; 1 point: 18–19.99; 2 points: 16–17.99; 3 points: <16 ALB (g/dL): 0 points: ≥4.0; 1 point: 3.5–3.9; 2 points: 3.0–3.4; 3 points: <3.0 Total iron binding capacity (mg/dL): 0 points: <250; 1 point: 200–249; 2 points: 150–199; 3 points: <150 (Suggested equivalent increments for serum transferrin are >200, 170–199, 140–169, <140 mg/dL, respectively) | [504] |
| Memorial Sloan Kettering Prognostic Score (MPS) | 0 points: ALB ≥ 4 g/dL and NLR ≤4; 1 point: ALB < 4 g/dL or NLR >4; 2 points: ALB <4 g/dL and NLR >4 | [505] |
| Nonalcoholic fatty liver disease (NAFLD) Fibrosis Score | −1.675 + 0.037 × Age (years) + 0.094 × BMI (kg/m2) + 1.13 × (impaired fasting glucose)/diabetes (yes = 1, no = 0) + 0.99 × Aspartattransaminase/Alaninaminotransferase ratio − 0.013 × Thrombocytes (109/L) − 0.66 × ALB (g/dL) | [506] |
| Naples Prognostic Score | 1 point for each of: ALB < 4 g/dL; Cholesterol ≤ 180 mg/dL; NLR > 2.96; Lymphocyte/Monocyte Ratio ≤ 4.44 | [507] |
| Neutrophil (Percentage)/ALB Ratio (NPAR) 3 | Neutrophil percentage (X% recorded as X)/ALB (g/L) Or Neutrophils (109/L)/ALB (g/dL) | [508,509] |
| New England Spinal Metastasis Score | Modified Bauer Score = 0 or 1 point if ≤2 or ≥3, respectively, of the following criteria: no visceral metastases; primary tumor is not lung cancer; primary tumor is breast, renal, lymphoma, or myeloma; single skeleton metastasis 1 point for each of: independent ambulatory function; ALB ≥ 3.5 g/dL | [150,510] |
| NOBLADS | 1 point for each of: Non-steroid anti-inflammatory drugs; no diarrhea; no abdominal tenderness; systolic blood pressure ≤ 100 mm Hg; antiplatelet drugs (non-aspirin); ALB < 3.0 g/dL; Charlson Comorbidity Index Score ≥ 2; syncope | [463,511] |
| Nutritional and inflammatory status (NIS) ratio | (C-reactive protein [mg/L] × alpha-1 acid glycoprotein [mg/L])/(ALB [g/L] × pre-ALB [mg/L]) | [512] |
| Platelet-ALB-Bilirubin (PALBI) grade | 2.02 × log10(bilirubin) (μmol/L) − 0.37 × [log10(bilirubin)]2 − 0.04 × ALB (g/L) − 3.48 × log10(Thrombocytes) + 1.01 × log10(Thrombocytes) | [399] |
| Prognostic Inflammation Score (PIS) 4 | 0 point: ALB: >40 g/L and NLR ≤ 3.43; 1 point: ALB: ≤40 g/L or NLR > 3.43; 2 points: ALB: ≤40 g/L and NLR > 3.43 | [513] |
| Prognostic Inflammatory Nutritional Index (PINI) | (alpha-1-acid glycoprotein [mg/L] + C-reactive protein [mg/L])/(ALB [g/dL] + pre-ALB [mg/L]) | [514] |
| Prognostic Nutritional Index = Onodera’s Prognostic Nutritional Index 5 | ALB (g/L) + 5 × Lymphocytes (109/L) | [515,516] |
| Red cell distribution width/ALB ratio | Red cell distribution width (%)/ALB (g/dL) | [517] |
| SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH) | 1 point for each of: Multiple lung lobes involvement on chest X-ray; ALB < 3.5 g/dL; Respiratory rate > 30 N/min; heart rate > 125 beats/min; confusion (acute) 2 points for each of: Systolic blood pressure < 90 mmHg; Oxygen saturation < 90%); potential pH < 7.35 | [518] |
| Systemic Inflammation Score (SIS) 6 | Score 0: ALB ≥ 4.0 g/dL and Lymphocyte/Monocyte Ratio ≥ 4.44; Score 1: ALB < 4.0 g/dL or Lymphocyte/Monocyte Ratio < 4.44; Score 2: ALB < 4.0 g/dL and Lymphocyte/Monocyte Ratio < 4.44 | [519] |
| SWOG Stage | Stage1: Serum ß2 microglobulin < 2.5 mg/L; Stage 2: 2.5 ≤ Serum ß2 microglobulin < 5.5 mg/L; Stage 3: Serum ß2 microglobulin ≥ 5.5 mg/L and ALB ≥ 30 g/L; Stage 4: Serum ß2 microglobulin ≥ 5.5 mg/L and ALB <30 g/L | [520] |
ALB = albumin; BMI = body mass index; INR = International normalized Ratio; NLR = neutrophil to lymphocyte ratio. 1 Other cut-off levels are also reported for AGS [521]. 2 In the Modified Glasgow Prognostic Score, 1 point for ALB < 35 g/L and C-reactive protein < 10 mg/L is omitted [491]. 3 The terms ”Neutrohil to ALB ratio” and “Neutrophil percentage to ALB ratio” are used interchangeably, and the same applies to the way they are computed (either as a number or as a percentage of neutrophils). Consequently, the two terms have been merged in this article. 4 There is also a score designated to the Prognostic Inflammation Score (PIS), which does not incorporate ALB [522]. 5 The Prognostic nutritional index (PNI), initially reported by [523], was previously calculated differently, but Onodera et al. [516] developed the present index in 1984 [422]. 6 Other cut-off levels are reported; e.g., for the Modified Systemic Inflammation score (mSIS) [524] or the Adapted Systemic Inflammation Score (aSIS) [435].
References
- Clayton, D.; Hills, M. Choise and Interpretation of Models. In Statistical Models in Epidemiology; Clayton, D., Hills, M., Eds.; Oxford Science Publications: Oxford, UK, 1993; pp. 271–281. [Google Scholar]
- Knaus, W.A.; Wagner, D.P.; Draper, E.A.; Zimmerman, J.E.; Bergner, M.; Bastos, P.G.; Sirio, C.A.; Murphy, D.J.; Lotring, T.; Damiano, A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991, 100, 1619–1636. [Google Scholar] [CrossRef]
- Goldwasser, P.; Feldman, J. Association of serum albumin and mortality risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef]
- Vincent, J.L.; Dubois, M.J.; Navickis, R.J.; Wilkes, M.M. Hypoalbuminemia in acute illness: Is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann. Surg. 2003, 237, 319–334. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.P.; Wolmarans, M.R.; Park, G.R. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parent. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Johnson, A.M. Low levels of plasma proteins: Malnutrition or inflammation? Clin. Chem. Lab. Med. 1999, 37, 91–96. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Zaccherini, G.; Bernardi, M. The role and indications of albumin in advanced liver disease. Acta Gastroenterol. Belg. 2019, 82, 301–308. [Google Scholar]
- Gavran, L.; Pavlovic, J.; Racic, M.; Ivkovic, N.; Tusek Bunc, K. Evaluation of biochemical markers effectiveness in elderly malnutrition assessment. Med. Glas. 2019, 16, 351–358. [Google Scholar] [CrossRef]
- Horino, T.; Tokunaga, R.; Miyamoto, Y.; Hiyoshi, Y.; Akiyama, T.; Daitoku, N.; Sakamoto, Y.; Yoshida, N.; Baba, H. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann. Gastroenterol. Surg. 2022, 6, 83–91. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Hsu, C.M.; Chang, G.H.; Tsai, M.S.; Lee, Y.C.; Huang, E.I.; Lai, C.H.; Fang, K.H. Advanced Lung Cancer Inflammation Index Predicts Survival Outcomes of Patients with Oral Cavity Cancer Following Curative Surgery. Front. Oncol. 2021, 11, 609314. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.T.; Yang, M.; Zhang, X.W.; Song, M.M.; Hu, C.L.; Ge, Y.Z.; Xie, H.L.; Liu, T.; Tang, M.; Zhang, Q.; et al. Association of Systemic Inflammation and Overall Survival in Elderly Patients with Cancer Cachexia—Results from a Multicenter Study. J. Inflamm. Res. 2021, 14, 5527–5540. [Google Scholar] [CrossRef]
- Mao, W.; Wang, K.; Wu, Y.; Ni, J.; Zhang, H.; Wang, Y.; Wu, Z.; Liu, R.; Geng, J.; Chen, S.; et al. Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index in Patients with Renal Cell Carcinoma Undergoing Laparoscopic Nephrectomy: A Multi-Institutional, Propensity Score Matching Cohort Study. Front. Nutr. 2021, 8, 781647. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ma, Y.; Kai, J.; Wang, J.; Yin, Z.; Xu, H.; Li, X.; Liang, X.; Wei, S.; Liang, X. A Low Advanced Lung Cancer Inflammation Index Predicts a Poor Prognosis in Patients with Metastatic Non-Small Cell Lung Cancer. Front. Mol. Biosci. 2021, 8, 784667. [Google Scholar] [CrossRef]
- Wu, H.; Ding, F.; Lin, M.; Shi, Z.; Mei, Z.; Chen, S.; Jiang, C.; Qiu, H.; Zheng, Z.; Chen, Y.; et al. Use of the Advanced Lung Cancer Inflammation Index as a Prognostic Indicator for Patients with Cholangiocarcinoma. Front. Surg. 2022, 9, 801767. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ye, F.; Li, Y.; Liu, A. Comparison and exploration of the prognostic value of the advanced lung cancer inflammation index, prognostic nutritional index, and systemic immune-inflammation index in newly diagnosed diffuse large B-cell lymphoma. Ann. Palliat. Med. 2021, 10, 9650–9659. [Google Scholar] [CrossRef]
- Han, Z.; Hu, Z.; Zhao, Q.; Xue, W.; Duan, G. The advanced lung cancer inflammation index predicts outcomes of patients with non-small cell lung cancer following video-assisted thoracic surgery. J. Int. Med. Res. 2021, 49, 3000605211062442. [Google Scholar] [CrossRef]
- Mountzios, G.; Samantas, E.; Senghas, K.; Zervas, E.; Krisam, J.; Samitas, K.; Bozorgmehr, F.; Kuon, J.; Agelaki, S.; Baka, S.; et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 2021, 6, 100254. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Liu, Y.; Ding, Z.; Si, Y.; Shi, F.; Liu, J.; Sun, L. Nomograms Based on the Advanced Lung Cancer Inflammation Index for the Prediction of Coronary Artery Disease and Calcification. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211060455. [Google Scholar] [CrossRef] [PubMed]
- Uysal, E.; Acar, Y.A. Features of patients with upper gastrointestinal bleeding and factors affecting the re-bleeding risk. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Marmo, R.; Soncini, M.; Bucci, C.; Zullo, A. Comparison of assessment tools in acute upper gastrointestinal bleeding: Which one for which decision. Scand. J. Gastroenterol. 2022, 57, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Wu, K.; Ou, X. Evaluation of Six Preendoscopy Scoring Systems to Predict Outcomes for Older Adults with Upper Gastrointestinal Bleeding. Gastroenterol. Res. Pract. 2022, 2022, 9334866. [Google Scholar] [CrossRef]
- Franco, M.C.; Jang, S.; Martins, B.D.C.; Stevens, T.; Jairath, V.; Lopez, R.; Vargo, J.J.; Barkun, A.; Maluf-Filho, F. Risk Stratification in Cancer Patients with Acute Upper Gastrointestinal Bleeding: Comparison of Glasgow-Blatchford, Rockall and AIMS65, and Development of a New Scoring System. Clin. Endosc. 2022, 55, 240. [Google Scholar] [CrossRef] [PubMed]
- Bardakçı, O.; Sıddıkoğlu, D.; Akdur, G.; Şimşek, G.; Atalay, Ü.; Das, M.; Akdur, O.; Beyazit, Y. Prediction of adverse outcomes using non-endoscopic scoring systems in patients over 80 years of age who present with the upper gastrointestinal bleeding in the emergency department. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shual, S.; Zhifang, Z.; Yuming, W.; Hong, J.; Chao, S.; Kui, J.; Bangmao, W. Clinical Scoring Systems in Predicting the Outcomes of Small Bowel Bleeding. Turk. J. Gastroenterol. 2021, 32, 493–499. [Google Scholar] [CrossRef]
- Sachan, A.; Dhibar, D.P.; Bhalla, A.; Prakash, A.; Taneja, S.; Sharma, V. Comparison of non-endoscopic scores for the prediction of outcomes in patients of upper gastrointestinal bleed in an emergency of a tertiary care referral hospital: A prospective cohort study. Arq. Gastroenterol. 2021, 58, 534–540. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Walline, J.H.; Yu, X.; Zhu, H. Comparing the Performance of the ABC, AIMS65, GBS, and pRS Scores in Predicting 90-day Mortality or Rebleeding Among Emergency Department Patients with Acute Upper Gastrointestinal Bleeding: A Prospective Multicenter Study. J. Transl. Int. Med. 2021, 9, 114–122. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, H.J.; Shin, K.H.; Kim, G.H.; Kim, H.H. Red Blood Cell Transfusion Volumes According to AIMS65 Scores in Patients with Peptic Ulcer Bleeding. Lab. Med. 2021, 53, 190–193. [Google Scholar] [CrossRef]
- Ak, R.; Hökenek, N.M. Comparison of AIMS65 and Glasgow Blatchford scores in predicting mortality in patients with upper gastrointestinal bleeding. Rev. Assoc. Med. Bras. (1992) 2021, 67, 766–770. [Google Scholar] [CrossRef]
- Simon, T.G.; Travis, A.C.; Saltzman, J.R. Initial Assessment and Resuscitation in Nonvariceal Upper Gastrointestinal Bleeding. Gastrointest. Endosc. Clin. N. Am. 2015, 25, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, R.; Wei, N.; Chen, H. Systematic review and meta-analysis of risk scores in prediction for the clinical outcomes in patients with acute variceal bleeding. Ann. Med. 2021, 53, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Oakland, K. Risk stratification in upper and upper and lower GI bleeding: Which scores should we use? Best Pract. Res. Clin. Gastroenterol. 2019, 42–43, 101613. [Google Scholar] [CrossRef]
- Alzoubaidi, D.; Lovat, L.B.; Haidry, R. Management of non-variceal upper gastrointestinal bleeding: Where are we in 2018? Frontline Gastroenterol. 2019, 10, 35–42. [Google Scholar] [CrossRef]
- Ebrahimi Bakhtavar, H.; Morteza Bagi, H.R.; Rahmani, F.; Shahsavari Nia, K.; Ettehadi, A. Clinical Scoring Systems in Predicting the Outcome of Acute Upper Gastrointestinal Bleeding; A Narrative Review. Emergency 2017, 5, e36. [Google Scholar]
- Beales, I. Recent advances in the management of peptic ulcer bleeding. F1000Res 2017, 6, 1763. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, R.; Mukarram, M.; Smith, C.A.; Thiruganasambandamoorthy, V. The Predictive Value of Preendoscopic Risk Scores to Predict Adverse Outcomes in Emergency Department Patients with Upper Gastrointestinal Bleeding: A Systematic Review. Acad. Emerg. Med. 2016, 23, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Tham, J.; Stanley, A. Clinical utility of pre-endoscopy risk scores in upper gastrointestinal bleeding. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 1161–1167. [Google Scholar] [CrossRef]
- Kariyama, K.; Nouso, K.; Toyoda, H.; Tada, T.; Hiraoka, A.; Tsuji, K.; Itobayashi, E.; Ishikawa, T.; Wakuta, A.; Oonishi, A.; et al. Utility of FIB4-T as a Prognostic Factor for Hepatocellular Carcinoma. Cancers 2019, 11, 203. [Google Scholar] [CrossRef]
- Yu, J.I.; Park, H.C.; Yoo, G.S.; Choi, C.; Choi, M.S.; Nam, H.; Baek, S.Y.; Park, M. Clinical importance of the absolute count of neutrophils, lymphocytes, monocytes, and platelets in newly diagnosed hepatocellular carcinoma. Sci. Rep. 2021, 11, 2614. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tsuji, K.; Matsui, T.; Kang, J.H.; Sakurai, Y.; Kodama, Y.; Minami, R.; Watanabe, K.; Katanuma, A. Potential of PALBI-T score as a prognostic model for hepatocellular carcinoma in alcoholic liver disease. JGH Open 2022, 6, 36–43. [Google Scholar] [CrossRef]
- Sonohara, F.; Yamada, S.; Tanaka, N.; Suenaga, M.; Takami, H.; Hayashi, M.; Niwa, Y.; Sugimoto, H.; Hattori, N.; Kanda, M.; et al. Perioperative and prognostic implication of albumin-bilirubin-TNM score in Child-Pugh class A hepatocellular carcinoma. Ann. Gastroenterol. Surg. 2019, 3, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Elshaarawy, O.; Alkhatib, A.; Elhelbawy, M.; Gomaa, A.; Allam, N.; Alsebaey, A.; Rewisha, E.; Waked, I. Validation of modified albumin-bilirubin-TNM score as a prognostic model to evaluate patients with hepatocellular carcinoma. World J. Hepatol. 2019, 11, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Tsuji, K.; Itobayashi, E.; Kariyama, K.; Ishikawa, T.; Tajiri, K.; Ochi, H.; et al. Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer 2017, 6, 204–215. [Google Scholar] [CrossRef]
- Harimoto, N.; Yoshizumi, T.; Sakata, K.; Nagatsu, A.; Motomura, T.; Itoh, S.; Harada, N.; Ikegami, T.; Uchiyama, H.; Soejima, Y.; et al. Prognostic significance of combined albumin-bilirubin and tumor-node-metastasis staging system in patients who underwent hepatic resection for hepatocellular carcinoma. Hepatol. Res. 2017, 47, 1289–1298. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Toyoda, H.; Tada, T.; Ueki, H.; Kaneto, M.; Aibiki, T.; Okudaira, T.; Kawakami, T.; et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 1031–1036. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Kudo, M. Newly Proposed ALBI Grade and ALBI-T Score as Tools for Assessment of Hepatic Function and Prognosis in Hepatocellular Carcinoma Patients. Liver Cancer 2019, 8, 312–325. [Google Scholar] [CrossRef]
- Saito, Y.; Nakai, T.; Ikeya, Y.; Kogawa, R.; Otsuka, N.; Wakamatsu, Y.; Kurokawa, S.; Ohkubo, K.; Nagashima, K.; Okumura, Y. Clinical significance of the albumin-bilirubin score in patients with heart failure undergoing cardiac resynchronization therapy. Heart Vessel. 2022, 37, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Young, L.B.; Tabrizian, P.; Sung, J.; Biederman, D.; Bishay, V.L.; Ranade, M.; Patel, R.S.; Nowakowski, F.S.; Fischman, A.M.; Lookstein, R.A.; et al. Survival Analysis Using ALBI Grade for Patients Treated with DEE-TACE for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2022, 33, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, A.; Takamura, M.; Sakai, N.; Watanabe, Y.; Arao, Y.; Kimura, N.; Setsu, T.; Abe, H.; Yokoo, T.; Kamimura, H.; et al. Longitudinal increase in albumin-bilirubin score is associated with non-malignancy-related mortality and quality of life in patients with liver cirrhosis. PLoS ONE 2022, 17, e0263464. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Yamashita, Y.I.; Arima, K.; Higashi, T.; Hayashi, H.; Imai, K.; Nitta, H.; Chikamoto, A.; Beppu, T.; Baba, H. Alteration of prognostic efficacy of albumin-bilirubin grade and Child-Pugh score according to liver fibrosis in hepatocellular carcinoma patients with Child-Pugh A following hepatectomy. Ann. Gastroenterol. Surg. 2022, 6, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.C.; Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lei, H.J.; Lee, R.C.; Hou, M.C.; et al. Dual hepatitis B and C-associated hepatocellular carcinoma: Clinical characteristics, outcome, and prognostic role of albumin-bilirubin grade. Int. J. Clin. Oncol. 2022, 27, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Fidan, E.; Fidan, S.; Merev, E.; Kazaz, N. The Relationship between albumin-Bilirubin grade and survival in hepatocelluler carcinoma patients treated with sorefanib. Niger. J. Clin. Pract. 2022, 25, 173–177. [Google Scholar] [CrossRef]
