Psychosocial Consequences of Hand Eczema—A Prospective Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Group
2.2. Disease Severity Assessment
2.3. Itch and Pain Assessment
2.4. QoL Assessment
2.5. Depression and Anxiety Assessment
2.6. Statistical Analysis
3. Results
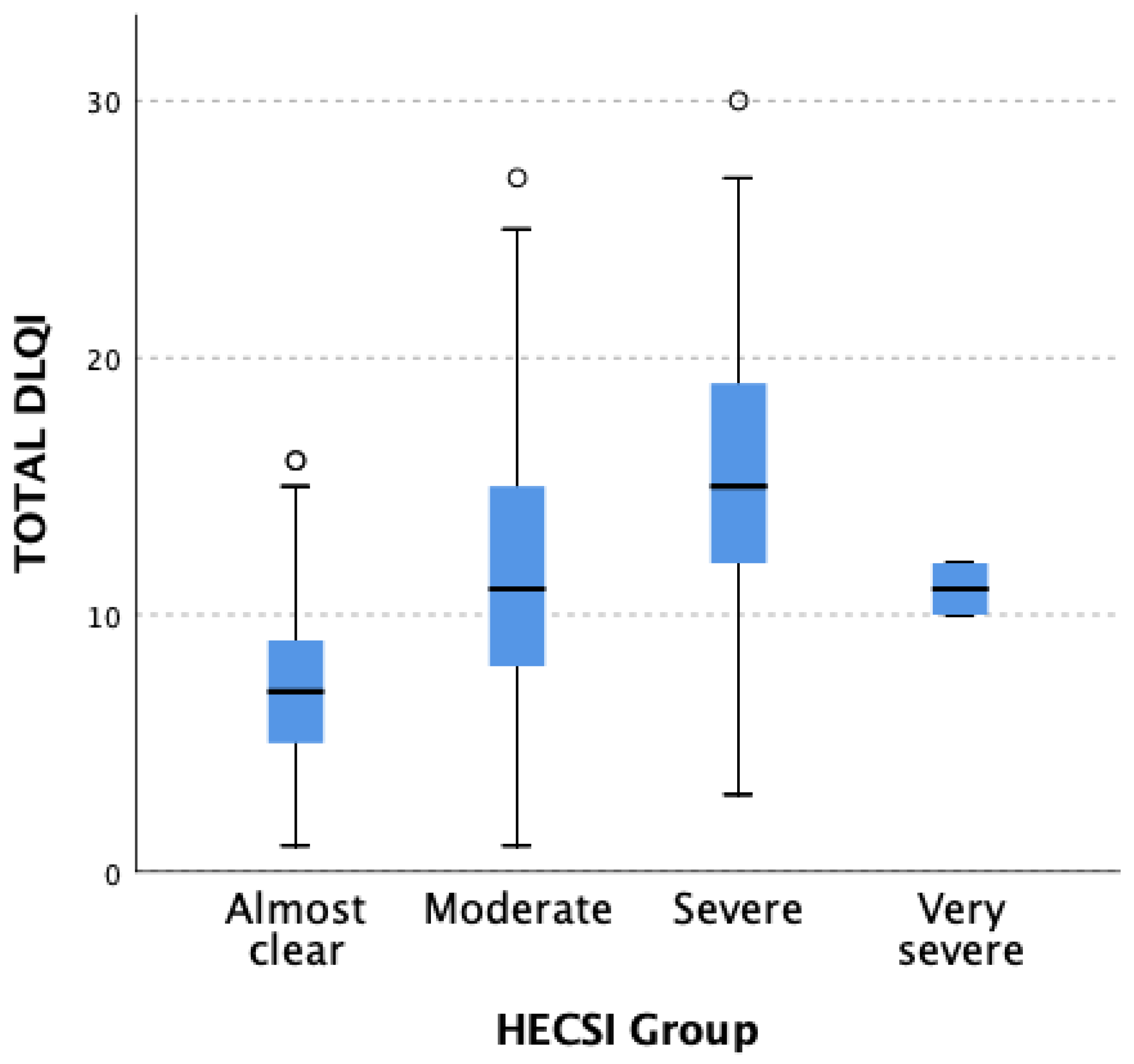
3.1. Disease Severity Assessment
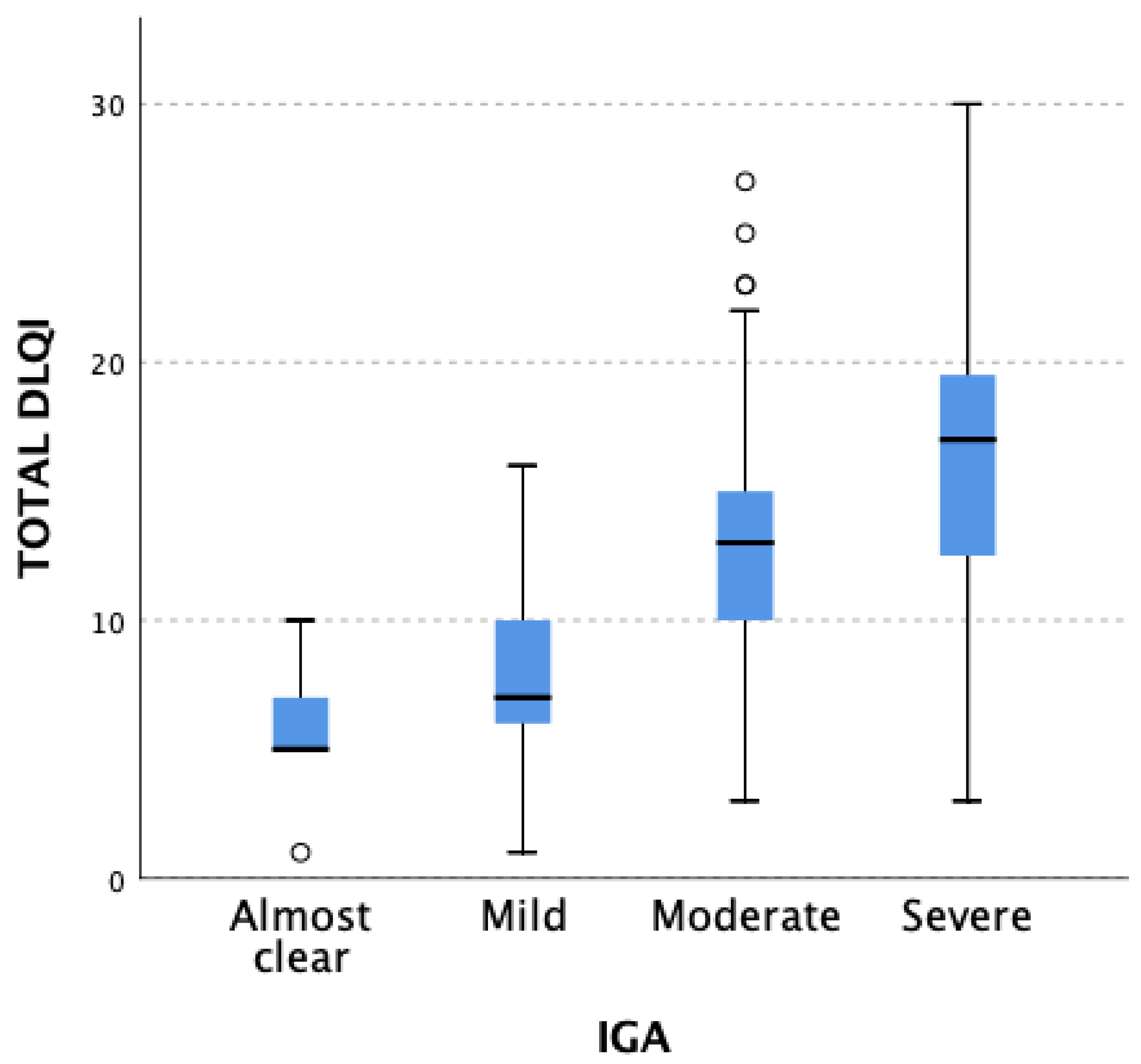
3.2. QoL Assessment
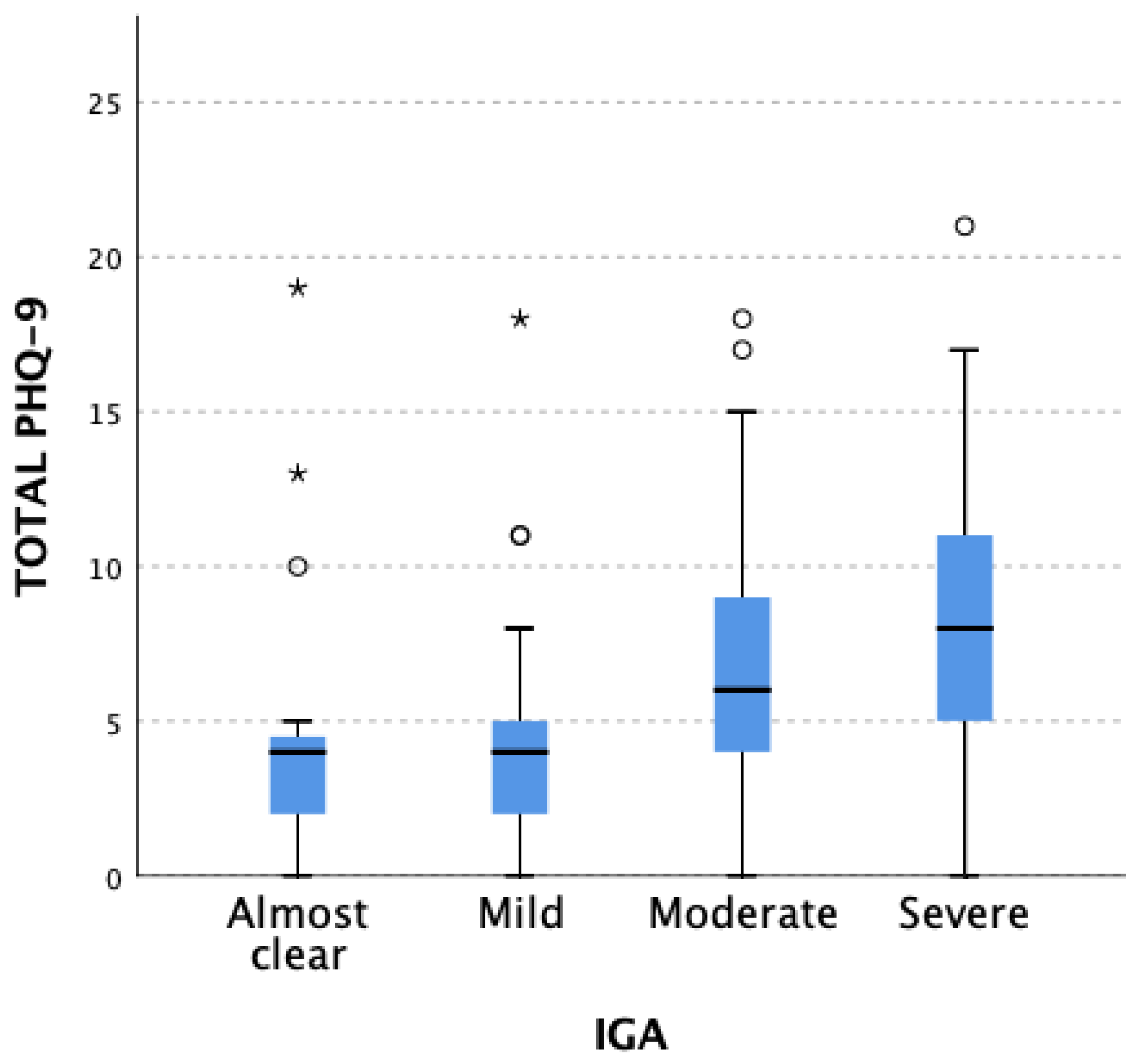
3.3. Depression Assessment (PHQ-9 and HADS-M (D))
3.3.1. PHQ-9
3.3.2. HADS-M: Depression (D)
3.4. Anxiety Assessment (GAD-7 and HADS-M (A))
3.4.1. GAD-7
3.4.2. HADS-M: Anxiety (A)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsner, P.; Agner, T. Hand Eczema: Treatment. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. S1), 13–21. [Google Scholar] [CrossRef]
- Zalewski, A.; Krajewski, P.K.; Szepietowski, J.C. Prevalence and Characteristics of Itch and Pain in Patients Suffering from Chronic Hand Eczema. J. Clin. Med. 2023, 12, 4198. [Google Scholar] [CrossRef]
- Zalewski, A.; Szepietowski, J.C. Topical and Systemic JAK Inhibitors in Hand Eczema—A Narrative Review. Expert Rev. Clin. Immunol. 2023, 19, 365–373. [Google Scholar] [CrossRef]
- Maden, S.; Ozbagcivan, O.; Onur Aysevener, B.E.; Aktan, S. Quality of Life, Anxiety, Depression, Social Anxiety and Avoidance in Patients with Chronic Hand Eczema. Ital. J. Dermatol. Venerol. 2021, 156, 562–569. [Google Scholar] [CrossRef]
- Ahmed, A.; Shah, R.; Papadopoulos, L.; Bewley, A. An Ethnographic Study into the Psychological Impact and Adaptive Mechanisms of Living with Hand Eczema. Clin. Exp. Dermatol. 2015, 40, 495–501. [Google Scholar] [CrossRef]
- Marron, S.E.; Tomas-Aragones, L.; Navarro-Lopez, J.; Gieler, U.; Kupfer, J.; Dalgard, F.J.; Lien, L.; Finlay, A.Y.; Poot, F.; Linder, D.; et al. The Psychosocial Burden of Hand Eczema: Data from a European Dermatological Multicentre Study. Contact Dermat. 2018, 78, 406–412. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Ballmer-Weber, B.K.; Gräni, N.; Spring, P.; Bircher, A.; Anliker, M.; Sonntag, A.K.; Piletta, P.; Huber, C.; Borradori, L.; et al. Medical, Psychological and Socio-Economic Implications of Chronic Hand Eczema: A Cross-Sectional Study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 628–637. [Google Scholar] [CrossRef]
