A Biopsychosocial Model Predicting Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Procedure
2.2. Instruments
2.3. Statistical Analyses
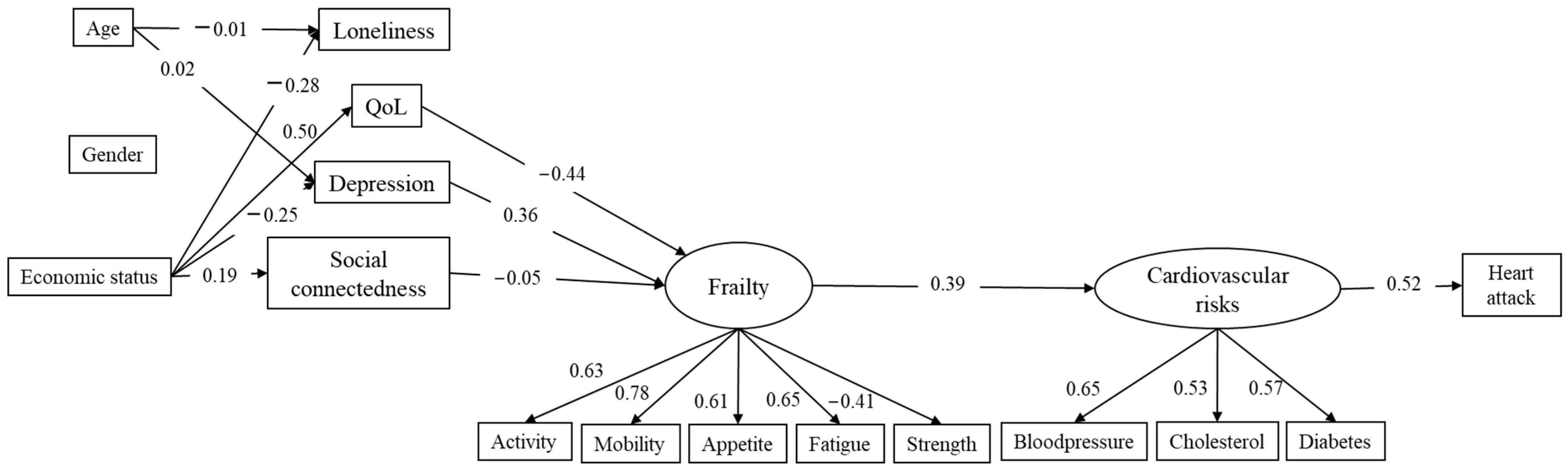
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Windecker, S. Fourth universal definition of myocardial infarction. Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Feng, B.; Li, H. Genetic Polymorphism of Matrix Metalloproteinase-9 and Susceptibility to Myocardial Infarction: A Meta-Analysis. Dis. Markers 2022, 2022, 5507153. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Raja, M.; Radha, D.; Gaur, T.A.; Geetha, J.; Sakthivadivel, V. Risk factors and inflammatory markers in acute coronary syndrome-ST elevation myocardial infarction (STEMI). Horm. Mol. Biol. Clin. Investig. 2023, 44, 115–120. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular risks associated with gender and aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Woudstra, L.; Biesbroek, P.S.; Emmens, R.W.; Heymans, S.; Juffermans, L.J.; van Rossum, A.C.; Niessen, H.W.; Krijnen, P.A. Lymphocytic myocarditis occurs with myocardial infarction and coincides with increased inflammation, hemorrhage and instability in coronary artery atherosclerotic plaques. Int. J. Cardiol. 2017, 232, 53–62. [Google Scholar] [CrossRef][Green Version]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective from Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.C.; O’Halloran, A.M. Tools for Assessing Frailty in Older People: General Concepts. Adv. Exp. Med. Biol. 2020, 1216, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Stubbs, B.; Solmi, M.; Luchini, C.; Manzato, E.; Sergi, G.; Manu, P.; Harris, T.; Fontana, L.; et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res. Rev. 2017, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wleklik, M.; Denfeld, Q.; Lisiak, M.; Czapla, M.; Kałużna-Oleksy, M.; Uchmanowicz, I. Frailty Syndrome in Older Adults with Cardiovascular Diseases–What Do We Know and What Requires Further Research? Int. J. Environ. Res. Public Health 2022, 19, 2234. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Forman, D.E. Applying frailty to guide myocardial infarction management: An important step towards precision medicine and personalized care for older adults. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [CrossRef]
- Mulero, J.; Zafrilla, P.; Martinez-Cacha, A. Oxidative stress, frailty and cognitive decline. J. Nutr. Health Aging 2011, 15, 756–760. [Google Scholar] [CrossRef]
- Curtis, A.B.; Karki, R.; Hattoum, A.; Sharma, U.C. Arrhythmias in Patients ≥80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018, 71, 2041–2057. [Google Scholar] [CrossRef]
- Uchmanowicz, I. Oxidative Stress, Frailty and Cardiovascular Diseases: Current Evidence. Adv. Exp. Med. Biol. 2020, 1216, 65–77. [Google Scholar] [CrossRef]
- Nadruz, W.; Kitzman, D., Jr.; Windham, B.G.; Kucharska-Newton, A.; Butler, K.; Palta, P.; Griswold, M.E.; Wagenknecht, L.E.; Heiss, G.; Solomon, S.D.; et al. Cardiovascular Dysfunction and Frailty Among Older Adults in the Community: The ARIC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K. Cardiac Senescence, Heart Failure, and Frailty: A Triangle in Elderly People. Keio J. Med. 2016, 65, 25–32. [Google Scholar] [CrossRef]