- Dong, D.; Shi, J.Y.; Shang, X.; Liu, B.; Xu, W.L.; Cui, G.Z.; Wang, N.Y. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib: A retrospective analysis. Medicine 2022, 101, e28680. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Troshina, G.; Santaniello, U.; Rosati, G.; Bombaci, F.; Birolo, G.; Nicolosi, A.; Saracco, G.M.; Ciancio, A. Long-Term Hepatocellular Carcinoma Development and Predictive Ability of Non-Invasive Scoring Systems in Patients with HCV-Related Cirrhosis Treated with Direct-Acting Antivirals. Cancers 2022, 14, 828. [Google Scholar] [CrossRef]
- Asai, Y.; Yamamoto, T.; Sato, Y. Risk assessment of micafungin-induced liver injury using spontaneous reporting system data and electronic medical records. J. Infect. Chemother. 2022, 28, 690–695. [Google Scholar] [CrossRef]
- Li, S.; Li, B.; Li, L.; Xu, F.; Yang, X.; Wang, W. A combination of portal vein stent insertion and endovascular iodine-125 seed-strip implantation, followed by transcatheter arterial chemoembolization with sorafenib for treatment of hepatocellular carcinoma-associated portal vein tumor thrombus. J. Contemp. Brachytherapy 2021, 13, 670–679. [Google Scholar] [CrossRef]
- Pang, Q.; Zhou, S.; Liu, S.; Liu, H.; Lu, Z. Prognostic role of preoperative albumin-bilirubin score in posthepatectomy liver failure and mortality: A systematic review and meta-analysis. Updates Surg. 2021, 74, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wei, Q.; He, Y.; Xie, Q.; Liang, Y.; Zhang, L.; Xia, Y.; Li, Y.; Chen, W.; Zhao, J.; et al. ALBI versus child-pugh in predicting outcome of patients with HCC: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; D’Alessio, A.; Rimassa, L.; Sharma, R.; Pinato, D.J. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021, 3, 100347. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zong, R.; Shi, Y.; Xu, K. Prognostic role of preoperative albumin-bilirubin grade on patients with hepatocellular carcinoma after surgical resection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 769–778. [Google Scholar] [CrossRef]
- Coombs, A.W.; Jordan, C.; Hussain, S.A.; Ghandour, O. Scoring systems for the management of oncological hepato-pancreato-biliary patients. Ann. Hepatobiliary Pancreat. Surg. 2022, 26, 17–30. [Google Scholar] [CrossRef]
- Yamamoto, Y. Evaluation of Liver Function and the Role of Biliary Drainage before Major Hepatic Resections. Visc. Med. 2021, 37, 10–17. [Google Scholar] [CrossRef]
- Marasco, G.; Alemanni, L.V.; Colecchia, A.; Festi, D.; Bazzoli, F.; Mazzella, G.; Montagnani, M.; Azzaroli, F. Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2011. [Google Scholar] [CrossRef]
- Marasco, G.; Colecchia, A.; Silva, G.; Rossini, B.; Eusebi, L.H.; Ravaioli, F.; Dajti, E.; Alemanni, L.V.; Colecchia, L.; Renzulli, M.; et al. Non-invasive tests for the prediction of primary hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 3326–3343. [Google Scholar] [CrossRef]
- Feng, D.; Wang, M.; Hu, J.; Li, S.; Zhao, S.; Li, H.; Liu, L. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases. Ann. Transl. Med. 2020, 8, 553. [Google Scholar] [CrossRef]
- Deng, M.; Ng, S.W.Y.; Cheung, S.T.; Chong, C.C.N. Clinical application of Albumin-Bilirubin (ALBI) score: The current status. Surgeon 2020, 18, 178–186. [Google Scholar] [CrossRef]
- Li, J.; Feng, D.; Pang, N.; Zhao, C.; Gao, L.; Liu, S.; Li, L. Controlling nutritional status score as a new indicator of overt hepatic encephalopathy in cirrhotic patients following transjugular intrahepatic portosystemic shunt. Clin. Nutr. 2022, 41, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Sanghvi, T.; Sharma, S.; Richardson, C.; Rubin, N.; Richards, M.; D’Souza, D.; Flanagan, S.; Golzarian, J. Local recurrence following complete radiologic response in patients treated with transarterial chemoembolization for hepatocellular carcinoma. Diagn. Interv. Imaging 2022, 103, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Takaya, H.; Namisaki, T.; Matsumoto, K.; Suzuki, J.; Murata, K.; Tsuji, Y.; Nakanishi, K.; Kaji, K.; Kitade, M.; Noguchi, R.; et al. Comparison of Ablation Area and Change in Functional Liver Reserve after Radiofrequency Ablation for Hepatocellular Carcinoma Using the arfa(®) and VIVA(®) Systems. J. Clin. Med. 2022, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Refardt, J.; den Hoed, C.M.; Langendonk, J.; Zandee, W.; Charehbili, A.; Feelders, R.A.; de Herder, W.W.; Brabander, T.; Hofland, H.J. Prognostic significance of hyperammonemia in neuroendocrine neoplasm patients with liver metastases. Endocr. Relat. Cancer 2022, 29, 241–250. [Google Scholar] [CrossRef]
- Young, S.; Rubin, N.; D’Souza, D.; Sharma, P.; Pontolillo, J.; Flanagan, S.; Golzarian, J.; Sanghvi, T. Inflammatory Scores: Correlation with Clinical Outcomes in Hepatocellular Carcinoma Patients Undergoing Transarterial Radioembolization. Cardiovasc. Intervent. Radiol. 2022, 45, 461–475. [Google Scholar] [CrossRef]
- Wang, Q.; Kesen, S.; Liljeroth, M.; Nilsson, H.; Zhao, Y.; Sparrelid, E.; Brismar, T.B. Quantitative evaluation of liver function with gadoxetic acid enhanced MRI: Comparison among signal intensity-, T1-relaxometry-, and dynamic-hepatocyte-specific-contrast-enhanced MRI- derived parameters. Scand. J. Gastroenterol. 2022. [Google Scholar] [CrossRef]
- Uchida, Y.; Uemura, H.; Tsuji, S.; Yamada, S.; Kouyama, J.I.; Naiki, K.; Sugawara, K.; Nakao, M.; Nakayama, N.; Imai, Y.; et al. Significance of furosemide in patients with cirrhosis treated with or without zinc acetate hydrate supplementation. Hepatol. Res. 2022, 52, 449–461. [Google Scholar] [CrossRef]
- Mallik, M.; Singhai, A.; Khadanga, S.; Ingle, V. The Significant Morbidity and Mortality Indicators in Patients of Cirrhosis. Cureus 2022, 14, e21226. [Google Scholar] [CrossRef] [PubMed]
- Yosef, T.; Ibrahim, W.A.; Matboli, M.; Swilam, A.A.; El-Nakeep, S. New stem cell autophagy surrogate diagnostic biomarkers in early-stage hepatocellular carcinoma in Egypt: A pilot study. World J. Hepatol. 2021, 13, 2137–2149. [Google Scholar] [CrossRef]
- Iwasa, M.; Shigefuku, R.; Eguchi, A.; Tamai, Y.; Takei, Y. Update on blood-based biomarkers for chronic liver diseases prognosis: Literature review and institutional experience. JGH Open 2021, 5, 1250–1256. [Google Scholar] [CrossRef]
- El-Khateeb, E.; Darwich, A.S.; Achour, B.; Athwal, V.; Rostami-Hodjegan, A. Review article: Time to revisit Child-Pugh score as the basis for predicting drug clearance in hepatic impairment. Aliment. Pharmacol. Ther. 2021, 54, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Qi, X.; Guo, X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine 2016, 95, e2877. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.A.; Hjortnaes, J.; Kranenburg, G.; de Heer, F.; Kluin, J. Mortality after cardiac surgery in patients with liver cirrhosis classified by the Child-Pugh score. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.C.; Yu, S.C.; Wang, R.C.; Lai, S.F.; Teng, C.J.; Lin, J.W.; Lin, W.L.; Huang, T.C. Investigating early progression of Hodgkin lymphoma in a two-center analysis. J. Formos. Med. Assoc. 2022, 121, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Lucijanić, M.; Huzjan Korunić, R.; Ivić, M.; Fazlić Džankić, A.; Jonjić, Ž.; Mitrović, Z.; Prka, Ž.; Piršić, M.; Jakšić, O.; Gaćina, P.; et al. Psoas muscle index at the time of diagnosis might reflect the prognosis of classical Hodgkin’s lymphoma patients. Wien Klin. Wochenschr. 2022, 134, 80–82. [Google Scholar] [CrossRef]
- Koyuncu, M.B.; Guler, E.; Koseci, T.; Ucar, M.A.; Tombak, A.; Akdeniz, A.; Tiftik, E.N.; Cavusoglu, C. Integration of low muscle mass into the IPS system and its prognostic significance in patients with Hodgkin’s lymphoma. Biomark. Med. 2022, 16, 57–67. [Google Scholar] [CrossRef]
- Rose, A.; Grajales-Cruz, A.; Lim, A.; Todd, A.; Bello, C.; Shah, B.; Chavez, J.; Pinilla-Ibartz, J.; Saeed, H.; Sandoval-Sus, J.; et al. Classical Hodgkin Lymphoma: Clinicopathologic Features, Prognostic Factors, and Outcomes From a 28-Year Single Institutional Experience. Clin. Lymphoma Myeloma Leuk. 2021, 21, 132–138. [Google Scholar] [CrossRef]
- Qin, J.Q.; Yin, H.; Wu, J.Z.; Chen, R.Z.; Xia, Y.; Wang, L.; Zhu, H.Y.; Fan, L.; Li, J.Y.; Liang, J.H.; et al. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in Hodgkin lymphoma. Leuk. Res. 2021, 105, 106580. [Google Scholar] [CrossRef]
- Potre, O.; Pescaru, M.; Sima, A.; Ionita, I.; Tudor, R.; Borsi, E.; Samfireag, M.; Potre, C. Evaluation of the Relapse Risk and Survival Rate in Patients with Hodgkin Lymphoma: A Monocentric Experience. Medicina 2021, 57, 1026. [Google Scholar] [CrossRef]
- Hutchings, M.; Radford, J.; Ansell, S.M.; Illés, Á.; Sureda, A.; Connors, J.M.; Sýkorová, A.; Shibayama, H.; Abramson, J.S.; Chua, N.S.; et al. Brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine in patients with advanced-stage, classical Hodgkin lymphoma: A prespecified subgroup analysis of high-risk patients from the ECHELON-1 study. Hematol. Oncol. 2021, 39, 185–195. [Google Scholar] [CrossRef]
- Adam, M.; Bekueretsion, Y.; Abubeker, A.; Tadesse, F.; Kwiecinska, A.; Howe, R.; Petros, B.; Jerkeman, M.; Gebremedhin, A. Clinical Characteristics and Histopathological Patterns of Hodgkin Lymphoma and Treatment Outcomes at a Tertiary Cancer Center in Ethiopia. JCO Glob. Oncol. 2021, 7, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Cuccaro, A.; Maiolo, E.; Bellesi, S.; D’Alò, F.; Fusco, D.; Colloca, G.; De Stefano, V.; Hohaus, S. Comorbidity assessment to determine prognosis in older adult patients with classical Hodgkin lymphoma. Hematol. Oncol. 2020, 38, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, M.Y.Y.; Fahmi, M.W.; El-Ashwah, S. Clinico-pathological significance of immunohistochemically marked tumor-associated macrophage in classic Hodgkin lymphoma. J. Egypt Natl. Canc. Inst. 2020, 32, 18. [Google Scholar] [CrossRef] [PubMed]
- Roth-Stefanski, C.T.; Rodrigues de Almeida, N.; Biagini, G.; Scatone, N.K.; Nerbass, F.B.; de Moraes, T.P. The Diagnosis of Protein Energy Wasting in Chronic Peritoneal Dialysis Patients Is Influenced by the Method of Calculating Muscle Mass. A Prospective, Multicenter Study. Front. Med. 2021, 8, 702749. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Nagy, E.; AbdAlBary, M.; El-Kannishy, G.; Sayed-Ahmed, N. Relation of protein energy wasting to carotid intima media thickness in hemodialysis patients. J. Hum. Hypertens. 2021, 35, 598–603. [Google Scholar] [CrossRef]
- Aniort, J.; Freist, M.; Piraud, A.; Philipponnet, C.; Hadj Abdelkader, M.; Garrouste, C.; Gentes, E.; Pereira, B.; Heng, A.E. Circulating 20S proteasome for assessing protein energy wasting syndrome in hemodialysis patients. PLoS ONE 2020, 15, e0236948. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, W.; Pan, D.; Sun, G. Predicational ability of phase angle on protein energy wasting in kidney disease patients with renal replacement therapy: A cross-sectional study. Food Sci. Nutr. 2021, 9, 3573–3579. [Google Scholar] [CrossRef]
- Tsai, M.T.; Tseng, W.C.; Ou, S.M.; Lee, K.H.; Yang, C.Y.; Tarng, D.C. Comparison of Simplified Creatinine Index and Systemic Inflammatory Markers for Nutritional Evaluation of Hemodialysis Patients. Nutrients 2021, 13, 1870. [Google Scholar] [CrossRef]
- Da, J.; Long, Y.; Li, Q.; Yang, X.; Yuan, J.; Zha, Y. Resting metabolic rate and its adjustments as predictors of risk protein-energy wasting in hemodialysis patients. Biosci. Rep. 2021, 41, BSR20210010. [Google Scholar] [CrossRef]
- Saitoh, M.; Ogawa, M.; Kondo, H.; Suga, K.; Takahashi, T.; Itoh, H.; Tabata, Y. Bioelectrical impedance analysis-derived phase angle as a determinant of protein-energy wasting and frailty in maintenance hemodialysis patients: Retrospective cohort study. BMC Nephrol. 2020, 21, 438. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of Ultrasound-Derived Metrics of the Quadriceps Muscle with Protein Energy Wasting in Hemodialysis Patients: A Multicenter Cross-Sectional Study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef]
- Leal Escobar, G.; Osuna Padilla, I.A.; Cano Escobar, K.B.; Moguel González, B.; Pérez Grovas, H.A.; Ruiz Ubaldo, S. Phase angle and mid arm circumference as predictors of protein energy wasting in renal replacement therapy patients. Nutr. Hosp. 2019, 36, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, G.; Karabudak, E.; Mandıroğlu, F. Relationship of dietary and serum zinc and leptin levels with protein energy wasting in haemodialysis patients. Int. Urol. Nephrol. 2020, 52, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, H.T.; Singh, M.; Milce, J.; Haidar, M.; Rieth, A.; Lebioda, A.; Kohnke, J. Management of Patients with Relapsed and/or Refractory Multiple Myeloma Treated with Novel Combination Therapies in Routine Clinical Practice in Germany. Adv. Ther. 2022, 39, 1247–1266. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Jiang, J.; Liu, P. The role of KIAA1191 in the necroptotic pathway of multiple myeloma. Ann. Hematol. 2022, 101, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Soekojo, C.Y.; Chung, T.H.; Furqan, M.S.; Chng, W.J. Genomic characterization of functional high-risk multiple myeloma patients. Blood Cancer J. 2022, 12, 24. [Google Scholar] [CrossRef]
- Sidana, S.; Kumar, S.; Fraser, R.; Estrada-Merly, N.; Giralt, S.; Agrawal, V.; Anderson, L.D., Jr.; Aljurf, M.; Banerjee, R.; Bashey, A.; et al. Impact of Induction Therapy with VRD versus VCD on Outcomes in Patients with Multiple Myeloma in Partial Response or Better Undergoing Upfront Autologous Stem Cell Transplantation. Transplant. Cell. Ther. 2022, 28, e81–e83.e89. [Google Scholar] [CrossRef]
- Schjesvold, F.H.; Dimopoulos, M.A.; Delimpasi, S.; Robak, P.; Coriu, D.; Legiec, W.; Pour, L.; Špička, I.; Masszi, T.; Doronin, V.; et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): A randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022, 9, e98–e110. [Google Scholar] [CrossRef]
- Kaddoura, M.; Binder, M.; Dingli, D.; Buadi, F.K.; Lacy, M.Q.; Gertz, M.A.; Dispenzieri, A.; Kapoor, P.; Hwa, L.; Fonder, A.; et al. Impact of achieving a complete response to initial therapy of multiple myeloma and predictors of subsequent outcome. Am. J. Hematol. 2022, 97, 267–273. [Google Scholar] [CrossRef]
- Shang, Y.; Jin, Y.; Liu, H.; Ding, L.; Tong, X.; Tu, H.; Zang, L.; Lin, C.; Hu, J.; Zhou, F. Evaluation of prognostic staging systems of multiple myeloma in the era of novel agents. Hematol. Oncol. 2022, 40, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Liu, M.; Wang, F.; Xiong, Y.; Tang, Z.; Li, Q.; Lu, Q.; Liang, S.; Niu, T.; et al. Myocardial Injury in Multiple Myeloma Patients with Preserved Left Ventricular Ejection Fraction: Noninvasive Left Ventricular Pressure-Strain Myocardial Work. Front. Cardiovasc. Med. 2021, 8, 782580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yin, P.; Hao, C.; Sun, C.; Chen, L.; Wang, S.; Hong, N. MRI-Based Bone Marrow Radiomics Nomogram for Prediction of Overall Survival in Patients with Multiple Myeloma. Front. Oncol. 2021, 11, 709813. [Google Scholar] [CrossRef]
- Gay, F.; Musto, P.; Rota-Scalabrini, D.; Bertamini, L.; Belotti, A.; Galli, M.; Offidani, M.; Zamagni, E.; Ledda, A.; Grasso, M.; et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): A randomised, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Bataille, R.; Annweiler, C.; Beauchet, O. Multiple myeloma international staging system: “staging” or simply “aging” system? Clin. Lymphoma Myeloma Leuk. 2013, 13, 635–637. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Bonello, F.; Cani, L.; D’Agostino, M. Risk Stratification Before and During Treatment in Newly Diagnosed Multiple Myeloma: From Clinical Trials to the Real-World Setting. Front. Oncol. 2022, 12, 830922. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Costa, L.J.; Usmani, S.Z. Defining and Managing High-Risk Multiple Myeloma: Current Concepts. J. Natl. Compr. Canc. Netw. 2020, 18, 1730–1737. [Google Scholar] [CrossRef]
- Lancman, G.; Tremblay, D.; Barley, K.; Barlogie, B.; Cho, H.J.; Jagannath, S.; Madduri, D.; Moshier, E.; Parekh, S.; Chari, A. The effect of novel therapies in high-molecular-risk multiple myeloma. Clin. Adv. Hematol. Oncol. 2017, 15, 870–879. [Google Scholar]