- Ergün, M.; Türel Ermertcan, A.; Oztürkcan, S.; Temeltaş, G.; Deveci, A.; Dinç, G. Sexual Dysfunction in Patients with Chronic Hand Eczema in the Turkish Population. J. Sex. Med. 2007, 4, 1684–1690. [Google Scholar] [CrossRef]
- Ruzicka, T.; Lynde, C.W.; Jemec, G.B.E.; Diepgen, T.; Berth-Jones, J.; Coenraads, P.J.; Kaszuba, A.; Bissonnette, R.; Varjonen, E.; Holló, P.; et al. Efficacy and Safety of Oral Alitretinoin (9-Cis Retinoic Acid) in Patients with Severe Chronic Hand Eczema Refractory to Topical Corticosteroids: Results of a Randomized, Double-Blind, Placebo-Controlled, Multicentre Trial. Br. J. Dermatol. 2008, 158, 808–817. [Google Scholar] [CrossRef]
- Held, E.; Skoet, R.; Johansen, J.D.; Agner, T. The Hand Eczema Severity Index (HECSI): A Scoring System for Clinical Assessment of Hand Eczema. A Study of Inter- and Intraobserver Reliability. Br. J. Dermatol. 2005, 152, 302–307. [Google Scholar] [CrossRef]
- Oosterhaven, J.A.F.; Schuttelaar, M.L.A. Responsiveness and Interpretability of the Hand Eczema Severity Index. Br. J. Dermatol. 2020, 182, 932–939. [Google Scholar] [CrossRef]
- Cheung, H.N.; Chan, Y.S.; Hsiung, N.H. Validation of the 5-D Itch Scale in Three Ethnic Groups and Exploring Optimal Cutoff Values Using the Itch Numerical Rating Scale. BioMed Res. Int. 2021, 2021, 7640314. [Google Scholar] [CrossRef]
- Chien, C.-W.; Bagraith, K.S.; Khan, A.; Deen, M.; Syu, J.-J.; Strong, J. Establishment of Cutpoints to Categorize the Severity of Chronic Pain Using Composite Ratings with Rasch Analysis. Eur. J. Pain 2017, 21, 82–91. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Salomon, J.; Finlay, A.Y. Dermatology Life Quality Index (DLQI): Polish Version. In Proceedings of the 11th International Congress European Society for Dermatology and Psychiatry, Giessen, Germany, 5–7 May 2005; p. 42. [Google Scholar]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)--a Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Barrett, A.; Hahn-Pedersen, J.; Kragh, N.; Evans, E.; Gnanasakthy, A. Patient-Reported Outcome Measures in Atopic Dermatitis and Chronic Hand Eczema in Adults. Patient 2019, 12, 445–459. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Levis, B.; Benedetti, A.; Thombs, B.D.; DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for Screening to Detect Major Depression: Individual Participant Data Meta-Analysis. BMJ 2019, 365, l1476. [Google Scholar] [CrossRef]
- Dajpratham, P.; Pukrittayakamee, P.; Atsariyasing, W.; Wannarit, K.; Boonhong, J.; Pongpirul, K. The Validity and Reliability of the PHQ-9 in Screening for Post-Stroke Depression. BMC Psychiatry 2020, 20, 291. [Google Scholar] [CrossRef]
- Plummer, F.; Manea, L.; Trepel, D.; McMillan, D. Screening for Anxiety Disorders with the GAD-7 and GAD-2: A Systematic Review and Diagnostic Metaanalysis. Gen. Hosp. Psychiatry 2016, 39, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, A.; Hüsing, P.; Gumz, A.; Wingenfeld, K.; Härter, M.; Schramm, E.; Löwe, B. Sensitivity to Change and Minimal Clinically Important Difference of the 7-Item Generalized Anxiety Disorder Questionnaire (GAD-7). J. Affect. Disord. 2020, 265, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Monahan, P.O.; Löwe, B. Anxiety Disorders in Primary Care: Prevalence, Impairment, Comorbidity, and Detection. Ann. Intern. Med. 2007, 146, 317–325. [Google Scholar] [CrossRef]
- Dziedzic, B.; Sarwa, P.; Kobos, E.; Sienkiewicz, Z.; Idzik, A.; Wysokiński, M.; Fidecki, W. Loneliness and Depression among Polish High-School Students. Int. J. Environ. Res. Public Health 2021, 18, 1706. [Google Scholar] [CrossRef]
- Majkowicz, M. Praktyczna ocena efektywności opieki paliatywnej—Wybrane techniki badawcze. In Ocena Jakości Opierki Paliatywnej w Teori i Praktyce; De Walden-Gałuszko, K., Majkowicz, M., Eds.; Akademia Medyczna Gdańsk: Gdańsk, Poland, 2000; pp. 21–42. [Google Scholar]
- Bingefors, K.; Lindberg, M.; Isacson, D. Quality of Life, Use of Topical Medications and Socio-Economic Data in Hand Eczema: A Swedish Nationwide Survey. Acta Derm. Venereol. 2011, 91, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.; Bingefors, K.; Meding, B.; Berg, M. Hand Eczema and Health-Related Quality of Life; a Comparison of EQ-5D and the Dermatology Life Quality Index (DLQI) in Relation to the Hand Eczema Extent Score (HEES). Contact Dermat. 2013, 69, 138–143. [Google Scholar] [CrossRef]