- Ooi, M.G.; de Mel, S.; Chng, W.J. Risk Stratification in Multiple Myeloma. Curr. Hematol. Malig. Rep. 2016, 11, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Morgan, G.J. Biologic frontiers in multiple myeloma: From biomarker identification to clinical practice. Clin. Cancer Res. 2014, 20, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Biran, N.; Jagannath, S.; Chari, A. Risk stratification in multiple myeloma, part 1: Characterization of high-risk disease. Clin. Adv. Hematol. Oncol. 2013, 11, 489–503. [Google Scholar]
- Kyrtsonis, M.C.; Maltezas, D.; Tzenou, T.; Koulieris, E.; Bradwell, A.R. Staging systems and prognostic factors as a guide to therapeutic decisions in multiple myeloma. Semin. Hematol. 2009, 46, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sahathevan, S.; Karupaiah, T.; Khor, B.H.; Sadu Singh, B.K.; Mat Daud, Z.A.; Fiaccadori, E.; Sabatino, A.; Chinna, K.; Abdul Gafor, A.H.; Bavanandan, S.; et al. Muscle Status Response to Oral Nutritional Supplementation in Hemodialysis Patients with Protein Energy Wasting: A Multi-Center Randomized, Open Label-Controlled Trial. Front. Nutr. 2021, 8, 743324. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.M.; Khor, B.H.; Sahathevan, S.; Sualeheen, A.; Chinna, K.; Gafor, A.H.A.; Goh, B.L.; Ahmad, G.; Morad, Z.; Daud, Z.A.M.; et al. Is malnutrition a determining factor of health-related quality of life in hemodialysis patients? A cross-sectional design examining relationships with a comprehensive assessment of nutritional status. Qual. Life Res. 2022, 31, 1441–1459. [Google Scholar] [CrossRef]
- Xavier, J.S.; de Góes, C.R.; Costa Borges, M.C.; Caramori, J.C.T.; Vogt, B.P. Handgrip strength thresholds are associated with malnutrition inflammation score (MIS) in maintenance hemodialysis patients. J. Ren. Nutr. 2022, 32, 739–743. [Google Scholar] [CrossRef]
- Sualeheen, A.; Khor, B.H.; Balasubramaniam, G.V.; Sahathevan, S.; Chinna, K.; Mat Daud, Z.A.; Khosla, P.; Abdul Gafor, A.H.; Karupaiah, T. Benchmarking Diet Quality to Assess Nutritional Risk in Hemodialysis Patients: Applying Adequacy and Moderation Metrics of the Hemodialysis-Healthy Eating Index. J. Ren. Nutr. 2022, 32, 726–738. [Google Scholar] [CrossRef]
- Sá Martins, V.; Adragão, T.; Aguiar, L.; Pinto, I.; Dias, C.; Figueiredo, R.; Lourenço, P.; Pascoal, T.; Pereira, J.; Pinheiro, T.; et al. Prognostic Value of the Malnutrition-inflammation Score in Hospitalization and Mortality on Long-term Hemodialysis. J. Ren. Nutr. 2022, 32, 569–577. [Google Scholar] [CrossRef]
- Torreggiani, M.; Fois, A.; Moio, M.R.; Chatrenet, A.; Mazé, B.; Lippi, F.; Vigreux, J.; Beaumont, C.; Santagati, G.; Paulin, N.; et al. Spontaneously Low Protein Intake in Elderly CKD Patients: Myth or Reality? Analysis of Baseline Protein Intake in a Large Cohort of Patients with Advanced CKD. Nutrients 2021, 13, 4371. [Google Scholar] [CrossRef]
- López-Cisneros, S.; González-Ortiz, A.; Ramos-Acevedo, S.; Espinosa-Cuevas, A. Is there a relationship between oral hygiene and nutritional status in peritoneal dialysis patients? Nutr. Hosp. 2021, 39, 355–364. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Zhang, H.; Hu, J.; Wang, J.; Zhou, H.; Liu, Y.; Liu, J.; Yi, B.; Zhang, W. A Prospective, Self-Controlled Pilot Study of the Efficacy of Roxadustat for Erythropoietin Hyporesponsiveness in Patients Requiring Chronic Ambulatory Peritoneal Dialysis. J. Ren. Nutr. 2022, 32, 595–604. [Google Scholar] [CrossRef]
- Chan, G.C.; Ng, J.K.; Chow, K.M.; Kwong, V.W.; Pang, W.F.; Cheng, P.M.; Law, M.C.; Leung, C.B.; Li, P.K.; Szeto, C.C. Impact of frailty and its inter-relationship with lean tissue wasting and malnutrition on kidney transplant waitlist candidacy and delisting. Clin. Nutr. 2021, 40, 5620–5629. [Google Scholar] [CrossRef] [PubMed]
- Jatupornpoonsub, T.; Thimachai, P.; Supasyndh, O.; Wongsawat, Y. EEG Delta/Theta Ratio and Microstate Analysis Originating Novel Biomarkers for Malnutrition-Inflammation Complex Syndrome in ESRD Patients. Front. Hum. Neurosci. 2021, 15, 795237. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, M.; Fois, A.; Njandjo, L.; Longhitano, E.; Chatrenet, A.; Esposito, C.; Fessi, H.; Piccoli, G.B. Toward an individualized determination of dialysis adequacy: A narrative review with special emphasis on incremental hemodialysis. Expert Rev. Mol. Diagn. 2021, 21, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Zsom, L.; Zsom, M.; Abdul Salim, S.; Fülöp, T. Subjective global assessment of nutrition, dialysis quality, and the theory of the scientific method in Nephrology practice. Art. Organs 2020, 44, 1021–1030. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Cuppari, L.; Campbell, K.L.; Avesani, C.M. Nutritional assessment of elderly patients on dialysis: Pitfalls and potentials for practice. Nephrol. Dial. Transplant. 2017, 32, 1780–1789. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Riella, M.C. Nutritional evaluation of patients receiving dialysis for the management of protein-energy wasting: What is old and what is new? J. Ren. Nutr. 2013, 23, 195–198. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Cai, X.; Xu, W.; Wang, D.; Wang, T.; Jia, Q.; Gong, H.; Sun, H.; Wu, Z.; et al. Prognostic Significance of a Novel Score Model Based on Preoperative Indicators in Patients with Breast Cancer Spine Metastases (BCSM). Cancer Manag. Res. 2020, 12, 11501–11513. [Google Scholar] [CrossRef] [PubMed]
- Massaad, E.; Hadzipasic, M.; Alvarez-Breckenridge, C.; Kiapour, A.; Fatima, N.; Schwab, J.H.; Saylor, P.; Oh, K.; Schoenfeld, A.J.; Shankar, G.M.; et al. Predicting tumor-specific survival in patients with spinal metastatic renal cell carcinoma: Which scoring system is most accurate? J. Neurosurg. Spine 2020, 33, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.H.; Baker, J.F. Retrospective evaluation of prognostic factors in metastatic spine disease: Serum albumin and primary tumour type are key. ANZ J. Surg. 2020, 90, 1070–1074. [Google Scholar] [CrossRef]
- Xiong, G.X.; Fisher, M.W.A.; Schwab, J.H.; Simpson, A.K.; Nguyen, L.; Tobert, D.G.; Balboni, T.A.; Shin, J.H.; Ferrone, M.L.; Schoenfeld, A.J. A Natural History of Patients Treated Operatively and Non-Operatively for Spinal Metastases Over 2 Years Following Treatment: Survival and Functional Outcomes. Spine 2022, 47, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Ferrone, M.L.; Blucher, J.A.; Agaronnik, N.; Nguyen, L.; Tobert, D.G.; Balboni, T.A.; Schwab, J.H.; Shin, J.H.; Sciubba, D.M.; et al. Prospective comparison of the accuracy of the New England Spinal Metastasis Score (NESMS) to legacy scoring systems in prognosticating outcomes following treatment of spinal metastases. Spine J. 2022, 22, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Ferrone, M.L.; Schwab, J.H.; Blucher, J.A.; Barton, L.B.; Tobert, D.G.; Chi, J.H.; Shin, J.H.; Kang, J.D.; Harris, M.B. Prospective validation of a clinical prediction score for survival in patients with spinal metastases: The New England Spinal Metastasis Score. Spine J. 2021, 21, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, S.Y.; Son, J.; Mok, S.; Park, S.C.; Chang, B.S. The Effect of Adding Biological Factors to the Decision-Making Process for Spinal Metastasis of Non-Small Cell Lung Cancer. J. Clin. Med. 2021, 10, 1119. [Google Scholar] [CrossRef]
- De la Garza Ramos, R.; Naidu, I.; Choi, J.H.; Pennington, Z.; Goodwin, C.R.; Sciubba, D.M.; Shin, J.H.; Yanamadala, V.; Murthy, S.; Gelfand, Y.; et al. Comparison of three predictive scoring systems for morbidity in oncological spine surgery. J. Clin. Neurosci. 2021, 94, 13–17. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Blucher, J.A.; Barton, L.B.; Schwab, J.H.; Balboni, T.A.; Chi, J.H.; Shin, J.H.; Kang, J.D.; Harris, M.B.; Ferrone, M.L. Design of the prospective observational study of spinal metastasis treatment (POST). Spine J. 2020, 20, 572–579. [Google Scholar] [CrossRef]
- Shi, D.D.; Chen, Y.H.; Lam, T.C.; Leonard, D.; Balboni, T.A.; Schoenfeld, A.; Skamene, S.; Cagney, D.N.; Chi, J.H.; Cho, C.H.; et al. Assessing the utility of a prognostication model to predict 1-year mortality in patients undergoing radiation therapy for spinal metastases. Spine J. 2018, 18, 935–940. [Google Scholar] [CrossRef]
- Pennington, Z.; Ehresman, J.; Cottrill, E.; Lubelski, D.; Lehner, K.; Feghali, J.; Ahmed, A.K.; Schilling, A.; Sciubba, D.M. To operate, or not to operate? Narrative review of the role of survival predictors in patient selection for operative management of patients with metastatic spine disease. J. Neurosurg. Spine 2021, 34, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.M.; Jin, J.L.; Cao, Y.X.; Guo, Y.L.; Wu, N.Q.; Zhu, C.G.; Dong, Q.; Sun, J.; Xu, R.X.; et al. NAFLD fibrosis score is correlated with PCSK9 and improves outcome prediction of PCSK9 in patients with chest pain: A cohort study. Lipids Health Dis. 2022, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Tustumi, F.; Dantas, A.C.B.; Miranda, B.C.J.; Pajecki, D.; De Cleva, R.; Santo, M.A.; Nahas, S.C. Obesity and severe steatosis: The importance of biochemical exams and scores. Arq. Bras. Cir. Dig. 2022, 34, e1626. [Google Scholar] [CrossRef] [PubMed]
- Qadri, S.; Ahlholm, N.; Lønsmann, I.; Pellegrini, P.; Poikola, A.; Luukkonen, P.K.; Porthan, K.; Juuti, A.; Sammalkorpi, H.; Penttilä, A.K.; et al. Obesity Modifies the Performance of Fibrosis Biomarkers in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, e2008–e2020. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, A.; Manuella, L.; Cannata, E.; Russo, G.; La Vignera, S.; Purrello, F.; Di Cataldo, A.; Sciacca, L. Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors. J. Clin. Med. 2022, 11, 878. [Google Scholar] [CrossRef]
- Duseja, A.; Singh, S.P.; Mehta, M.; Shalimar; Venkataraman, J.; Mehta, V.; Devadas, K.; Kar, S.K.; Goyal, O.; Nagral, A.; et al. Clinicopathological Profile and Outcome of a Large Cohort of Patients with Nonalcoholic Fatty Liver Disease from South Asia: Interim Results of the Indian Consortium on Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2022, 20, 166–173. [Google Scholar] [CrossRef]
- Chun, H.S.; Lee, M.; Lee, H.A.; Oh, S.Y.; Baek, H.J.; Moon, J.W.; Kim, Y.J.; Lee, J.; Kim, H.; Kim, H.Y.; et al. Association of Physical Activity with Risk of Liver Fibrosis, Sarcopenia, and Cardiovascular Disease in Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 358–369. [Google Scholar] [CrossRef]
- Choi, I.Y.; Chang, Y.; Kang, G.; Jung, H.S.; Shin, H.; Wild, S.H.; Byrne, C.D.; Ryu, S. Low heart rate variability from 10-s electrocardiograms is associated with development of non-alcoholic fatty liver disease. Sci. Rep. 2022, 12, 1062. [Google Scholar] [CrossRef]
- Al-Karaghouli, M.; Fuentes, S.; Davyduke, T.; Ma, M.; Abraldes, J.G. Impact of statin treatment on non-invasive tests based predictions of fibrosis in a referral pathway for NAFLD. BMJ Open Gastroenterol. 2022, 9, e000798. [Google Scholar] [CrossRef]
- Ouzan, D.; Mosnier, A.; Penaranda, G.; Daviaud, I.; Joly, H.; Muntlak, M.; Cohen, J.M. Prospective screening for significant liver fibrosis by fibrosis-4 in primary care patients without known liver disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e986–e991. [Google Scholar] [CrossRef]
- Khayyat, Y.M. Determination of “indeterminate score” measurements in lean nonalcoholic fatty liver disease patients from western Saudi Arabia. World J. Hepatol. 2021, 13, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Sessa, A.; Allaire, M.; Lebray, P.; Medmoun, M.; Tiritilli, A.; Iaria, P.; Cadranel, J.F. From congestive hepatopathy to hepatocellular carcinoma, how can we improve patient management? JHEP Rep. 2021, 3, 100249. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Perseghin, G. Advances in fibrosis biomarkers in nonalcoholic fatty liver disease. Adv. Clin. Chem. 2022, 106, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Huang, D.Q.; Loomba, R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol. Res. 2022, 52, 497–507. [Google Scholar] [CrossRef]
- Roeb, E. Diagnostic and Therapy of Nonalcoholic Fatty Liver Disease: A Narrative Review. Visc. Med. 2022, 38, 126–132. [Google Scholar] [CrossRef]
- Noorian, S.; Patel, A.; Ashkar, C.; Saab, S. Identifying Advanced Fibrosis in NAFLD Using Noninvasive Tests: A Systematic Review of Sequential Algorithms. J. Clin. Gastroenterol. 2022, 56, 266–272. [Google Scholar] [CrossRef]
- Liu, M.; Mei, K.; Tan, Z.; Huang, S.; Liu, F.; Deng, C.; Ma, J.; Yu, P.; Liu, X. Liver Fibrosis Scores and Hospitalization, Mechanical Ventilation, Severity, and Death in Patients with COVID-19: A Systematic Review and Dose-Response Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2022, 2022, 7235860. [Google Scholar] [CrossRef]
- Han, S.; Choi, M.; Lee, B.; Lee, H.W.; Kang, S.H.; Cho, Y.; Ahn, S.B.; Song, D.S.; Jun, D.W.; Lee, J.; et al. Accuracy of Noninvasive Scoring Systems in Assessing Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gut Liver 2022, 16, 952–963. [Google Scholar] [CrossRef]
- Liu, C.H.; Ampuero, J.; Pavlides, M.; Wong, V.W.; Fan, J.G.; Bai, L.; Li, H.; Wu, D.B.; Zhou, L.Y.; Du, L.Y.; et al. Simple non-invasive scoring systems and histological scores in predicting mortality in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1754–1768. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Fagoonee, S.; Pellicano, R.; Abenavoli, L.; Dumitrascu, D.L. Noninvasive biomarkers in predicting nonalcoholic steatohepatitis and assessing liver fibrosis: Systematic review and meta-analysis. Panminerva Med. 2021, 63, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, P.; Hu, L.; Deng, L. Serum levels of SIRT3 and other inflammatory factors are associated with clinical outcomes and prognosis in severe community-acquired pneumonia in adults: A prospective study. Medicine 2021, 100, e26721. [Google Scholar] [CrossRef] [PubMed]
- Vetrici, M.A.; Mokmeli, S.; Bohm, A.R.; Monici, M.; Sigman, S.A. Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial. J. Inflamm. Res. 2021, 14, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Manosuthi, W.; Kaewkungwal, J.; Silachamroon, U.; Mansanguan, C.; Kamolratanakul, S.; Pitisuttithum, P. Etiology, Clinical Course, and Outcomes of Pneumonia in the Elderly: A Retrospective and Prospective Cohort Study in Thailand. Am. J. Trop. Med. Hyg. 2021, 104, 2009–2016. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Ye, L.; Yu, K.; Luo, Z.; Liang, C.; Cao, J.; Wu, X.; Li, S.; Zhu, L.; et al. Predictors of mortality for hospitalized young adults aged less than 60 years old with severe COVID-19: A retrospective study. J. Thorac. Dis. 2021, 13, 3628–3642. [Google Scholar] [CrossRef] [PubMed]
- Lazar Neto, F.; Marino, L.O.; Torres, A.; Cilloniz, C.; Meirelles Marchini, J.F.; Garcia de Alencar, J.C.; Palomeque, A.; Albacar, N.; Brandão Neto, R.A.; Souza, H.P.; et al. Community-acquired pneumonia severity assessment tools in patients hospitalized with COVID-19: A validation and clinical applicability study. Clin. Microbiol. Infect. 2021, 27, 1037.e1031–1037.e1038. [Google Scholar] [CrossRef]
- García Clemente, M.M.; Herrero Huertas, J.; Fernández Fernández, A.; De La Escosura Muñoz, C.; Enríquez Rodríguez, A.I.; Pérez Martínez, L.; Gómez Mañas, S.; Iscar Urrutia, M.; López González, F.J.; Madrid Carbajal, C.J.; et al. Assessment of risk scores in COVID-19. Int. J. Clin. Pract. 2021, 75, e13705. [Google Scholar] [CrossRef]
- Feng, C.M.; Wang, X.M.; Li, M.D.; Xu, Z.; Hua, D.X.; Cheng, J.Y.; Zheng, L.; Zhao, H.; Fu, L. Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: A prospective cohort study. BMC Pulm. Med. 2021, 21, 393. [Google Scholar] [CrossRef]
- Chkhis, A.; Abdulrazzaq, N.; Mokhtar, S.; Jasmi, A.A. Efficacy of High-Dose Nebulized Interferon α 2b in Severe COVID-19 Pneumonia. Turk. Thorac. J. 2021, 22, 199–204. [Google Scholar] [CrossRef]
- Fan, G.; Tu, C.; Zhou, F.; Liu, Z.; Wang, Y.; Song, B.; Gu, X.; Wang, Y.; Wei, Y.; Li, H.; et al. Comparison of severity scores for COVID-19 patients with pneumonia: A retrospective study. Eur. Respir. J. 2020, 56, 2002113. [Google Scholar] [CrossRef]
- Suk, C.W.; Hsu, S.C.; Chen, C.Y.; Hsieh, H.L.; Kuo, H.T.; Hsu, Y.P.; Sue, Y.M.; Chen, T.H.; Lin, F.Y.; Shih, C.M.; et al. Point of Care eGFR and the Prediction of Outcomes in Pneumonia. Sci. Rep. 2019, 9, 8478. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Alharahsheh, B.; Garg, N.; Guha, P. Evaluating risk stratification scoring systems to predict mortality in patients with COVID-19. BMJ Health Care Inform. 2021, 28, e100389. [Google Scholar] [CrossRef] [PubMed]
- Uwaezuoke, S.N.; Ayuk, A.C. Prognostic scores and biomarkers for pediatric community-acquired pneumonia: How far have we come? Pediatr. Health Med. Ther. 2017, 8, 9–18. [Google Scholar] [CrossRef]
- Marti, C.; Garin, N.; Grosgurin, O.; Poncet, A.; Combescure, C.; Carballo, S.; Perrier, A. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit. Care 2012, 16, R141. [Google Scholar] [CrossRef] [PubMed]
- Ewig, S.; Woodhead, M.; Torres, A. Towards a sensible comprehension of severe community-acquired pneumonia. Intensive Care Med. 2011, 37, 214–223. [Google Scholar] [CrossRef]
- Brown, S.M.; Dean, N.C. Defining and predicting severe community-acquired pneumonia. Curr. Opin. Infect. Dis. 2010, 23, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S. Making sense of scoring systems in community acquired pneumonia. Respirology 2009, 14, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cabre, M. Pneumonia in the elderly. Curr. Opin. Pulm. Med. 2009, 15, 223–229. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kitano, Y.; Imai, K.; Kinoshita, S.; Sato, H.; Shiraishi, Y.; Mima, K.; Hayashi, H.; Yamashita, Y.I.; Baba, H. Clinical significance of preoperative inflammation-based score for the prognosis of patients with hepatocellular carcinoma who underwent hepatectomy. Surg. Today 2022, 52, 1008–1015. [Google Scholar] [CrossRef]
- Asakura, R.; Yanagimoto, H.; Ajiki, T.; Tsugawa, D.; Mizumoto, T.; So, S.; Urade, T.; Nanno, Y.; Fukushima, K.; Gon, H.; et al. Prognostic impact of inflammation-based scores for extrahepatic cholangiocarcinoma. Dig. Surg. 2022, 39, 65–74. [Google Scholar] [CrossRef]
- Ulutaş, F.; Çobankara, V.; Karasu, U.; Kaymaz, S.; Albayrak Yaşar, C.; Dündar Ok, Z. Evaluation of the controlling nutritional status score and prognostic nutritional index in patients with familial Mediterranean fever. Eur. J. Rheumatol. 2022, 9, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kojima, I.; Tanaka, S.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kimura, Y.; Ishiyama, D.; Maetani, Y.; Kusumi, H.; Terao, Y.; et al. What is the optimal nutritional assessment tool for predicting decline in the activity of daily living among older patients with heart failure? Heart Vessel. 2022, 37, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, Z.; Chen, L.; Yang, X.; Xu, J.; Cui, H.; Zhu, M. Predictive Value of Geriatric Nutritional Risk Index in Older Adult Cancer Patients. J. Nutr. Health Aging 2022, 26, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, M.; Zhou, X.; Xiang, Y.; Zhang, S.; Feng, W.; Liu, Y. Prognostic Significance of Preoperative Albumin to Alkaline Phosphatase Ratio in Patients with Glioblastoma. J. Cancer 2021, 12, 5950–5959. [Google Scholar] [CrossRef]
- Qu, F.; Li, Z.; Lai, S.; Zhong, X.; Fu, X.; Huang, X.; Li, Q.; Liu, S.; Li, H. Construction and Validation of a Serum Albumin-to-Alkaline Phosphatase Ratio-Based Nomogram for Predicting Pathological Complete Response in Breast Cancer. Front. Oncol. 2021, 11, 681905. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Winther-Larsen, A. Pretreatment Albumin-to-Alkaline Phosphatase Ratio Is a Prognostic Marker in Lung Cancer Patients: A Registry-Based Study of 7077 Lung Cancer Patients. Cancers 2021, 13, 6133. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.W.; Zhu, G.Q.; Li, N.; Qian, X.L.; Chong, H.H.; Yang, C.; Zeng, M.S. A multidimensional nomogram combining imaging features and clinical factors to predict the invasiveness and metastasis of combined hepatocellular cholangiocarcinoma. Ann. Transl. Med. 2021, 9, 1518. [Google Scholar] [CrossRef]
- Wu, J.; You, K.; Jiang, Y.; Shen, T.; Song, J.; Chen, C.; Liu, Y. Prognostic role of pretreatment albumin-to-alkaline phosphatase ratio in locally advanced laryngeal and hypopharyngeal cancer: Retrospective cohort study. J. Cancer 2021, 12, 6182–6188. [Google Scholar] [CrossRef]
- Yoshino, M.; Ishihara, H.; Ishiyama, Y.; Tachibana, H.; Toki, D.; Yamashita, K.; Kobayashi, H.; Fukuda, H.; Yoshida, K.; Takagi, T.; et al. Albumin-to-Alkaline Phosphatase Ratio as a Novel Prognostic Marker of Nivolumab Monotherapy for Previously Treated Metastatic Renal Cell Carcinoma. In Vivo 2021, 35, 2855–2862. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, D.; Li, S.; Zhang, W.; Li, Y.; Wang, X.; Zhou, J.; Wen, Z. Albumin-To-Alkaline Phosphatase Ratio as a Novel and Promising Prognostic Biomarker in Patients Undergoing Esophagectomy for Carcinoma: A Propensity Score Matching Study. Front. Oncol. 2021, 11, 764076. [Google Scholar] [CrossRef]
- Al-Rajhi, N.; Mohammed, S.F.; Khoja, H.A.; Al-Dehaim, M.; Ghebeh, H. Prognostic markers compared to CD3+TIL in locally advanced nasopharyngeal carcinoma. Medicine 2021, 100, e27956. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Peng, N.; Hu, C.; Zhong, L.; Zhong, M.; Zou, Y. The albumin-to-alkaline phosphatase ratio as an independent predictor of future non-alcoholic fatty liver disease in a 5-year longitudinal cohort study of a non-obese Chinese population. Lipids Health Dis. 2021, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, F.; Yang, J.; Xia, T.; Jia, Z.; Shen, J.; Xu, C.; Feng, J.; Lu, Y. Decreased albumin-to-alkaline phosphatase ratio predicted poor survival of resectable gastric cancer patients. J. Gastrointest. Oncol. 2021, 12, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, Y.; Chen, Y.; Zhou, X. Prognostic effect of albumin-to-alkaline phosphatase ratio on patients with hepatocellular carcinoma: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, S.; Lu, L.; Huang, L.; Li, S. Diagnostic value of combined prealbumin-to-fibrinogen and albumin-to-fibrinogen ratios in Hp-negative gastric cancer. Int. J. Biol. Markers 2022, 37, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Z.; Wen, D.; Ma, L.; You, C. Prognostic value of albumin-fibrinogen ratio in subarachnoid hemorrhage patients. Medicine 2021, 100, e25764. [Google Scholar] [CrossRef]
- Liao, Y.C.; Ying, H.Q.; Huang, Y.; Luo, Y.R.; Xiong, C.F.; Nie, R.W.; Li, X.J.; Cheng, X.X. Role of Chronic Inflammatory Ratios in Predicting Recurrence of Resected Patients with Stage I-III Mucinous Colorectal Adenocarcinoma. Cancer Manag. Res. 2021, 13, 3455–3464. [Google Scholar] [CrossRef]
- Liao, Y.C.; Fu, M.; Wang, X.F.; Cheng, X.X. Combined fibrinogen-to-pre-albumin ratio and carbohydrate antigen 19-9 score is a promising metric to predict progression of metastatic colorectal mucinous adenocarcinoma. J. Clin. Lab. Anal. 2021, 35, e23757. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Sun, S.; Lin, B.; Liu, W.; Lai, W.; Wu, Y.; Lin, Z.; Jiang, Y.; Cao, Y.; et al. Novel inflammatory markers in the blood of patients with knee synovitis. J. Int. Med. Res. 2021, 49, 3000605211029557. [Google Scholar] [CrossRef]
- Claps, F.; Rai, S.; Mir, M.C.; van Rhijn, B.W.G.; Mazzon, G.; Davis, L.E.; Valadon, C.L.; Silvestri, T.; Rizzo, M.; Ankem, M.; et al. Prognostic value of preoperative albumin-to-fibrinogen ratio (AFR) in patients with bladder cancer treated with radical cystectomy. Urol. Oncol. 2021, 39, 835.e839–835.e817. [Google Scholar] [CrossRef]
- Cao, F.; Chen, X.; Huang, G.; Liu, W.; Zhou, N.; Yuan, H.; Zou, M. The Albumin-to-Fibrinogen Ratio Independently Predicts Acute Kidney Injury in Infants with Ventricular Septal Defect Undergoing Cardiac Surgery with Cardiopulmonary Bypass. Front. Pediatr. 2021, 9, 682839. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lin, D.; Chen, Q.; Zheng, B.; Liang, M.; Chen, C.; Zheng, W. Prognostic Value of Combined Detection of Preoperative Albumin-to-Fibrinogen Ratio and Neutrophil-to-Lymphocyte Ratio in Operable Esophageal Squamous Cell Carcinoma Patients without Neoadjuvant Therapy. Cancer Manag. Res. 2021, 13, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, S.; Chen, H.; Hu, Z.; Li, S. Diagnostic Values of the Prealbumin-to-Fibrinogen, Albumin-to-Fibrinogen, and Monocyte-to-Lymphocyte Ratios in Gastric Cancer. Ann. Clin. Lab. Sci. 2021, 51, 385–392. [Google Scholar] [PubMed]
- Li, S.; Zhang, D.; Zeng, S.; Wu, T.; Wang, Y.; Zhang, H.; Wang, B.; Hu, X. Prognostic Value of Preoperative Albumin-to-Fibrinogen Ratio in Patients with Bladder Cancer. J. Cancer 2021, 12, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.; Han, P.; Cui, B. Research Progress of Preoperative FPR, FAR or AFR in Patients with Colorectal Cancer. Cancer Manag. Res. 2021, 13, 1791–1801. [Google Scholar] [CrossRef]
- Shang, G.; Fei, Z.; Xu, H.; Wang, Y.; Xiang, S. Globulin and albumin to globulin ratio precisely diagnose periprosthetic joint infection and determine the timing of second-stage reimplantation. J. Orthop. Surg. Res. 2022, 17, 12. [Google Scholar] [CrossRef]
- Xie, H.L.; Zhang, Q.; Ruan, G.T.; Ge, Y.Z.; Hu, C.L.; Song, M.M.; Song, C.H.; Zhang, X.; Zhang, X.W.; Li, X.R.; et al. Evaluation and Validation of the Prognostic Value of Serum Albumin to Globulin Ratio in Patients with Cancer Cachexia: Results From a Large Multicenter Collaboration. Front. Oncol. 2021, 11, 707705. [Google Scholar] [CrossRef]
- Utsumi, M.; Kitada, K.; Tokunaga, N.; Narusaka, T.; Hamano, R.; Miyasou, H.; Tsunemitsu, Y.; Otsuka, S.; Inagaki, M. Preoperative Albumin-to-Globulin Ratio Predicts Prognosis in Hepatocellular Carcinoma: A Cohort Study Including Non-Hepatitis Virus-Infected Patients. Dig. Surg. 2021, 38, 307–315. [Google Scholar] [CrossRef]
- Pai, A.Y.; Sy, J.; Kim, J.; Kleine, C.E.; Edward, J.; Hsiung, J.T.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Association of serum globulin with all-cause mortality in incident hemodialysis patients. Nephrol. Dial. Transplant. 2022, 37, 1993–2003. [Google Scholar] [CrossRef]
- Liu, H.; Tang, K.; Chen, Z.; Li, Z.; Meng, X.; Xia, D. Comparison and development of preoperative systemic inflammation markers-based models for the prediction of unfavorable pathology in newly diagnosed clinical T1 renal cell carcinoma. Pathol. Res. Pract. 2021, 225, 153563. [Google Scholar] [CrossRef]
- Chung, J.W.; Ha, Y.S.; Kim, S.W.; Park, S.C.; Kang, T.W.; Jeong, Y.B.; Park, S.W.; Park, J.; Yoo, E.S.; Kwon, T.G.; et al. The prognostic value of the pretreatment serum albumin to globulin ratio for predicting adverse pathology in patients undergoing radical prostatectomy for prostate cancer. Investig. Clin. Urol. 2021, 62, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Kawai, T.; Nakagawa, T.; Nakamura, Y.; Kamei, J.; Obinata, D.; Yamaguchi, K.; Kaneko, T.; Kakutani, S.; Tokunaga, M.; et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: A multicenter retrospective study. Sci. Rep. 2021, 11, 15623. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, Y.; Kawahara, S.; Kakuta, S.; Onodera, A.; Hara, K.; Kazama, K.; Numata, M.; Aoyama, T.; Tamagawa, A.; Tamagawa, H.; et al. Low Preoperative Albumin-to-Globulin Ratio Is a Marker of Poor Prognosis in Patients with Esophageal Cancer. In Vivo 2021, 35, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.; Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Otsuka, S.; Todaka, A.; Fukutomi, A.; et al. Predictive factors of survival in patients with borderline resectable pancreatic cancer who received neoadjuvant therapy. Pancreatology 2021, 21, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Kong, T.; Zhu, X.; Zhen, Y.; Li, H.; Chen, D.; Yuan, S.; Zhang, D.; Jiao, H.; Li, X.; et al. A Clinical Prognostic Model Based on Preoperative Hematological and Clinical Parameters Predicts the Progression of Primary WHO Grade II Meningioma. Front. Oncol. 2021, 11, 748586. [Google Scholar] [CrossRef]
- Wang, Y.T.; Kuo, L.T.; Lai, C.H.; Tsai, Y.H.; Lee, Y.C.; Hsu, C.M.; Liao, C.T.; Kang, C.J.; Huang, E.I.; Tsai, M.S.; et al. Low Pretreatment Albumin-to-Globulin Ratios Predict Poor Survival Outcomes in Patients with Head and Neck Cancer: A Systematic Review and Meta-analysis. J. Cancer 2023, 14, 281–289. [Google Scholar] [CrossRef]
- Xia, Z.; Fu, X.; Yuan, X.; Li, J.; Wang, H.; Sun, J.; Wu, J.; Tang, L. Serum albumin to globulin ratio prior to treatment as a potential non-invasive prognostic indicator for urological cancers. Front. Nutr. 2022, 9, 1012181. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Mosquera-Rojas, M.D.; Hernandez-Bustamante, E.A.; Alarcón-Braga, E.A.; Herrera-Añazco, P.; Benites-Zapata, V.A. Prognostic value of albumin-to-globulin ratio in COVID-19 patients: A systematic review and meta-analysis. Heliyon 2022, 8, e09457. [Google Scholar] [CrossRef]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Viscuso, P.; Casale, P.; De Berardinis, E.; Di Pierro, G.B.; Cattarino, S.; Giorgino, G.; Rosati, D.; et al. Prognostic Value of Albumin to Globulin Ratio in Non-Metastatic and Metastatic Prostate Cancer Patients: A Meta-Analysis and Systematic Review. Int. J. Mol. Sci. 2022, 23, 11501. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Z.; Wang, G.; Zhou, Y.; Tian, L. Low Pretreatment Albumin-to-Globulin Ratio Predicts Poor Prognosis in Gastric Cancer: Insight From a Meta-Analysis. Front. Oncol. 2020, 10, 623046. [Google Scholar] [CrossRef]
- Lv, G.Y.; An, L.; Sun, X.D.; Hu, Y.L.; Sun, D.W. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: A meta-analysis. Clin. Chim. Acta 2018, 476, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Xie, Q.; Jia, J.; Liu, X.; Sun, J.; Chen, J.; Yi, L. Prognostic Value of Albumin/Globulin Ratio in Survival and Lymph Node Metastasis in Patients with Cancer: A Systematic Review and Meta-analysis. J. Cancer 2018, 9, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pan, H.; Liang, W.; Xiao, D.; Chen, X.; Guo, M.; He, J. Prognostic Effect of Albumin-to-Globulin Ratio in Patients with solid tumors: A Systematic Review and Meta-analysis. J. Cancer 2017, 8, 4002–4010. [Google Scholar] [CrossRef]
- Spencer, E.; Rosengrave, P.; Williman, J.; Shaw, G.; Carr, A.C. Circulating protein carbonyls are specifically elevated in critically ill patients with pneumonia relative to other sources of sepsis. Free Radic. Biol. Med. 2022, 179, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Sacha, G.L.; Chen, A.Y.; Palm, N.M.; Wang, X.; Li, M.; Duggal, A. Clinical Utilization of Stress Dose Hydrocortisone in Adult Patients with Septic Shock: A Retrospective Observational Study at a Large Academic Medical Center. J. Pharm. Pract. 2023, 36, 606–613. [Google Scholar] [CrossRef]
- Laupland, K.B.; Ramanan, M.; Shekar, K.; Kirrane, M.; Clement, P.; Young, P.; Edwards, F.; Bushell, R.; Tabah, A. Is intensive care unit mortality a valid survival outcome measure related to critical illness? Anaesth. Crit. Care Pain Med. 2022, 41, 100996. [Google Scholar] [CrossRef]
- Zhang, A.T.; Tan, S.X.; Pillay, P.S.; Stewart, D. A critical decision point: Short- and long-term outcomes of older surgical patients admitted to a Queensland intensive care unit. Australas J. Ageing 2022, 41, e32–e40. [Google Scholar] [CrossRef]
- Yen, C.L.; Fan, P.C.; Kuo, G.; Lee, C.C.; Chen, J.J.; Lee, T.H.; Tu, Y.R.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Prognostic Performance of Existing Scoring Systems among Critically Ill Patients Requiring Continuous Renal Replacement Therapy: An Observational Study. J. Clin. Med. 2021, 10, 4592. [Google Scholar] [CrossRef]
- Sathe, N.A.; Zelnick, L.R.; Mikacenic, C.; Morrell, E.D.; Bhatraju, P.K.; McNeil, J.B.; Kosamo, S.; Hough, C.L.; Liles, W.C.; Ware, L.B.; et al. Identification of persistent and resolving subphenotypes of acute hypoxemic respiratory failure in two independent cohorts. Crit. Care 2021, 25, 336. [Google Scholar] [CrossRef]
- Salaveria, K.; Smith, S.; Liu, Y.H.; Bagshaw, R.; Ott, M.; Stewart, A.; Law, M.; Carter, A.; Hanson, J. The Applicability of Commonly Used Severity of Illness Scores to Tropical Infections in Australia. Am. J. Trop. Med. Hyg. 2021, 106, 257–267. [Google Scholar] [CrossRef]
- Lecompte-Osorio, P.; Pearson, S.D.; Pieroni, C.H.; Stutz, M.R.; Pohlman, A.S.; Lin, J.; Hall, J.B.; Htwe, Y.M.; Belvitch, P.G.; Dudek, S.M.; et al. Bedside estimates of dead space using end-tidal CO(2) are independently associated with mortality in ARDS. Crit. Care 2021, 25, 333. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wen, Z.; Wang, J.; Zhang, P.; Gong, Q.; Ge, S.; Duan, J. Prediction of Short-Term Mortality with Renal Replacement Therapy in Patients with Cardiac Surgery-Associated Acute Kidney Injury. Front. Cardiovasc. Med. 2021, 8, 738947. [Google Scholar] [CrossRef]
- Birudaraju, D.; Hamal, S.; Tayek, J.A. Solumedrol Treatment for Severe Sepsis in Humans with a Blunted Adrenocorticotropic Hormone-Cortisol Response: A Prospective Randomized Double-Blind Placebo-Controlled Pilot Clinical Trial. J. Intensive Care Med. 2022, 37, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zha, M.L.; Zhang, W.Q.; Hu, S.Q.; Chen, H.L. APACHE scoring system and pressure injury risk for intensive care patients: A systematic review and meta-analysis. Wound Repair Regen. 2022, 30, 498–508. [Google Scholar] [CrossRef]
- Niewiński, G.; Starczewska, M.; Kański, A. Prognostic scoring systems for mortality in intensive care units—The APACHE model. Anaesthesiol. Intensive Ther. 2014, 46, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.E.; Kramer, A.A. Outcome prediction in critical care: The Acute Physiology and Chronic Health Evaluation models. Curr. Opin. Crit. Care 2008, 14, 491–497. [Google Scholar] [CrossRef]