- Mollerup, A.; Veien, N.K.; Johansen, J.D. An Analysis of Gender Differences in Patients with Hand Eczema - Everyday Exposures, Severity, and Consequences. Contact Dermat. 2014, 71, 21–30. [Google Scholar] [CrossRef]
- Apfelbacher, C.; Molin, S.; Weisshaar, E.; Bauer, A.; Elsner, P.; Mahler, V.; Weiss, M.; Ruzicka, T.; Diepgen, T.L. Characteristics and Provision of Care in Patients with Chronic Hand Eczema: Updated Data from the CARPE Registry. Acta Derm. Venereol. 2014, 94, 163–167. [Google Scholar] [CrossRef]
- Cortesi, P.A.; Scalone, L.; Belisari, A.; Bonamonte, D.; Cannavò, S.P.; Cristaudo, A.; De Pità, O.; Gallo, R.; Giannetti, A.; Gola, M.; et al. Cost and Quality of Life in Patients with Severe Chronic Hand Eczema Refractory to Standard Therapy with Topical Potent Corticosteroids. Contact Dermat. 2014, 70, 158–168. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Chen, Y.; Zhang, C.; Chen, L.; Lu, Y.; Li, L.; Shi, W. Comparison of Five Clinical Scores for Assessment of Chronic Hand Eczema. J. Dermatol. 2019, 46, e433–e434. [Google Scholar] [CrossRef]
- Ruppert, L.; Apfelbacher, C.; Molin, S.; Bauer, A.; Mahler, V.; Schmitt, J.; Elsner, P.; Diepgen, T.L.; Weisshaar, E. Itching in Patients with Chronic Hand Eczema: Data from the CARPE Registry. Dermatology 2014, 229, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Torisu-Itakura, H.; Anderson, P.; Piercy, J.; Pike, J.; Sakamoto, A.; Kabashima, K. Impact of Itch and Skin Pain on Quality of Life in Adult Patients with Atopic Dermatitis in Japan: Results from a Real-World, Point-in-Time, Survey of Physicians and Patients. Curr. Med. Res. Opin. 2022, 38, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Moberg, C.; Alderling, M.; Meding, B. Hand Eczema and Quality of Life: A Population-Based Study. Br. J. Dermatol. 2009, 161, 397–403. [Google Scholar] [CrossRef]
- Gieler, U.; Gieler, T.; Peters, E.M.J.; Linder, D. Skin and Psychosomatics - Psychodermatology Today. J. Dtsch. Dermatol. Ges. 2020, 18, 1280–1298. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.E.; Krajewski, P.K.; Szczęch, J.; Szepietowski, J.C. Depression and Anxiety in Hidradenitis Suppurativa Patients: A Cross-Sectional Study among Polish Patients. Postep. Dermatol. Alergol. 2023, 40, 35–39. [Google Scholar] [CrossRef]