- Sakallaris, B.R.; Jastremski, C.A.; Von Rueden, K.T. Clinical decision support systems for outcome measurement and management. AACN Clin. Issues 2000, 11, 351–362. [Google Scholar] [CrossRef]
- Wong, D.T.; Knaus, W.A. Predicting outcome in critical care: The current status of the APACHE prognostic scoring system. Can. J. Anaesth. 1991, 38, 374–383. [Google Scholar] [CrossRef]
- Sahin, G.K.; Gulen, M.; Acehan, S.; Firat, B.T.; Isikber, C.; Kaya, A.; Segmen, M.S.; Simsek, Y.; Sozutek, A.; Satar, S. Do biomarkers have predictive value in the treatment modality of the patients diagnosed with bowel obstruction? Rev. Assoc. Med. Bras. (1992) 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Peng, X.; Huang, Y.; Fu, H.; Zhang, Z.; He, A.; Luo, R. Prognostic Value of Blood Urea Nitrogen to Serum Albumin Ratio in Intensive Care Unit Patients with Lung Cancer. Int. J. Gen. Med. 2021, 14, 7349–7359. [Google Scholar] [CrossRef]
- Küçükceran, K.; Ayrancı, M.K.; Girişgin, A.S.; Koçak, S.; Dündar, Z.D. The role of the BUN/albumin ratio in predicting mortality in COVID-19 patients in the emergency department. Am. J. Emerg. Med. 2021, 48, 33–37. [Google Scholar] [CrossRef]
- Huang, D.; Yang, H.; Yu, H.; Wang, T.; Chen, Z.; Liang, Z.; Yao, R. Blood Urea Nitrogen to Serum Albumin Ratio (BAR) Predicts Critical Illness in Patients with Coronavirus Disease 2019 (COVID-19). Int. J. Gen. Med. 2021, 14, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Lee, S.H.; Yun, S.J.; Kim, K. Comparison of IVC diameter ratio, BUN/creatinine ratio and BUN/albumin ratio for risk prediction in emergency department patients. Am. J. Emerg. Med. 2021, 47, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Akahane, J.; Ushiki, A.; Kosaka, M.; Ikuyama, Y.; Matsuo, A.; Hachiya, T.; Yoshiike, F.; Koyama, S.; Hanaoka, M. Blood urea nitrogen-to-serum albumin ratio and A-DROP are useful in assessing the severity of Pneumocystis pneumonia in patients without human immunodeficiency virus infection. J. Infect. Chemother. 2021, 27, 707–714. [Google Scholar] [CrossRef]
- He, T.; Li, G.; Xu, S.; Guo, L.; Tang, B. Blood Urea Nitrogen to Serum Albumin Ratio in the Prediction of Acute Kidney Injury of Patients with Rib Fracture in Intensive Care Unit. Int. J. Gen. Med. 2022, 15, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Kim, K.; Yun, S.J.; Lee, S.H. Predictive performance of blood urea nitrogen to serum albumin ratio in elderly patients with gastrointestinal bleeding. Am. J. Emerg. Med. 2021, 41, 152–157. [Google Scholar] [CrossRef]
- Ata, F.; As, A.K.; Engin, M.; Kat, N.K.; Ata, Y.; Turk, T. Can blood urea Nitrogen-to-Albumin ratio predict mortality in patients with moderate-to-severe COVID-19 pneumonia hospitalized in the intensive care unit? Rev. Assoc. Med. Brasileira (1992) 2021, 67, 1421–1426. [Google Scholar] [CrossRef]
- Allameh, F.; Montazeri, S.; Shahabi, V.; Hojjati, S.A.; Alinejad Khorram, A.; Razzaghi, Z.; Dadkhahfar, S. Assessment of the Prognostic Effect of Blood Urea Nitrogen to Serum Albumin Ratio in Patients with Fournier’s Gangrene in a Referral Center. Urol. J. 2021, 19, 325–328. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Al-Kassab-Córdova, A.; Mosquera-Rojas, M.D.; Ulloque-Badaracco, R.R.; Huayta-Cortez, M.A.; Maita-Arauco, S.H.; Herrera-Añazco, P.; Benites-Zapata, V.A. Fibrinogen-to-Albumin Ratio and Blood Urea Nitrogen-to-Albumin Ratio in COVID-19 Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 150. [Google Scholar] [CrossRef]
- Milas, G.P.; Issaris, V.; Papavasileiou, V. Blood urea nitrogen to albumin ratio as a predictive factor for pneumonia: A meta-analysis. Respir. Med. Res. 2022, 81, 100886. [Google Scholar] [CrossRef]
- Özcan, S.; Dönmez, E.; Yavuz Tuğrul, S.; Şahin, İ.; İnce, O.; Ziyrek, M.; Varol, S.; Karahan, S.; Okuyan, E. The Prognostic Value of C-Reactive Protein/Albumin Ratio in Acute Pulmonary Embolism. Rev. Investig. Clin. 2022, 74, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, M.; Sakurai, Y.; Narusaka, T.; Tokunaga, N.; Kitada, K.; Hamano, R.; Tsunemitsu, Y.; Miyasou, H.; Otsuka, S.; Inagaki, M. C-reactive protein to albumin ratio predicts difficult laparoscopic cholecystectomy in patients with acute cholecystitis diagnosed according to the Tokyo Guidelines 2018. Asian J. Endosc. Surg. 2022, 15, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lucijanić, M.; Stojić, J.; Atić, A.; Čikara, T.; Osmani, B.; Barišić-Jaman, M.; Andrilović, A.; Bistrović, P.; Zrilić Vrkljan, A.; Lagančić, M.; et al. Clinical and prognostic significance of C-reactive protein to albumin ratio in hospitalized coronavirus disease 2019 (COVID-19) patients: Data on 2309 patients from a tertiary center and validation in an independent cohort. Wien. Klin. Wochenschr. 2022, 134, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Shi, M.; Song, J.; Niu, X.; Niu, J.; Wei, S.; Li, D.; Bai, Y.; Sun, K. The Prognostic Significance of C-Reactive Protein to Albumin Ratio in Newly Diagnosed Acute Myeloid Leukaemia Patients. Cancer Manag. Res. 2022, 14, 303–316. [Google Scholar] [CrossRef]
- Demirci, G.; Demir, A.R.; Uygur, B.; Bulut, U.; Avci, Y.; Tükenmez Karakurt, S.; Memiç Sancar, K.; Aktemur, T.; Ersoy, B.; Celik, O.; et al. C-reactive protein to albumin ratio provides important long-term prognostic information in patients undergoing endovascular abdominal aortic repair. Vascular 2023, 31, 270–278. [Google Scholar] [CrossRef]
- Ak, R.; Doğanay, F.; Yilmaz, E. Comparison of C-reactive protein and C-reactive protein-to-albumin ratio in predicting mortality among geriatric coronavirus disease 2019 patients. Rev. Assoc. Med. Brasileira (1992) 2022, 68, 82–86. [Google Scholar] [CrossRef]
- Baldemir, R.; Alagoz, A. The Relationship Between Mortality, Nutritional Status, and Laboratory Parameters in Geriatric Chronic Obstructive Pulmonary Disease Patients. Cureus 2021, 13, e20526. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Hua, Q.; Wu, J.; Wang, Y. Diagnostic Value of Systemic Inflammatory Response Index for Catheter-Related Bloodstream Infection in Patients Undergoing Haemodialysis. J. Immunol. Res. 2022, 2022, 7453354. [Google Scholar] [CrossRef]
- Zavalaga-Zegarra, H.J.; Palomino-Gutierrez, J.J.; Ulloque-Badaracco, J.R.; Mosquera-Rojas, M.D.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Hernandez, A.V. C-Reactive Protein-to-Albumin Ratio and Clinical Outcomes in COVID-19 Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 186. [Google Scholar] [CrossRef]
- He, D.; Yang, Y.; Yang, Y.; Tang, X.; Huang, K. Prognostic significance of preoperative C-reactive protein to albumin ratio in non-small cell lung cancer patients: A meta-analysis. Front. Surg. 2022, 9, 1056795. [Google Scholar] [CrossRef]
- Liao, C.K.; Yu, Y.L.; Lin, Y.C.; Hsu, Y.J.; Chern, Y.J.; Chiang, J.M.; You, J.F. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2021, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Fan, Y.; Gao, Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: A meta-analysis. Medicine 2020, 99, e20595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, G.L. C-reactive protein to albumin ratio predicts the outcome in renal cell carcinoma: A meta-analysis. PLoS ONE 2019, 14, e0224266. [Google Scholar] [CrossRef]
- Zhou, Q.P.; Li, X.J. C-Reactive Protein to Albumin Ratio in Colorectal Cancer: A Meta-Analysis of Prognostic Value. Dose Response 2019, 17, 1559325819889814. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhou, Y.; Chen, Q.; Yu, Z.; Gu, H.; Lin, P.; Li, Y.; Liu, C. Prognostic Role of Pretreatment C-Reactive Protein to Albumin Ratio in Urological Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 879803. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lou, Y.; Wang, L. Prognostic value of C-reactive protein to albumin ratio in metastatic colorectal cancer: A systematic review and meta-analysis. Medicine 2021, 100, e27783. [Google Scholar] [CrossRef]
- Luan, C.W.; Yang, H.Y.; Tsai, Y.T.; Hsieh, M.C.; Chou, H.H.; Chen, K.S. Prognostic Value of C-Reactive Protein-to-Albumin Ratio in Head and Neck Cancer: A Meta-Analysis. Diagnostics 2021, 11, 403. [Google Scholar] [CrossRef]
- Lin, N.; Li, J.; Ke, Q.; Wang, L.; Cao, Y.; Liu, J. Clinical Significance of C-Reactive Protein to Albumin Ratio in Patients with Hepatocellular Carcinoma: A Meta-Analysis. Dis. Markers 2020, 2020, 4867974. [Google Scholar] [CrossRef]
- Dalmiglio, C.; Brilli, L.; Campanile, M.; Ciuoli, C.; Cartocci, A.; Castagna, M.G. CONUT Score: A New Tool for Predicting Prognosis in Patients with Advanced Thyroid Cancer Treated with TKI. Cancers 2022, 14, 724. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, J.; Hashimoto, T.; Suhara, M.; Sato, O. Relationship between the Controlling Nutritional Status Score and Infrainguinal Bypass Surgery Outcomes in Patients with Chronic Limb-threatening Ischemia. Ann. Vasc. Dis. 2021, 14, 334–340. [Google Scholar] [CrossRef]
- Ikegami, T.; Nishikawa, H.; Goto, M.; Matsui, M.; Asai, A.; Ushiro, K.; Ogura, T.; Takeuchi, T.; Nakamura, S.; Kakimoto, K.; et al. The Relationship between the SARC-F Score and the Controlling Nutritional Status Score in Gastrointestinal Diseases. J. Clin. Med. 2022, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Fujio, A.; Usuda, M.; Hara, Y.; Kakizaki, Y.; Okada, K.; Miyata, G.; Unno, M.; Kamei, T. Usefulness of Preoperative Controlling Nutritional Status in Predicting Prolonged Hospitalization and Incidence of Postoperative Delirium for Elderly Hepatectomy with Hepatocellular Carcinoma. Tohoku J. Exp. Med. 2022, 256, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wang, M.; Qin, T.; Qin, R. How can we better predict the prognosis of patients with pancreatic cancer undergoing surgery using an immune-nutritional scoring system? Surgery 2022, 172, 291–302. [Google Scholar] [CrossRef]
- Campanelli, M.; Bianciardi, E.; Benavoli, D.; Bagaglini, G.; Lisi, G.; Gentileschi, P. Laparoscopic Banded One Anastomosis Gastric Bypass: A Single-Center Series. J. Obes. 2022, 2022, 4942052. [Google Scholar] [CrossRef]
- Spoletini, G.; Ferri, F.; Mauro, A.; Mennini, G.; Bianco, G.; Cardinale, V.; Agnes, S.; Rossi, M.; Avolio, A.W.; Lai, Q. CONUT Score Predicts Early Morbidity after Liver Transplantation: A Collaborative Study. Front. Nutr. 2021, 8, 793885. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, R.; Sun, X.; Lv, J.; Chen, S. Association of Controlling Nutritional Status Score with Adverse Outcomes in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Angiology 2023, 74, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Lu, C.; Yu, L. Prognostic role of controlling nutritional status score in hematological malignancies. Hematology 2022, 27, 653–658. [Google Scholar] [CrossRef]
- Xue, W.; Hu, X.; Zhang, Y. The Association of Controlling Nutritional Status (CONUT) Score with Survival in Patients with Surgically Treated Renal Cell Carcinoma and Upper Tract Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 1907–1916. [Google Scholar] [CrossRef]
- Peng, L.; Meng, C.; Li, J.; You, C.; Du, Y.; Xiong, W.; Xia, Z.; Cao, D.; Li, Y. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 801–810. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Q.; Fei, L.; Wang, J.; Wang, C.; Yu, L. Prognostic impact of the controlling nutritional status score in patients with hematologic malignancies: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 952802. [Google Scholar] [CrossRef]
- Chen, J.; Song, P.; Peng, Z.; Liu, Z.; Yang, L.; Wang, L.; Zhou, J.; Dong, Q. The Controlling Nutritional Status (CONUT) Score and Prognosis in Malignant Tumors: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 3146–3163. [Google Scholar] [CrossRef] [PubMed]
- Arero, G.; Arero, A.G.; Mohammed, S.H.; Vasheghani-Farahani, A. Prognostic Potential of the Controlling Nutritional Status (CONUT) Score in Predicting All-Cause Mortality and Major Adverse Cardiovascular Events in Patients with Coronary Artery Disease: A Meta-Analysis. Front. Nutr. 2022, 9, 850641. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Du, C.; Meng, C.; Li, J.; You, C.; Li, X.; Zhao, P.; Cao, D.; Li, Y. Controlling Nutritional Status Score Before Receiving Treatment as a Prognostic Indicator for Patients with Urothelial Cancer: An Exploration Evaluation Methods. Front. Oncol. 2021, 11, 702908. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zou, W.; Sun, Y. Prognostic Value of Pretreatment Controlling Nutritional Status Score for Patients with Pancreatic Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 770894. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Luo, J.J.; Baldinger, B. The controlling nutritional status score and clinical outcomes in patients with heart failure: Pool analysis of observational studies. Front. Cardiovasc. Med. 2022, 9, 961141. [Google Scholar] [CrossRef]
- Ma, K.; Li, J.; Shen, G.; Zheng, D.; Xuan, Y.; Lu, Y.; Li, W. Development and Validation of a Risk Nomogram Model for Predicting Contrast-Induced Acute Kidney Injury in Patients with Non-ST-Elevation Acute Coronary Syndrome Undergoing Primary Percutaneous Coronary Intervention. Clin. Interv. Aging 2022, 17, 65–77. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, J.; Han, X. Fibrinogen-to-Albumin Ratio is Associated with All-Cause Mortality in Cancer Patients. Int. J. Gen. Med. 2021, 14, 4867–4875. [Google Scholar] [CrossRef]
- Onder, E.N.A.; Ertan, P. Fibrinogen-to-Albumin Ratio in Familial Mediterranean Fever: Association with Subclinical Inflammation. Klin. Padiatr. 2021, 233, 292–298. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, G.; Hu, F.; Wu, H. Fibrinogen/albumin ratio index is an independent predictor of recurrence-free survival in patients with intrahepatic cholangiocarcinoma following surgical resection. World J. Surg. Oncol. 2021, 19, 218. [Google Scholar] [CrossRef]
- Lin, G.; Hu, M.; Song, J.; Xu, X.; Liu, H.; Qiu, L.; Zhu, H.; Xu, M.; Geng, D.; Yang, L.; et al. High Fibrinogen to Albumin Ratio: A Novel Marker for Risk of Stroke-Associated Pneumonia? Front. Neurol. 2021, 12, 747118. [Google Scholar] [CrossRef]
- Kuyumcu, M.S.; Aydın, O. Fibrinogen-to-albumin ratio may be a predictor for ascending aortic aneurysm. Rev. Assoc. Med. Bras. (1992) 2021, 67, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, C.; Fu, B.; Han, D. Association between fibrinogen-to-albumin ratio and the presence and severity of coronary artery disease in patients with acute coronary syndrome. BMC Cardiovasc. Disord. 2021, 21, 588. [Google Scholar] [CrossRef] [PubMed]
- Çekiç, D.; Emir Arman, M.; Cihad Genç, A.; İşsever, K.; Yıldırım, İ.; Bilal Genç, A.; Dheir, H.; Yaylacı, S. Predictive role of FAR ratio in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14931. [Google Scholar] [CrossRef]
- Afşin, A.; Tibilli, H.; Hoşoğlu, Y.; Asoğlu, R.; Süsenbük, A.; Markirt, S.; Tuna, V.D. Fibrinogen-to-albumin ratio predicts mortality in COVID-19 patients admitted to the intensive care unit. Adv. Respir. Med. 2021, 89, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, W.; Zha, B.; Shi, J.; Wu, G.; Ding, H. Fibrinogen to Albumin Ratio as an Independent Risk Factor for Type 2 Diabetic Kidney Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Deng, H.; Lei, B.; Chen, L.; Zhang, X.; Sha, D. The prognostic value of fibrinogen to albumin ratio in malignant tumor patients: A meta-analysis. Front. Oncol. 2022, 12, 985377. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Huang, Y.T.; Chang, Y.J.; Yu, C.H.; Wang, L.K.; Wu, C.Y.; Liu, P.H.; Chiu, S.F.; Sun, C.K. Association between Fibrinogen-to-Albumin Ratio and Prognosis of Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1678. [Google Scholar] [CrossRef]
- Doi, S.; Migita, K.; Ueno, M.; Yasuda, S.; Aoki, S.; Fujimoto, K.; Ishikawa, H. The Prognostic Significance of the Geriatric Nutritional Risk Index in Colorectal Cancer Patients. Nutr. Cancer 2022, 74, 2838–2845. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, R.; Xu, Q.; Xu, S.; Zuo, S.; Qiu, J.; Zhong, X.; Tan, R.; Liu, Y. High Blood Cu/Zn Ratio is Associated with Nutritional Risk in Patients Undergoing Maintenance Hemodialysis. Biol. Trace Elem. Res. 2022, 200, 4977–4987. [Google Scholar] [CrossRef]
- Nakanishi, N.; Kaikita, K.; Ishii, M.; Kuyama, N.; Tabata, N.; Ito, M.; Yamanaga, K.; Fujisue, K.; Hoshiyama, T.; Kanazawa, H.; et al. Malnutrition-associated high bleeding risk with low thrombogenicity in patients undergoing percutaneous coronary intervention. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1227–1235. [Google Scholar] [CrossRef]
- Akazawa, N.; Kishi, M.; Hino, T.; Tsuji, R.; Tamura, K.; Hioka, A.; Moriyama, H. Relationship between muscle mass and fraction of intramuscular adipose tissue of the quadriceps in older inpatients. PLoS ONE 2022, 17, e0263973. [Google Scholar] [CrossRef] [PubMed]
- Machiba, Y.; Mori, K.; Shoji, T.; Nagata, Y.; Uedono, H.; Nakatani, S.; Ochi, A.; Tsuda, A.; Morioka, T.; Yoshida, H.; et al. Nutritional disorder evaluated by geriatric nutritional risk index predicts death after hospitalization for infection in patients undergoing maintenance hemodialysis. J. Ren. Nutr. 2022, 32, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Uei, H.; Nakanishi, K. Association Among Geriatric Nutritional Risk Index and Functional Prognosis in Elderly Patients with Osteoporotic Vertebral Compression Fractures. Indian J. Orthop. 2022, 56, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T. Is modified creatinine index really superior to geriatric nutritional risk index for predicting malnutrition and clinical outcomes in hemodialysis patients? J. Ren. Nutr. 2022, 32, 772–773. [Google Scholar] [CrossRef]
- Kusahana, E.; Uchiyama, K.; Yamaguchi, N.; Hirashima, M.; Togashi, T.; Yamamoto, Y.; Imai, M.; Ashida, M.; Yamamura, K.; Nakayama, T.; et al. Self-assessment sheet submission rate predicts technique survival in patients initiating peritoneal dialysis. Nephrology 2022, 27, 501–509. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, F. Prognostic impact of geriatric nutritional risk index on patients with urological cancers: A meta-analysis. Front. Oncol. 2022, 12, 1077792. [Google Scholar] [CrossRef]
- Nakagawa, N.; Maruyama, K.; Hasebe, N. Utility of Geriatric Nutritional Risk Index in Patients with Chronic Kidney Disease: A Mini-Review. Nutrients 2021, 13, 3688. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, P.; Li, X.; Luan, S.; Xiao, X.; Gu, Y.; Shang, Q.; Zhang, H.; Yang, Y.; Zeng, X.; et al. Prognostic Value of Geriatric Nutritional Risk Index in Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 831283. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, L.; Tang, P.; Guo, R. Geriatric Nutritional Risk Index and Survival of Patients with Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 906711. [Google Scholar] [CrossRef]
- Shen, F.; Ma, Y.; Guo, W.; Li, F. Prognostic Value of Geriatric Nutritional Risk Index for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung 2022, 200, 661–669. [Google Scholar] [CrossRef]
- Mao, Y.; Lan, J. Prognostic value of the geriatric nutritional index in colorectal cancer patients undergoing surgical intervention: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1066417. [Google Scholar] [CrossRef]
- Lu, W.; Shen, J.; Zou, D.; Li, P.; Liu, X.; Jian, Y. Predictive role of preoperative geriatric nutritional risk index for clinical outcomes in surgical gastric cancer patients: A meta-analysis. Front. Surg. 2022, 9, 1020482. [Google Scholar] [CrossRef]
- Liu, G.; Zou, C.; Jie, Y.; Wang, P.; Wang, X.; Fan, Y. Predictive Value of Geriatric Nutritional Risk Index in Patients with Lower Extremity Peripheral Artery Disease: A Meta-Analysis. Front. Nutr. 2022, 9, 903293. [Google Scholar] [CrossRef]
- He, L.; Li, Y.; Qu, L.; Zhang, F. Prognostic and clinicopathological value of the geriatric nutritional risk index in gastric cancer: A meta-analysis of 5,834 patients. Front. Surg. 2022, 9, 1087298. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Yang, R.; Jin, J.; Liu, D.; Li, W. Prognostic Value of the Geriatric Nutritional Risk Index in Non-Small Cell Lung Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 794862. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Aggerholm-Pedersen, N.; Sandfeld-Paulsen, B. Inflammation-scores as prognostic markers of overall survival in lung cancer: A register-based study of 6,210 Danish lung cancer patients. BMC Cancer 2022, 22, 63. [Google Scholar] [CrossRef]
- Kasahara, K.; Enomoto, M.; Udo, R.; Tago, T.; Mazaki, J.; Ishizaki, T.; Yamada, T.; Nagakawa, Y.; Katsumata, K.; Tsuchida, A. Prognostic value of preoperative high-sensitivity modified Glasgow prognostic score in advanced colon cancer: A retrospective observational study. BMC Cancer 2022, 22, 20. [Google Scholar] [CrossRef]
- Furukawa, K.; Onda, S.; Yanagaki, M.; Taniai, T.; Hamura, R.; Haruki, K.; Shirai, Y.; Tsunematsu, M.; Sakamoto, T.; Ikegami, T. Significance of intra/post-operative prognostic scoring system in hepatectomy for colorectal liver metastases. Ann. Gastroenterol. Surg. 2022, 6, 159–168. [Google Scholar] [CrossRef]
- Sato, R.; Oikawa, M.; Kakita, T.; Okada, T.; Abe, T.; Yazawa, T.; Tsuchiya, H.; Akazawa, N.; Yoshimachi, S.; Okano, H.; et al. Impact of Sarcopenia on Postoperative Complications in Obstructive Colorectal Cancer Patients Who Received Stenting as a Bridge to Curative Surgery. J. Anus Rectum Colon 2022, 6, 40–51. [Google Scholar] [CrossRef]
- Woodley, N.; Rogers, A.D.G.; Turnbull, K.; Slim, M.A.M.; Ton, T.; Montgomery, J.; Douglas, C. Prognostic scores in laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2022, 279, 3705–3715. [Google Scholar] [CrossRef]
- Inokuchi, Y.; Ishida, A.; Hayashi, K.; Kaneta, Y.; Watanabe, H.; Kano, K.; Furuta, M.; Takahashi, K.; Fujikawa, H.; Yamada, T.; et al. Feasibility of gastric endoscopic submucosal dissection in elderly patients aged ≥ 80 years. World J. Gastrointest. Endosc. 2022, 14, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Nishikawa, H.; Goto, M.; Asai, A.; Ushiro, K.; Ogura, T.; Takeuchi, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; et al. Prognostic Impact of the SARC-F Score in Gastrointestinal Advanced Cancers. Cancers 2022, 14, 10. [Google Scholar] [CrossRef]
- Ali, W.A.S.; Hui, P.; Ma, Y.; Wu, Y.; Zhang, Y.; Chen, Y.; Hong, S.; Yang, Y.; Huang, Y.; Zhao, Y.; et al. Determinants of survival in advanced non-small cell lung cancer patients treated with anti-PD-1/PD-L1 therapy. Ann. Transl. Med. 2021, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Tsai, Y.T.; Chen, K.Y.; Yap, W.K.; Luan, C.W. Utility of High-Sensitivity Modified Glasgow Prognostic Score in Cancer Prognosis: A Systemic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Fan, K.; Gao, M.Q.; Pang, B. Prognostic Value of Glasgow Prognostic Score in Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Pathol. Oncol. Res. 2022, 28, 1610109. [Google Scholar] [CrossRef]
- Xu, S.; Song, L.; Liu, X. Prognostic Value of Pretreatment Glasgow Prognostic Score/Modified Glasgow Prognostic Score in Ovarian Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 1968–1975. [Google Scholar] [CrossRef]
- Tong, T.; Guan, Y.; Xiong, H.; Wang, L.; Pang, J. A Meta-Analysis of Glasgow Prognostic Score and Modified Glasgow Prognostic Score as Biomarkers for Predicting Survival Outcome in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 1541. [Google Scholar] [CrossRef]
- Nie, D.; Zhang, L.; Wang, C.; Guo, Q.; Mao, X. A high Glasgow prognostic score (GPS) or modified Glasgow prognostic score (mGPS) predicts poor prognosis in gynecologic cancers: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 301, 1543–1551. [Google Scholar] [CrossRef]
- Dolan, R.D.; McMillan, D.C. The prevalence of cancer associated systemic inflammation: Implications of prognostic studies using the Glasgow Prognostic Score. Crit. Rev. Oncol. Hematol. 2020, 150, 102962. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Yang, W.X.; Dou, W.C.; Shao, Y.X.; Li, X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 6163–6173. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Cai, J.; Chen, L.; Yao, J.; Zhang, Y.; Xu, W.; Geng, L.; Yang, M.; Chen, P.; et al. Prognostic Value of the Glasgow Prognostic Score or Modified Glasgow Prognostic Score for Patients with Colorectal Cancer Receiving Various Treatments: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2018, 51, 1237–1249. [Google Scholar] [CrossRef]
- Yang, C.; Ren, G.; Yang, Q. Prognostic value of preoperative modified Glasgow prognostic score in surgical non-small cell lung cancer: A meta-analysis. Front. Surg. 2022, 9, 1094973. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Xu, Y.; Zheng, Y.; Li, X.; Gao, Y. Pre-treatment Glasgow prognostic score and modified Glasgow prognostic score may be potential prognostic biomarkers in urological cancers: A systematic review and meta-analysis. Ann. Transl. Med. 2019, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zhao, L.; Wang, H.Y.; Zhou, H.N.; Ju, J.; Du, H.; Che, G.W. Gustave Roussy Immune Score based on a three-category risk assessment scale serves as a novel and effective prognostic indicator for surgically resectable early-stage non-small-cell lung cancer: A propensity score matching retrospective cohort study. Int. J. Surg. 2020, 84, 25–40. [Google Scholar] [CrossRef]
- Tian, S.; Cao, Y.; Duan, Y.; Liu, Q.; Peng, P. Gustave Roussy Immune Score as a Novel Prognostic Scoring System for Colorectal Cancer Patients: A Propensity Score Matching Analysis. Front. Oncol. 2021, 11, 737283. [Google Scholar] [CrossRef]
- Feng, J.F.; Wang, L.; Yang, X.; Chen, S. Gustave Roussy Immune Score (GRIm-Score) is a prognostic marker in patients with resectable esophageal squamous cell carcinoma. J. Cancer 2020, 11, 1334–1340. [Google Scholar] [CrossRef]
- SchnÖller, L.; KÄsmann, L.; Taugner, J.; Abdo, R.; Eze, C.; Manapov, F. Prognostic Role of Lung Immune Scores for Prediction of Survival in Limited-stage Small Cell Lung Cancer. In Vivo 2021, 35, 929–935. [Google Scholar] [CrossRef]
- Lenci, E.; Cantini, L.; Pecci, F.; Cognigni, V.; Agostinelli, V.; Mentrasti, G.; Lupi, A.; Ranallo, N.; Paoloni, F.; Rinaldi, S.; et al. The Gustave Roussy Immune (GRIm)-Score Variation Is an Early-on-Treatment Biomarker of Outcome in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Pembrolizumab. J. Clin. Med. 2021, 10, 1005. [Google Scholar] [CrossRef]
- Minami, S.; Ihara, S.; Komuta, K. Gustave Roussy Immune Score and Royal Marsden Hospital Prognostic Score Are Prognostic Markers for Extensive Disease of Small Cell Lung Cancer. World J. Oncol. 2020, 11, 98–105. [Google Scholar] [CrossRef]
- Al Darazi, G.; Martin, E.; Delord, J.P.; Korakis, I.; Betrian, S.; Estrabaut, M.; Poublanc, M.; Gomez-Roca, C.; Filleron, T. Improving patient selection for immuno-oncology phase 1 trials: External validation of six prognostic scores in a French Cancer Center. Int. J. Cancer 2021, 148, 2502–2511. [Google Scholar] [CrossRef]
- Minami, S.; Ihara, S.; Komuta, K. Gustave Roussy Immune Score Is a Prognostic Factor for Chemotherapy-Naive Pulmonary Adenocarcinoma with Wild-Type Epidermal Growth Factor Receptor. World J. Oncol. 2019, 10, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ihara, S.; Ikuta, S.; Komuta, K. Gustave Roussy Immune Score and Royal Marsden Hospital Prognostic Score Are Biomarkers of Immune-Checkpoint Inhibitor for Non-Small Cell Lung Cancer. World J. Oncol. 2019, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Bigot, F.; Castanon, E.; Baldini, C.; Hollebecque, A.; Carmona, A.; Postel-Vinay, S.; Angevin, E.; Armand, J.P.; Ribrag, V.; Aspeslagh, S.; et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 2017, 84, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lin, Y.H.; Lo, W.C.; Cheng, P.C.; Hsu, W.L.; Chen, Y.C.; Shueng, P.W.; Hsieh, C.H.; Liao, L.J. Nutritional status at diagnosis is prognostic for pharyngeal cancer patients: A retrospective study. Eur. Arch. Otorhinolaryngol. 2022, 279, 3671–3678. [Google Scholar] [CrossRef]
- Ogasawara, N.; Kikuchi, D.; Tanaka, M.; Ochiai, Y.; Okamura, T.; Hayasaka, J.; Suzuki, Y.; Mitsunaga, Y.; Nomura, K.; Odagiri, H.; et al. Comprehensive risk evaluation for metachronous carcinogenesis after endoscopic submucosal dissection of superficial pharyngeal squamous cell carcinoma. Esophagus 2022, 19, 460–468. [Google Scholar] [CrossRef]
- Kurashina, R.; Ando, K.; Inoue, M.; Izumi, K.; Maruyama, R.; Mitani, K.; Takenobu, H.; Haruta, M.; Iizuka, T.; Kamijo, T.; et al. Platelet-to-Lymphocyte Ratio Predicts the Efficacy of Pembrolizumab in Patients with Urothelial Carcinoma. Anticancer Res. 2022, 42, 1131–1136. [Google Scholar] [CrossRef]
- Gao, X.; Lin, B.; Lin, Q.; Ye, T.; Zhou, T.; Hu, M.; Zhu, H.; Lu, F.; Chen, W.; Xia, P.; et al. A HALP score-based prediction model for survival of patients with the upper tract urothelial carcinoma undergoing radical nephroureterectomy. Bosn. J. Basic Med. Sci. 2022, 22, 280–290. [Google Scholar] [CrossRef]
- Ekinci, F.; Balcik, O.Y.; Oktay, E.; Erdogan, A.P. HALP Score as a New Prognostic Index in Metastatic Renal Cell Cancer. J. Coll. Physicians Surg. Pak. 2022, 32, 313–318. [Google Scholar] [CrossRef]
- Yalav, O.; Topal, U.; Unal, A.G.; Eray, I.C. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann. Ital. Chir. 2021, 92, 283–292. [Google Scholar]
- Cay, F.; Duran, A. Predictive Factors of Success in Sleeve Gastrectomy: One-Year Follow-up and the Significance of HALP Score. J. Coll. Physicians Surg. Pak. 2021, 31, 1406–1411. [Google Scholar] [CrossRef]
- Dagmura, H.; Daldal, E.; Okan, I. The Efficacy of Hemoglobin, Albumin, Lymphocytes, and Platelets as a Prognostic Marker for Survival in Octogenarians and Nonagenarians Undergoing Colorectal Cancer Surgery. Cancer Biother. Radiopharm. 2022, 37, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Akbas, A.; Koyuncu, S.; Hacım, N.A.; Dasiran, M.F.; Kasap, Z.A.; Okan, I. Can HALP (Hemoglobin, Albumin, Lymphocytes, and Platelets) Score Differentiate Between Malignant and Benign Causes of Acute Mechanic Intestinal Obstruction? Cancer Biother. Radiopharm. 2022, 37, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Chen, J.; Wu, J.; Yang, L.; Guo, X.; Shao, J.; Xu, H.; Shen, A. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann. Transl. Med. 2021, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, X.; Ai, J.; Yang, L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int. Immunopharmacol. 2023, 114, 109496. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.L.; Zhang, F.; Zhao, Y.L.; Zhang, L.J.; Xie, Z.Y.; Huang, K.Z.; Ouyang, X.X.; Wu, X.X.; Xu, X.W.; Li, L.J. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Sci. Rep. 2020, 10, 18128. [Google Scholar] [CrossRef]
- Tayyar, A.T.; Kozalı, S.; Yetkin Yildirim, G.; Karakus, R.; Yuksel, I.T.; Erel, O.; Neselioglu, S.; Eroglu, M. Role of ischemia-modified albumin in the evaluation of oxidative stress in intrahepatic cholestasis of pregnancy. J. Matern. Fetal Neonatal Med. 2019, 32, 3836–3840. [Google Scholar] [CrossRef]
- Hakkoymaz, H.; Nazik, S.; Seyithanoğlu, M.; Güler, Ö.; Şahin, A.R.; Cengiz, E.; Yazar, F.M. The value of ischemia-modified albumin and oxidative stress markers in the diagnosis of acute appendicitis in adults. Am. J. Emerg. Med. 2019, 37, 2097–2101. [Google Scholar] [CrossRef]
- Du, L.; Ma, J.; He, D.; Zhang, X. Serum ischaemia-modified albumin might be a potential biomarker for oxidative stress in amnestic mild cognitive impairment. Psychogeriatrics 2019, 19, 150–156. [Google Scholar] [CrossRef]
- Das, S.; Hussain, M.S.; Maras, J.S.; Kumar, J.; Shasthry, S.M.; Nayak, S.; Arora, V.; Vijayaraghavan, R.; Sharma, S.; Maiwall, R.; et al. Modification Patterns of Urinary Albumin Correlates with Serum Albumin and Outcome in Severe Alcoholic Hepatitis. J. Clin. Gastroenterol. 2019, 53, e243–e252. [Google Scholar] [CrossRef]
- Yavuz, F.; Biyik, M.; Asil, M.; Dertli, R.; Demir, A.; Polat, H.; Uysal, S.; Ataseven, H. Serum ischemic modified albumin (IMA) concentration and IMA/albumin ratio in patients with hepatitis B-related chronic liver diseases. Turk. J. Med. Sci. 2017, 47, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Türkön, H.; Gökmen, F.; Çakir, D.; Sehitoğlu, M.H.; Reşorlu, H.; Döner, D.; Eşsizoğlu, E.; Akbal, A.; Kizilkaya, B.; Uysal, S. Increased Levels of Serum Ischemia Modified Albumin in Patients with Ankylosing Spondylitis. Clin. Lab. 2016, 62, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Inci, A.; Olmaz, R.; Sarı, F.; Coban, M.; Ellidag, H.Y.; Sarıkaya, M. Increased oxidative stress in diabetic nephropathy and its relationship with soluble Klotho levels. Hippokratia 2016, 20, 198–203. [Google Scholar] [PubMed]
- Türedi, S.; Şahin, A.; Akça, M.; Demir, S.; Reis Köse, G.D.; Çekiç, A.B.; Yıldırım, M.; Yuluğ, E.; Menteşe, A.; Türkmen, S.; et al. Ischemia-modified albumin and the IMA/albumin ratio in the diagnosis and staging of hemorrhagic shock: A randomized controlled experimental study. Ulus. Travma Acil Cerrahi Derg. 2020, 26, 153–162. [Google Scholar] [CrossRef]
- Kumar, P.A.; Subramanian, K. The Role of Ischemia Modified Albumin as a Biomarker in Patients with Chronic Liver Disease. J. Clin. Diagn. Res. 2016, 10, Bc09-12. [Google Scholar] [CrossRef]
- Xuan, J.; Peng, J.; Wang, S.; Cai, Y. Prognostic significance of Naples prognostic score in non-small-cell lung cancer patients with brain metastases. Future Oncol. 2022, 18, 1545–1555. [Google Scholar] [CrossRef]
- Pian, G.; Oh, S.Y. Comparison of nutritional and immunological scoring systems predicting prognosis in T1-2N0 colorectal cancer. Int. J. Color. Dis. 2022, 37, 179–188. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, H.; Kang, W.; Liu, H.; Ma, F.; Ma, S.; Li, Y.; Jin, P.; Tian, Y. Prognostic Impact of Preoperative Naples Prognostic Score in Gastric Cancer Patients Undergoing Surgery. Front. Surg. 2021, 8, 617744. [Google Scholar] [CrossRef]