- Fabrazzo, M.; Cipolla, S.; Signoriello, S.; Camerlengo, A.; Calabrese, G.; Giordano, G.M.; Argenziano, G.; Galderisi, S. A Systematic Review on Shared Biological Mechanisms of Depression and Anxiety in Comorbidity with Psoriasis, Atopic Dermatitis, and Hidradenitis Suppurativa. Eur. Psychiatry 2021, 64, e71. [Google Scholar] [CrossRef] [PubMed]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Farzanfar, D.; Dowlati, Y.; French, L.E.; Lowes, M.A.; Alavi, A. Inflammation: A Contributor to Depressive Comorbidity in Inflammatory Skin Disease. Skin Pharmacol. Physiol. 2018, 31, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Kappelmann, N.; Ye, Z.; Lamers, F.; Moser, S.; Jones, P.B.; Burgess, S.; Penninx, B.W.J.H.; Khandaker, G.M. Association of Inflammation with Depression and Anxiety: Evidence for Symptom-Specificity and Potential Causality from UK Biobank and NESDA Cohorts. Mol. Psychiatry 2021, 26, 7393–7402. [Google Scholar] [CrossRef]
- Thorlacius, L.; Cohen, A.D.; Gislason, G.H.; Jemec, G.B.E.; Egeberg, A. Increased Suicide Risk in Patients with Hidradenitis Suppurativa. J. Invest. Dermatol. 2018, 138, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Johanne Peters, E.M.; Sabat, R.; Sterry, W.; Schneider-Burrus, S. Depression Is a Frequent Co-Morbidity in Patients with Acne Inversa. J. Dtsch. Dermatol. Ges. 2013, 11, 743–749. [Google Scholar] [CrossRef]
- Zachariae, R.; Zachariae, H.; Blomqvist, K.; Davidsson, S.; Molin, L.; Mørk, C.; Sigurgeirsson, B. Quality of Life in 6497 Nordic Patients with Psoriasis. Br. J. Dermatol. 2002, 146, 1006–1016. [Google Scholar] [CrossRef]
- Kouris, A.; Christodoulou, C.; Stefanaki, C.; Livaditis, M.; Tsatovidou, R.; Kouskoukis, C.; Petridis, A.; Kontochristopoulos, G. Quality of Life and Psychosocial Aspects in Greek Patients with Psoriasis: A Cross-Sectional Study. An. Bras. Dermatol. 2015, 90, 841–845. [Google Scholar] [CrossRef]