- Lieto, E.; Auricchio, A.; Tirino, G.; Pompella, L.; Panarese, I.; Del Sorbo, G.; Ferraraccio, F.; De Vita, F.; Galizia, G.; Cardella, F. Naples Prognostic Score Predicts Tumor Regression Grade in Resectable Gastric Cancer Treated with Preoperative Chemotherapy. Cancers 2021, 13, 4676. [Google Scholar] [CrossRef]
- Kano, K.; Yamada, T.; Yamamoto, K.; Komori, K.; Watanabe, H.; Takahashi, K.; Fujikawa, H.; Aoyama, T.; Numata, M.; Tamagawa, H.; et al. The Impact of Pretherapeutic Naples Prognostic Score on Survival in Patients with Locally Advanced Esophageal Cancer. Ann. Surg. Oncol. 2021, 28, 4530–4539. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Peng, F.; Wang, X.; Wang, M.; Zhu, F.; Xiong, G.; Qin, R. Prognostic significance of preoperative Naples prognostic score on short- and long-term outcomes after pancreatoduodenectomy for ampullary carcinoma. Hepatobiliary Surg. Nutr. 2021, 10, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Zager, Y.; Mullin, G.; Cohen, M.; Dan, A.; Nevler, A.; Gutman, M.; Horesh, N. Naples Prognostic Score to Predict Postoperative Complications after Colectomy for Diverticulitis. Am. Surg. 2023, 89, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liu, J.; Li, Y.; Li, C.; Liu, Q.; Ji, S.; Zhu, S. Evaluation of Predictive Values of Naples Prognostic Score in Patients with Unresectable Stage III Non-Small Cell Lung Cancer. J. Inflamm. Res. 2021, 14, 6129–6141. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Yagi, K.; Okumura, Y.; Aikou, S.; Yamashita, H.; Seto, Y. Survival Prediction Capabilities of Preoperative Inflammatory and Nutritional Status in Esophageal Squamous Cell Carcinoma Patients. World J. Surg. 2022, 46, 639–647. [Google Scholar] [CrossRef]
- Feng, J.F.; Zhao, J.M.; Chen, S.; Chen, Q.X. Naples Prognostic Score: A Novel Prognostic Score in Predicting Cancer-Specific Survival in Patients with Resected Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 652537. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, J.; Xue, Y.; Zhang, Q.; Xu, Q.; Ji, K.; Yin, R. The Neutrophil-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Heart Failure. J. Inflamm. Res. 2022, 15, 701–713. [Google Scholar] [CrossRef]
- He, H.M.; Zhang, S.C.; He, C.; You, Z.B.; Luo, M.Q.; Lin, M.Q.; Lin, X.Q.; Zhang, L.W.; Lin, K.Y.; Guo, Y.S. Association between neutrophil percentage-to-albumin ratio and contrast-associated acute kidney injury in patients without chronic kidney disease undergoing percutaneous coronary intervention. J. Cardiol. 2022, 79, 257–264. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, D.; Li, Y.; Dai, Z.; Xiang, S.; Chen, Z.; Zhu, W. Neutrophil Albumin Ratio is Associated with All-Cause Mortality in Stroke Patients: A Retrospective Database Study. Int. J. Gen. Med. 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Dai, K.; Li, Z.; Luo, Y.; Xiong, Q.; Xiong, Y.; Song, Z.; Xiong, W. Neutrophil percentage-to-albumin ratio and monocyte-to-lymphocyte ratio as predictors of free-wall rupture in patients with acute myocardial infarction. J. Clin. Lab. Anal. 2022, 36, e24136. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, W.; Pan, X.; Liu, M.; Zhong, Z.; Huang, Q.; Li, T. The relationship between platelet to albumin ratio and disease activity in axial spondyloarthritis patients. Mod. Rheumatol. 2022, 32, 974–979. [Google Scholar] [CrossRef]
- Stefanescu, S.; Cocoș, R.; Turcu-Stiolica, A.; Shelby, E.S.; Matei, M.; Subtirelu, M.S.; Meca, A.D.; Stanciulescu, E.C.; Popescu, S.O.; Biciusca, V.; et al. Prediction of Treatment Outcome with Inflammatory Biomarkers after 2 Months of Therapy in Pulmonary Tuberculosis Patients: Preliminary Results. Pathogens 2021, 10, 789. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wu, S.; Ni, Q.; Chen, P. Association Between the Neutrophil Percentage-to-Albumin Ratio and Outcomes in Cardiac Intensive Care Unit Patients. Int. J. Gen. Med. 2021, 14, 4933–4943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, T.; Tian, X.; Lyu, P.; Wang, J.; Cao, Y. High Neutrophil Percentage-To-Albumin Ratio Can Predict Occurrence of Stroke-Associated Infection. Front. Neurol. 2021, 12, 705790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Zhang, S.; Wang, C.; Zou, C.; Li, A. Neutrophil-to-Albumin Ratio as a Biomarker of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2021, 147, e453–e458. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Zhou, Y.; Liu, M.; Li, S.; Bao, Y.; Jiang, W.; Tang, S.; Li, F.; Xue, H.; et al. A Nomogram for Predicting Acute Respiratory Failure after Cervical Traumatic Spinal Cord Injury Based on Admission Clinical Findings. Neurocrit. Care 2022, 36, 421–433. [Google Scholar] [CrossRef]
- Hou, D.; Wang, C.; Luo, Y.; Ye, X.; Han, X.; Feng, Y.; Zhong, P.; Wu, D. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int. J. Neurosci. 2021, 131, 1203–1208. [Google Scholar] [CrossRef]
- Pang, Q.; Liu, S.; Wang, L.; Pan, H.; Wang, C.; Zhou, L.; Lu, Y.; Liu, H. The Significance of Platelet-Albumin-Bilirubin (PALBI) Grade in Hepatocellular Carcinoma Patients Stratified According to Platelet Count. Cancer Manag. Res. 2020, 12, 12811–12822. [Google Scholar] [CrossRef]
- Wang, Z.X.; Peng, W.; Zhang, X.Y.; Wen, T.F.; Li, C. Prognostic significance of postoperative change of PALBI grade for patients with hepatocellular carcinoma after hepatectomy. Medicine 2021, 100, e24476. [Google Scholar] [CrossRef]
- Huang, Z.; Zuo, M.; Ni, J.; Gu, Y.; Zhang, T.; Jiang, Y.; Zhuo, S.; An, C.; Huang, J. Assessment in the Survival Outcome after Transarterial Chemoembolization Combined with Cryoablation for Hepatocellular Carcinoma (Diameter > 4 cm) Based on the Albumin-Bilirubin Grade and Platelet-Albumin-Bilirubin grade: A Preliminary Study. Cancer Manag. Res. 2020, 12, 1373–1385. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, F.; Huang, K.; Zou, Y.; Liu, Y. Development and Validation of a Nomogram Based on Noninvasive Liver Reserve and Fibrosis (PALBI and FIB-4) Model to Predict Posthepatectomy Liver Failure Grade B-C in Patients with Hepatocellular Carcinoma. J. Oncol. 2021, 2021, 6665267. [Google Scholar] [CrossRef]
- Zhong, B.Y.; Wang, W.S.; Shen, J.; Du, H.; Zhang, S.; Li, W.C.; Yin, Y.; Yang, J.; Ni, C.F.; Zhu, X.L. Single-Centre Retrospective Training Cohort Using Artificial Intelligence for Prognostic Prediction of Encephalopathy, Mortality, and Liver Dysfunction after Early TIPS Creation. Cardiovasc. Intervent. Radiol. 2021, 44, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.Y.; Tang, H.H.; Wang, W.S.; Shen, J.; Zhang, S.; Li, W.C.; Yin, Y.; Yang, J.; Liu, F.; Ni, C.F.; et al. Performance of artificial intelligence for prognostic prediction with the albumin-bilirubin and platelet-albumin-bilirubin for cirrhotic patients with acute variceal bleeding undergoing early transjugular intrahepatic portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 2021, 33, e153–e160. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, C.; Xie, F.; Wang, Z.; Wen, T. Combination of albumin-bilirubin grade and clinically significant portal hypertension predicts the prognosis of patients with hepatocellular carcinoma after liver resection. Biosci. Trends 2021, 15, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yuan, J.; Tang, B.; Zhang, F.; Lu, S.; Chen, R.; Zhang, L.; Ren, Z.; Yin, X. Albumin-bilirubin index and platelet-albumin-bilirubin index contribute to identifying survival benefit candidates in patients with hepatocellular carcinoma and Child-Pugh grade A undergoing transcatheter arterial chemoembolization with sorafenib treatment. Ann. Transl. Med. 2021, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, R.; Zhang, M.; Liu, W.; Li, H.; Li, D. Prognostic Value of Platelet-Albumin-Bilirubin Grade in Child-Pugh A and B Patients with Hepatocellular Carcinoma: A Meta-Analysis. Front. Oncol. 2022, 12, 914997. [Google Scholar] [CrossRef]
- Yılmaz, F.; Keleş, M.; Bora, F. Relationship between the prognostic nutritional index and resistant hypertension in patients with essential hypertension. Clin. Exp. Hypertens. 2022, 44, 326–333. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, C.; Kong, W.; Zhang, Y.; Pan, D. Prognostic nutrition index is associated with the all-cause mortality in sepsis patients: A retrospective cohort study. J. Clin. Lab. Anal. 2022, 36, e24297. [Google Scholar] [CrossRef]
- Kalayci, T.; Kartal, M. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, serum albumin and prognostic nutritional index as predictors of morbidity in super-elderly patients operated on for acute appendicitis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 820–827. [Google Scholar] [CrossRef]
- Isoda, K.; Tsuji, S.; Harada, Y.; Yoshimura, M.; Nakabayashi, A.; Sato, M.; Nagano, H.; Kim, D.S.; Hashimoto, J.; Ohshima, S. Potential of the Prognostic Nutritional Index to Determine the Risk Factor for Severe Infection in Elderly Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2023, 33, 88–95. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Yuan, J.; Wang, C.; An, J.; Ma, X. Correlations of preoperative systematic immuno-inflammatory index and prognostic nutrition index with a prognosis of patients after radical gastric cancer surgery. Surgery 2022, 172, 150–159. [Google Scholar] [CrossRef]
- Sasaki, T.; Nishiwada, S.; Nakagawa, K.; Nagai, M.; Terai, T.; Hokuto, D.; Yasuda, S.; Matsuo, Y.; Doi, S.; Sho, M. Integrative analysis identifies activated anti-tumor immune microenvironment in lung metastasis of pancreatic cancer. Int. J. Clin. Oncol. 2022, 27, 948–957. [Google Scholar] [CrossRef]
- Chen, T.; Liang, G.; Xiang, Z.; He, J.; Xu, X.; Tang, M. Prognostic value of prognostic nutritional index and its variations in advanced non-small-cell lung cancer patients treated with anlotinib monotherapy. J. Clin. Lab. Anal. 2022, 36, e24300. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, H.; Wang, D.; Wang, Y.; Chen, Q.; Chen, S.; Chen, M. Early Warning Models to Predict the 90-Day Urinary Tract Infection Risk after Radical Cystectomy and Urinary Diversion for Patients with Bladder Cancer. Front. Surg. 2021, 8, 782029. [Google Scholar] [CrossRef]
- Qu, Z.; Lu, Y.J.; Feng, J.W.; Chen, Y.X.; Shi, L.Q.; Chen, J.; Rambaran, N.; Duan, Y.F.; He, X.Z. Preoperative Prognostic Nutritional Index and Neutrophil-to-Lymphocyte Ratio Predict Survival Outcomes of Patients with Hepatocellular Carcinoma after Curative Resection. Front. Oncol. 2021, 11, 823054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, Y. Low Prognostic Nutritional Index Predicts Adverse Outcomes in Patients with Heart Failure: A Systematic Review and Meta-analysis. Angiology 2023, 33197231159680. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H. The Prognostic Value of Prognostic Nutritional Index in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2023, 75, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Cao, W.; Gao, X.; Tang, M.; Zhu, D.; Liu, W. Pretreatment “prognostic nutritional index” as an indicator of outcome in lung cancer patients receiving ICI-based treatment: Systematic review and meta-analysis. Medicine 2022, 101, e31113. [Google Scholar] [CrossRef]
- Ren, W.; Wang, H.; Xiang, T.; Liu, G. Prognostic Role of Preoperative Onodera’s Prognostic Nutritional Index (OPNI) in Gastrointestinal Stromal Tumors: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2022. [Google Scholar] [CrossRef]
- Ni, L.; Huang, J.; Ding, J.; Kou, J.; Shao, T.; Li, J.; Gao, L.; Zheng, W.; Wu, Z. Prognostic Nutritional Index Predicts Response and Prognosis in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823087. [Google Scholar] [CrossRef]
- Meng, C.; Gan, L.; Li, K.; Yi, F.; Peng, L.; Li, J.; Li, Y. Prognostic nutritional index before surgical treatment may serve as a prognostic biomarker for patients with upper tract urothelial carcinoma: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 972034. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Z.; Wang, Z.; Wu, C.; Huang, X.; Tian, B. Prognostic role of the prognostic nutritional index in patients with pancreatic cancer who underwent curative resection without preoperative neoadjuvant treatment: A systematic review and meta-analysis. Front. Surg. 2022, 9, 992641. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.I.; Kim, S.J.; Cho, D.S. Prognostic nutritional index as a prognostic factor for renal cell carcinoma: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0271821. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.; Mo, C.; Zhu, P.; Fan, X.; Tang, T. The prognostic significance of prognostic nutritional index in gastrointestinal stromal tumors: A systematic review and meta-analysis. Medicine 2022, 101, e32067. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Chiu, C.C.; Hsu, C.W.; Ho, C.N.; Ko, C.C.; Chen, I.W.; Sun, C.K. Association of preoperative prognostic nutritional index with risk of postoperative delirium: A systematic review and meta-analysis. Front. Med. 2022, 9, 1017000. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kang, W.; Ma, F.; Liu, H.; Ma, S.; Li, Y.; Jin, P.; Hu, H.; Tian, Y. Modified Systemic Inflammation Score Is an Independent Predictor of Long-Term Outcome in Patients Undergoing Surgery for Adenocarcinoma of the Esophagogastric Junction. Front. Surg. 2021, 8, 622821. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Kunisaki, C.; Sato, S.; Tanaka, Y.; Sato, K.; Watanabe, J.; Takeda, K.; Kosaka, T.; Akiyama, H.; Endo, I. Feasibility of esophagectomy for esophageal cancer in elderly patients: A case-control study. Langenbecks Arch. Surg. 2021, 406, 2687–2697. [Google Scholar] [CrossRef]
- Morkavuk, Ş.B.; Çulcu, S.; Esen, E.; Ünal, A.E. The diagnostic value of modified systemic ınflammation score in predicting post-operative outcomes of cutaneous melanoma patients who underwent ısolated limb perfusion. World J. Surg. Oncol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Lin, J.X.; Huang, Y.Q.; Wang, Z.K.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. Prognostic importance of dynamic changes in systemic inflammatory markers for patients with gastric cancer. J. Surg. Oncol. 2021, 124, 282–292. [Google Scholar] [CrossRef]
- Komune, N.; Sato, K.; Hongo, T.; Miyazaki, M.; Masuda, S.; Koike, K.; Uchi, R.; Tsuchihashi, N.A.; Noda, T.; Kogo, R.; et al. Prognostic Significance of Systemic Inflammatory Response in Cases of Temporal Bone Squamous Cell Carcinoma. Laryngoscope 2021, 131, 1782–1789. [Google Scholar] [CrossRef]
- Inokuchi, S.; Itoh, S.; Yoshizumi, T.; Morinaga, A.; Toshima, T.; Takeishi, K.; Nagao, Y.; Harada, N.; Ikegami, T.; Shimokawa, M.; et al. Prognostic significance of systemic inflammation score in patients who undergo hepatic resection for hepatocellular carcinoma. Langenbecks Arch. Surg. 2021, 406, 773–779. [Google Scholar] [CrossRef]
- Inagaki, K.; Kanda, M.; Nakanishi, K.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; et al. Accurate Prediction of Prognosis after Radical Resection of Gastric Cancer by the Modified Systemic Inflammation Score; a Multicenter Dataset Analysis. World J. Surg. 2021, 45, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Ataş, H.; Korukluoğlu, B.; Çomçali, B.; Yakşi, N.; Saylam, B.; Tez, M. Can preoperative modified systemic inflammation score (mSIS) be used to predict malignancy in persistent nondiagnostic thyroid nodules? Turk. J. Med. Sci. 2021, 51, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, Q.; Cai, L.; Sun, X.; Xie, X.; Sun, P. Systemic Inflammatory Score predicts Overall Survival in patients with Cervical Cancer. J. Cancer 2021, 12, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, D.; Baba, Y.; Akiyama, T.; Okadome, K.; Iwatsuki, M.; Iwagami, S.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Adapted systemic inflammation score as a novel prognostic marker for esophageal squamous cell carcinoma patients. Ann. Gastroenterol. Surg. 2021, 5, 669–676. [Google Scholar] [CrossRef]
- Liu, S.; Yu, X.; Ye, F.; Jiang, L. Can the systemic inflammation score be used to predict prognosis in gastric cancer patients undergoing surgery? A systematic review and meta-analysis. Front. Surg. 2022, 9, 971326. [Google Scholar] [CrossRef]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef]
- Klein, S. The myth of serum albumin as a measure of nutritional status. Gastroenterol. 1990, 99, 1845–1846. [Google Scholar] [CrossRef]
- Fleck, A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc. Nutr. Soc. 1989, 48, 347–354. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. NEJM 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Franch-Arcas, G. The meaning of hypoalbuminaemia in clinical practice. Clin. Nutr. 2001, 20, 265–269. [Google Scholar] [CrossRef]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Marcason, W. Should Albumin and Prealbumin Be Used as Indicators for Malnutrition? J. Acad. Nutr. Diet 2017, 117, 1144. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group, A.S.P.E.N. Malnutrition Task Force, A.S.P.E.N. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Acad. Nutr. Diet 2012, 112, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; Committee, A.M. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Lee, J.L.; Oh, E.S.; Lee, R.W.; Finucane, T.E. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: A systematic review. Am. J. Med. 2015, 128, 1023.e1021–1023.e1022. [Google Scholar] [CrossRef]
- Rigaud, D.; Hassid, J.; Meulemans, A.; Poupard, A.T.; Boulier, A. A paradoxical increase in resting energy expenditure in malnourished patients near death: The king penguin syndrome. Am. J. Clin. Nutr. 2000, 72, 355–360. [Google Scholar] [CrossRef]
- Ferrer, R.; Mateu, X.; Maseda, E.; Yebenes, J.C.; Aldecoa, C.; De Haro, C.; Ruiz-Rodriguez, J.C.; Garnacho-Montero, J. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev. Clin. Pharmacol. 2018, 11, 125–137. [Google Scholar] [CrossRef]
- Glassford, N.J.; Bellomo, R. Albumin Administration in Sepsis—The Case For and Against. ICU Manag. Pract. 2017, 17, 36–43. [Google Scholar]
- Kaysen, G.A. Biochemistry and biomarkers of inflamed patients: Why look, what to assess. Clin. J. Am. Soc. Nephrol. 2009, 4 (Suppl. S1), S56–S63. [Google Scholar] [CrossRef]
- Arroyo, V.; Garcia-Martinez, R.; Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 2014, 61, 396–407. [Google Scholar] [CrossRef]
- Magnussen, B.; Gradel, K.O.; Jensen, T.G.; Kolmos, H.J.; Pedersen, C.; Vinholt, P.J.; Lassen, A.T. Association between hypoalbuminaemia and mortality in patients with community-acquired bacteraemia is primarily related to acute disorders. PLoS ONE 2016, 11, e0160466. [Google Scholar] [CrossRef] [PubMed]
- Gradel, K.O.; Vinholt, P.J.; Magnussen, B.; Pedersen, C.; Jensen, T.G.; Kolmos, H.J.; Lassen, A.T. Hypoalbuminaemia as a marker of trans-capillary leakage in community-acquired bacteraemia patients. Epidemiol. Infect. 2018, 146, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Gradel, K.O.; Póvoa, P.; Vinholt, P.J.; Magnussen, B.; Pedersen, C.; Jensen, T.G.; Kolmos, H.J.; Lassen, A.T. Real-life data patterns of C-reactive protein and albumin level trajectories around bacteraemia. Biomark. Med. 2018, 12, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Garvik, O.S.; Vinholt, P.J.; Pedersen, C.; Jensen, T.G.; Kolmos, H.J.; Lassen, A.T.; Gradel, K.O. C-reactive protein and albumin kinetics after antibiotic therapy in community-acquired bloodstream infection. Int. J. Infect. Dis. 2020, 95, 50–58. [Google Scholar] [CrossRef]
- Garvik, O.S.; Póvoa, P.; Magnussen, B.; Vinholt, P.J.; Pedersen, C.; Jensen, T.G.; Kolmos, H.J.; Lassen, A.T.; Gradel, K.O. C-reactive protein and albumin kinetics before community-acquired bloodstream infections—A Danish population-based cohort study. Epidemiol. Infect. 2020, 148, e38. [Google Scholar] [CrossRef]
- Gradel, K.O.; Póvoa, P.; Garvik, O.S.; Vinholt, P.J.; Nielsen, S.L.; Jensen, T.G.; Chen, M.; Dessau, R.B.; Møller, J.K.; Coia, J.E.; et al. Longitudinal trajectory patterns of plasma albumin and C-reactive protein levels around diagnosis, relapse, bacteraemia, and death of acute myeloid leukaemia patients. BMC Cancer 2020, 20, 249. [Google Scholar] [CrossRef]
- Gradel, K.O.; Engberg, H.; Zampieri, F.G.; Póvoa, P.; Simonsen, S.F.; Vinholt, P.J.; Garvik, O.S.; Ljungdalh, P.S.; Frederiksen, H. Contributing factors to the plasma albumin level at diagnosis of hematological malignancy. Hosp. Pract. 2020, 48, 223–229. [Google Scholar] [CrossRef]
- Gradel, K.O.; Larsen, T.S.; Frederiksen, H.; Vinholt, P.J.; Iachina, M.; Póvoa, P.; Zampieri, F.G.; Nielsen, S.L.; Dessau, R.B.; Møller, J.K.; et al. Impact of C-reactive protein and albumin levels on short, medium, and long term mortality in patients with diffuse large B-cell lymphoma. Ann. Med. 2022, 54, 713–722. [Google Scholar] [CrossRef]
- Jafri, S.H.; Shi, R.; Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013, 13, 158. [Google Scholar] [CrossRef]
- Miura, K.; Konishi, J.; Miyake, T.; Makita, M.; Hojo, A.; Masaki, Y.; Uno, M.; Ozaki, J.; Yoshida, C.; Niiya, D.; et al. A host-dependent prognostic model for elderly patients with diffuse large B-cell lymphoma. Oncologist 2017, 22, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, C.; Sun, F.; Gong, W. A New Index AGR-PLR Score (APS) for Patients with Esophageal Squamous Cell Carcinoma Undergoing Radical Esophagectomy. Cancer Manag. Res. 2021, 13, 6129–6139. [Google Scholar] [CrossRef]
- Saltzman, J.R.; Tabak, Y.P.; Hyett, B.H.; Sun, X.; Travis, A.C.; Johannes, R.S. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest. Endosc. 2011, 74, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Dai, Q.; Xu, M.; Xu, X.; Xia, W. Prognostic value of the albumin-globulin ratio and albumin-globulin score in patients with multiple myeloma. J. Int. Med. Res. 2021, 49, 300060521997736. [Google Scholar] [CrossRef]
- Russolillo, N.; Forchino, F.; Conci, S.; Mele, C.; Langella, S.; Ruzzenente, A.; Scoleri, I.; Giuliante, F.; Guglielmi, A.; Ferrero, A. Validation of the albumin-indocyanine green evaluation model in patients with resected hepatocellular carcinoma and comparison with the albumin-bilirubin score. J. Hepatobiliary Pancreat. Sci. 2019, 26, 51–57. [Google Scholar] [CrossRef]
- Tomassini, F.; Giglio, M.C.; De Simone, G.; Montalti, R.; Troisi, R.I. Hepatic function assessment to predict post-hepatectomy liver failure: What can we trust? A systematic review. Updates Surg. 2020, 72, 925–938. [Google Scholar] [CrossRef]
- Afifi, N.; M Medhat, B.; Abdel Ghani, A.M.; Mohamed Ali Hassan, H.G.E.; Behiry, M.E. Value of Albumin-Fibrinogen Ratio and CRP-Albumin Ratio as Predictor Marker of Disease Activity in Egyptian RA Patients, Correlated with Musculoskeletal Sonography. Open Access Rheumatol. 2020, 12, 241–248. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.; Quan, B.; Li, M.; Lu, S.; Li, J.; Chen, R.; Yin, X. The Prognostic Value of the Albumin to Gamma-Glutamyltransferase Ratio in Patients with Hepatocellular Carcinoma Undergoing Radiofrequency Ablation. Dis. Markers 2021, 2021, 3514827. [Google Scholar] [CrossRef]
- Beamer, N.; Coull, B.M.; Sexton, G.; de Garmo, P.; Knox, R.; Seaman, G. Fibrinogen and the albumin-globulin ratio in recurrent stroke. Stroke 1993, 24, 1133–1139. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Osaki, Y.; Oka, H.; Urano, F.; Kudo, M.; Matsunaga, T. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin. Gastroenterol. Hepatol. 2006, 4, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Berhane, S.; Teng, M.; Cox, T.; Tada, T.; Toyoda, H.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Johnson, P.J. Biomarker-based prognosis in hepatocellular carcinoma: Validation and extension of the BALAD model. Br. J. Cancer 2014, 110, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886.e876. [Google Scholar] [CrossRef]
- Xu, H.J.; Ma, Y.; Deng, F.; Ju, W.B.; Sun, X.Y.; Wang, H. The prognostic value of C-reactive protein/albumin ratio in human malignancies: An updated meta-analysis. Onco. Targets Ther. 2017, 10, 3059–3070. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Li, B.; Lin, S.; Liu, B.; Li, Y.; Zhu, Q.; Wu, Y.; Yang, Y.; Tang, S.; Meng, F.; et al. Development and Validation of CAGIB Score for Evaluating the Prognosis of Cirrhosis with Acute Gastrointestinal Bleeding: A Retrospective Multicenter Study. Adv. Ther. 2019, 36, 3211–3220. [Google Scholar] [CrossRef]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Zhao, Z.; Yu, Y.; Xie, R.; Yang, K.; Xu, D.; Li, L.; Lin, J.; Zheng, L.; Zhang, C.; Xu, X.; et al. Prognostic value of the creatinine-albumin ratio in acute pancreatitis debridement. BMC Surg. 2020, 20, 322. [Google Scholar] [CrossRef]
- Zhao, T.J.; Yang, Q.K.; Bi, L.D.; Li, J.; Tan, C.Y.; Miao, Z.L. Prognostic value of DCTA scoring system in heart failure. Herz 2021, 46, 243–252. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, E.Y. Expanded A-DROP Score: A New Scoring System for the Prediction of Mortality in Hospitalized Patients with Community-acquired Pneumonia. Sci. Rep. 2018, 8, 14588. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, K.; Nouso, K.; Hiraoka, A.; Wakuta, A.; Oonishi, A.; Kuzuya, T.; Toyoda, H.; Tada, T.; Tsuji, K.; Itobayashi, E.; et al. EZ-ALBI Score for Predicting Hepatocellular Carcinoma Prognosis. Liver Cancer 2020, 9, 734–743. [Google Scholar] [CrossRef]
- Ozdemir, M.; Yurtdas, M.; Asoglu, R.; Yildirim, T.; Aladag, N.; Asoglu, E. Fibrinogen to albumin ratio as a powerful predictor of the exaggerated morning blood pressure surge in newly diagnosed treatment-naive hypertensive patients. Clin. Exp. Hypertens. 2020, 42, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.; Wu, C.; Wang, W.; Jin, W.; Gao, H.; Li, H.; Zhang, S.; Xu, J.; Zhang, W.; et al. Prognostic value of gamma-glutamyltransferase-to-albumin ratio in patients with pancreatic ductal adenocarcinoma following radical surgery. Cancer Med. 2019, 8, 572–584. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.W.; Benjamin, I.S.; Ferguson, J.C.; McKay, A.J.; Mackenzie, I.; O’Neill, J.; Blumgart, L.H. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br. J. Surg. 1978, 65, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.H.; Imrie, C.W.; Carter, D.C. Biliary surgery in the same admission for gallstone-associated acute pancreatitis. Br. J. Surg. 1981, 68, 758–761. [Google Scholar] [CrossRef]
- Blamey, S.L.; Imrie, C.W.; O’Neill, J.; Gilmour, W.H.; Carter, D.C. Prognostic factors in acute pancreatitis. Gut 1984, 25, 1340–1346. [Google Scholar] [CrossRef]
- Leese, T.; Shaw, D. Comparison of three Glasgow multifactor prognostic scoring systems in acute pancreatitis. Br. J. Surg. 1988, 75, 460–462. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Pinato, D.J.; Stebbing, J.; Ishizuka, M.; Khan, S.A.; Wasan, H.S.; North, B.V.; Kubota, K.; Sharma, R. A novel and validated prognostic index in hepatocellular carcinoma: The inflammation based index (IBI). J. Hepatol. 2012, 57, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.H.; Li, A.J.; Tang, E.J.; Dan, X.; Chen, Y.; Zhang, Y.; Tang, M.; Xiao, Y.H.; Deng, X.X.; Li, H.G.; et al. Prognostic Value of the Combination of Preoperative Hemoglobin, Lymphocyte, Albumin, and Neutrophil in Patients with Locally Advanced Colorectal Cancer. Med. Sci. Monit. 2016, 22, 4986–4991. [Google Scholar] [CrossRef]
- Tomita, M.; Ayabe, T.; Maeda, R.; Nakamura, K. The Inflammatory Prognostic Index Predicts Cancer-Specific Outcomes of Patients with Resected Non-Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 2867–2870. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.L.; Feng, R.; Li, J.T.; Tian, Y.; Wang, T. Validation and refinement of the Age, Comorbidities, and Albumin Index in elderly patients with diffuse large B-cell lymphoma: An effective tool for comprehensive geriatric assessment. Oncologist 2018, 23, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ding, S.; Luo, H.; Chai, X. International Normalized Ratio to Albumin Ratio (PTAR): An Objective Risk Stratification Tool in Patients with Sepsis. Int. J. Gen. Med. 2021, 14, 1829–1841. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. NEJM 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Foucan, L.; Merault, H.; Velayoudom-Cephise, F.L.; Larifla, L.; Alecu, C.; Ducros, J. Impact of protein energy wasting status on survival among Afro-Caribbean hemodialysis patients: A 3-year prospective study. Springerplus 2015, 4, 452. [Google Scholar] [CrossRef][Green Version]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Nakazawa, N.; Sohda, M.; Yamaguchi, A.; Watanabe, T.; Saito, H.; Ubukata, Y.; Kuriyama, K.; Sano, A.; Sakai, M.; Yokobori, T.; et al. An Elevated Serum Lactate Dehydrogenase-to-albumin Ratio Is a Useful Poor Prognostic Predictor of Nivolumab in Patients with Gastric Cancer. Anticancer Res. 2021, 41, 3925–3931. [Google Scholar] [CrossRef]
- Kokulu, K.; Sert, E.T. The role of the lactate/albumin ratio in predicting survival outcomes in patients resuscitated after out-of-hospital cardiac arrest: A preliminary report. Am. J. Emerg. Med. 2021, 50, 670–674. [Google Scholar] [CrossRef]
- Liang, X.; Yao, S.; Lu, P.; Ma, Y.; Xu, H.; Yin, Z.; Hu, J.; Liu, Y.; Wei, S. The Prognostic Value of New Index (LANR) Composed of Pre-operative Lymphocytes, Albumin, and Neutrophils in Patients with Resectable Colorectal Cancer. Front. Oncol. 2021, 11, 610264. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Lebenthal, J.M.; Zheng, J.; Glare, P.A.; O’Reilly, E.M.; Yang, A.C.; Epstein, A.S. Prognostic value of the Memorial Sloan Kettering Prognostic Score in metastatic pancreatic adenocarcinoma. Cancer 2021, 127, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, is an Independent Predictor of Long-term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis. Colon Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Cui, H.; Ding, X.; Li, W.; Chen, H.; Li, H. The Neutrophil Percentage to Albumin Ratio as a New Predictor of In-Hospital Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Med. Sci. Monit. 2019, 25, 7845–7852. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Song, S.; He, X.; Shi, X.; Li, Z.; Song, J. NARFIB: A Novel Prognostic Score Based on the Neutrophil-to-Albumin Ratio and Fibrinogen Can Predict the Prognosis of Gastrointestinal Stromal Tumors. Cancer Manag. Res. 2020, 12, 11183–11190. [Google Scholar] [CrossRef]
- Bauer, H.C.; Wedin, R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop. Scand. 1995, 66, 143–146. [Google Scholar] [CrossRef]
- Aoki, T.; Nagata, N.; Shimbo, T.; Niikura, R.; Sakurai, T.; Moriyasu, S.; Okubo, H.; Sekine, K.; Watanabe, K.; Yokoi, C.; et al. Development and Validation of a Risk Scoring System for Severe Acute Lower Gastrointestinal Bleeding. Clin. Gastroenterol. Hepatolog. 2016, 14, 1562–1570.e1562. [Google Scholar] [CrossRef]
- Alexandre, J.; Gross-Goupil, M.; Falissard, B.; Nguyen, M.L.; Gornet, J.M.; Misset, J.L.; Goldwasser, F. Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann. Oncol. 2003, 14, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Jin, C.; Zheng, H.M.; Zhou, K.; Shi, B.B.; Zhang, Q.; Zheng, F.Y.; Lin, F. A novel prognostic inflammation score predicts outcomes in patients with ovarian cancer. Clin. Chim. Acta 2016, 456, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.D.; Vieira de Melo, C.Y.; Amorim, A.C.; Cipriano Torres Dde, O.; Dos Santos, A.C. Association Between Nutritional Status, Inflammatory Condition, and Prognostic Indexes with Postoperative Complications and Clinical Outcome of Patients with Gastrointestinal Neoplasia. Nutr. Cancer 2016, 68, 1108–1114. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.Y.; You, J.; Hu, W.Q.; Wang, S.F.; Chen, P.; Yang, F.; Shi, L.; Zhao, W.; Zong, L. Onodera’s Prognostic Nutritional Index is a novel and useful prognostic marker for gastrointestinal stromal tumors. World J. Gastrointest. Surg. 2021, 13, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar] [PubMed]
- Ni, Q.; Wang, X.; Wang, J.; Chen, P. The red blood cell distribution width-albumin ratio: A promising predictor of mortality in heart failure patients—A cohort study. Clin. Chim. Acta 2022, 527, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.G.; Wolfe, R.; Whitby, M.; Fine, M.J.; Fuller, A.J.; Stirling, R.; Wright, A.A.; Ramirez, J.A.; Christiansen, K.J.; Waterer, G.W.; et al. SMART-COP: A tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin. Infect. Dis. 2008, 47, 375–384. [Google Scholar] [CrossRef]
- Chang, Y.; An, H.; Xu, L.; Zhu, Y.; Yang, Y.; Lin, Z.; Xu, J. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br. J. Cancer 2015, 113, 626–633. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Hussein, M.A.; Barlogie, B.; Durie, B.G.; Crowley, J.J. A new staging system for multiple myeloma patients based on the Southwest Oncology Group (SWOG) experience. Br. J. Haematol. 2003, 122, 441–450. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, N.; Wang, K.; Hu, Q.; Sun, S.; Xu, Z.; Yu, J.; Wang, C.; Chen, S.; Xu, B.; et al. Combination of Albumin-Globulin Score and Sarcopenia to Predict Prognosis in Patients with Renal Cell Carcinoma Undergoing Laparoscopic Nephrectomy. Front. Nutr. 2021, 8, 731466. [Google Scholar] [CrossRef]
- Seong, M.K. Prognostic Inflammation Score in Surgical Patients with Colorectal Cancer. J. Korean Med. Sci. 2015, 30, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Lin, J.P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.; Zheng, C.H.; et al. Prognostic importance of the preoperative modified systemic inflammation score for patients with gastric cancer. Gastric Cancer 2019, 22, 403–412. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).