- Remröd, C.; Sjöström, K.; Svensson, Å. Subjective Stress Reactivity in Psoriasis - a Cross Sectional Study of Associated Psychological Traits. BMC Dermatol. 2015, 15, 6. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A.; Nedoszytko, B.; Żmijewski, M.A.; Cubała, W.J.; Slominski, A.T. The Brain-Skin Axis in Psoriasis-Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int. J. Mol. Sci. 2022, 23, 669. [Google Scholar] [CrossRef]
- Pollo, C.F.; Miot, H.A.; Matos, T.D.d.S.; de Souza, J.M.; Jorge, M.F.S.; Miot, L.D.B.; Meneguin, S. Prevalence and Factors Associated with Depression and Anxiety in Patients with Psoriasis. J. Clin. Nurs. 2021, 30, 572–580. [Google Scholar] [CrossRef]
- Rønnstad, A.T.M.; Halling-Overgaard, A.-S.; Hamann, C.R.; Skov, L.; Egeberg, A.; Thyssen, J.P. Association of Atopic Dermatitis with Depression, Anxiety, and Suicidal Ideation in Children and Adults: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2018, 79, 448–456.e30. [Google Scholar] [CrossRef]
- Patel, K.R.; Immaneni, S.; Singam, V.; Rastogi, S.; Silverberg, J.I. Association between Atopic Dermatitis, Depression, and Suicidal Ideation: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2019, 80, 402–410. [Google Scholar] [CrossRef]
- Matsunaga, M.C.; Yamauchi, P.S. IL-4 and IL-13 Inhibition in Atopic Dermatitis. J. Drugs Dermatol. 2016, 15, 925–929. [Google Scholar]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Gadkari, A.; Worm, M.; Soong, W.; Blauvelt, A.; Eckert, L.; Wu, R.; Ardeleanu, M.; Graham, N.M.H.; Pirozzi, G.; et al. Dupilumab Therapy Provides Clinically Meaningful Improvement in Patient-Reported Outcomes (PROs): A Phase IIb, Randomized, Placebo-Controlled, Clinical Trial in Adult Patients with Moderate to Severe Atopic Dermatitis (AD). J. Am. Acad. Dermatol. 2016, 75, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Meding, B.; Wrangsjö, K.; Järvholm, B. Fifteen-Year Follow-up of Hand Eczema: Persistence and Consequences. Br. J. Dermatol. 2005, 152, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Boehm, D.; Schmid-Ott, G.; Finkeldey, F.; John, S.M.; Dwinger, C.; Werfel, T.; Diepgen, T.L.; Breuer, K. Anxiety, Depression and Impaired Health-Related Quality of Life in Patients with Occupational Hand Eczema. Contact Dermat. 2012, 67, 184–192. [Google Scholar] [CrossRef]
- Szepietowska, M.; Dąbrowska, A.; Dziasek, S.; Lisicki, B.; Skinderowicz, K.; Wilczynski, B.; Krajewski, P.K.; Szepietowski, J.C. Perception of Hand Eczema among Adolescents in Poland: A Cross-Sectional Study. Contact Dermat. 2023, 89, 68–70. [Google Scholar] [CrossRef]
- Kouris, A.; Armyra, K.; Christodoulou, C.; Katoulis, A.; Potouridou, I.; Tsatovidou, R.; Rigopoulos, D.; Kontochristopoulos, G. Quality of Life, Anxiety, Depression and Obsessive-Compulsive Tendencies in Patients with Chronic Hand Eczema. Contact Dermat. 2015, 72, 367–370. [Google Scholar] [CrossRef]
- Niemeier, V.; Nippesen, M.; Kupfer, J.; Schill, W.-B.; Gieler, U. Psychological Factors Associated with Hand Dermatoses: Which Subgroup Needs Additional Psychological Care? Br. J. Dermatol. 2002, 146, 1031–1037. [Google Scholar] [CrossRef]
| Characteristics | Whole Population (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| Age, years (mean ± SD) | 46.0 ± 17.23 | 46.6 ± 18.27 | 36.9 ± 13.2 | NS |
| Disease duration, months (mean ± SD) | 42.5 ± 60.84 | 30.85 ± 40.34 | 27.7 ± 7.1 | NS |
| Previous treatment | 71 (71.0%) | 47 (78.3%) | 24 (60.0%) | 0.048 |
| Systemic treatment | 28 (28.0%) | 17 (28.3%) | 11 (27.5%) | NS |
| History of atopy/allergy | 45 (45.0%) | 26 (43.3%) | 19 (47.5%) | NS |
| Diagnosed allergic contact background | 14 (14.0%) | 8 (13.3%) | 6 (15.0%) | NS |
| Previous patch testing | 27 (27.0%) | 13 (21.7%) | 14 (35.0%) | NS |
| Itch in last 3 days | 81 (81.0%) | 28 (70.0%) | 53 (88.3%) | 0.022 |
| Pain in last 3 days | 53 (53.0%) | 16 (40.0%) | 37 (61.7%) | 0.033 |
| Lesion location | ||||
| Only hands | 65 (65.0%) | 38 (63.3%) | 27 (67.5%) | NS |
| Hands and feet | 23 (23.0%) | 15 (25.0%) | 8 (20.0%) | NS |
| Disseminated lesions | 12 (12.0%) | 7 (11.7%) | (12.5%) | NS |
| DLQI Group (Effect on Patients QoL) | Total Group Size/ Frequency (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| no effect | 2 (2.0%) | 1 (1.7%) | 1 (2.5%) | 0.023 |
| small | 16 (16.0%) | 6 (10.0%) | 10 (25.0%) | |
| moderate | 33 (33.0%) | 16 (26.7%) | 17 (42.5%) | |
| very large | 39 (39.0%) | 28 (46.7%) | 11 (27.5%) | |
| extremely large | 10 (10.0%) | 9 (15.0%) | 1 (2.5%) |
| Depression Severity Group (According to PHQ-9) | Total Group Size/ Frequency (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| mild | 43 (43.0%) | 24 (40.0%) | 19 (47.5%) | NS |
| moderate | 35 (35.0%) | 19 (31.7%) | 16 (40.0%) | |
| moderately severe | 13 (13.0%) | 10 (16.7%) | 3 (7.5%) | |
| severe | 9 (9.0%) | 7 (11.7%) | 2 (5.0%) |
| Depressive Disorder Severity Group (According to HADS-M) | Total Group Size/ Frequency (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| no disorders | 80 (80.0%) | 44 (73.3%) | 36 (90.0%) | NS |
| borderline states | 14 (14.0%) | 11 (18.3%) | 3 (7.5%) | |
| disorders | 6 (6.0%) | 5 (8.3%) | 1 (2.5%) |
| Anxiety Severity Group (According to GAD-7) | Total Group Size/ Frequency (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| mild | 43 (43.0%) | 22 (36.7%) | 21 (52.5%) | NS |
| moderate | 39 (39.0%) | 26 (43.3%) | 13 (32.5%) | |
| severe | 18 (18.0%) | 12 (20.0%) | 6 (15.0%) |
| Anxiety Disorder Severity Groups (According to HADS-M) | Total Group Size/ Frequency (n = 100) | Females (n = 60) | Males (n = 40) | p |
|---|---|---|---|---|
| no disorders | 80 (80.0%) | 41 (68.3%) | 39 (97.5%) | 0.001 |
| borderline states | 14 (14.0%) | 15 (25.0%) | 0 (0.0%) | |
| disorders | 6 (6.0%) | 4 (6.7%) | 1 (2.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, A.; Krajewski, P.K.; Szepietowski, J.C. Psychosocial Consequences of Hand Eczema—A Prospective Cross-Sectional Study. J. Clin. Med. 2023, 12, 5741. https://doi.org/10.3390/jcm12175741
Zalewski A, Krajewski PK, Szepietowski JC. Psychosocial Consequences of Hand Eczema—A Prospective Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(17):5741. https://doi.org/10.3390/jcm12175741
Chicago/Turabian StyleZalewski, Adam, Piotr K. Krajewski, and Jacek C. Szepietowski. 2023. "Psychosocial Consequences of Hand Eczema—A Prospective Cross-Sectional Study" Journal of Clinical Medicine 12, no. 17: 5741. https://doi.org/10.3390/jcm12175741
APA StyleZalewski, A., Krajewski, P. K., & Szepietowski, J. C. (2023). Psychosocial Consequences of Hand Eczema—A Prospective Cross-Sectional Study. Journal of Clinical Medicine, 12(17), 5741. https://doi.org/10.3390/jcm12175741