- Boccardi, V.; Mecocci, P. The Importance of Cellular Senescence in Frailty and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1216, 79–86. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, H.; Chen, Q.; Wang, X.; Zou, J.; Hao, Q.; Yang, M.; Wu, J. Is frailty a prognostic factor for adverse outcomes in older patients with acute coronary syndrome? Aging Clin. Exp. Res. 2020, 32, 1435–1442. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, D.; Peng, J.; Wu, Q.; Yao, Y.; Ding, M.; Wang, J. Prevalence and adverse outcomes of frailty in older patients with acute myocardial infarction after percutaneous coronary interventions: A systematic review and meta-analysis. Clin. Cardiol. 2023, 46, 5–12. [Google Scholar] [CrossRef]
- Patel, A.; Goodman, S.G.; Yan, A.T.; Alexander, K.P.; Wong, C.L.; Cheema, A.N.; Udell, J.A.; Kaul, P.; D’Souza, M.; Hyun, K.; et al. Frailty and Outcomes After Myocardial Infarction: Insights from the CONCORDANCE Registry. J. Am. Heart Assoc. 2018, 7, e009859. [Google Scholar] [CrossRef]
- Lurie, I.; Myers, V.; Goldbourt, U.; Gerber, Y. Perceived social support following myocardial infarction and long-term development of frailty. Eur. J. Prev. Cardiol. 2015, 22, 1346–1353. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Palmer, K.M.; Galluzzo, L.; Giampaoli, S.; Marengoni, A.; Bernabei, R.; Onder, G. Hypertension and frailty: A systematic review and meta-analysis. BMJ Open 2018, 8, e024406. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Veronese, N.; Fontana, L.; De Rui, M.; Bolzetta, F.; Zambon, S.; Corti, M.; Baggio, G.; Toffanello, E.D.; Crepaldi, G.; et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: The Pro. VA study. Eur. Geriatr. Med. 2015, 65, 976–983. [Google Scholar]
- Gale, C.R.; Cooper, C.; Sayer, A.A. Framingham cardiovascular disease risk scores and incident frailty: The English longitudinal study of ageing. Age 2014, 36, 9692. [Google Scholar] [CrossRef]
- Ramsay, S.E.; Arianayagam, D.; Whincup, P.H.; Lennon, L.T.; Cryer, J.; Papacosta, A.O.; Iliffe, S.; Wannamethee, S.G. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart 2015, 101, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Corbin, A.L.; Goveas, J.S. Depression and frailty in later life: A systematic review. Clin. Interv. Aging 2015, 10, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Santosa, A.; Rosengren, A.; Ramasundarahettige, C.; Rangarajan, S.; Gulec, S.; Chifamba, J.; Lear, S.A.; Poirier, P.; Yeates, K.E.; Yusuf, R.; et al. Psychosocial Risk Factors and Cardiovascular Disease and Death in a Population-Based Cohort From 21 Low-, Middle-, and High-Income Countries. JAMA Netw. Open 2021, 4, e2138920. [Google Scholar] [CrossRef]
- Gale, C.R.; Cooper, C.; Deary, I.J.; Aihie Sayer, A. Psychological well-being and incident frailty in men and women: The English Longitudinal Study of Ageing. Psychol. Med. 2014, 44, 697–706. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Smit, A.P.; van Dam, C.; Schuster, N.A.; de Breij, S.; Holwerda, T.J.; Huisman, M.; Dent, E.; Andrew, M.K. Frailty Combined with Loneliness or Social Isolation: An Elevated Risk for Mortality in Later Life. J. Am. Geriatr. Soc. 2020, 68, 2587–2593. [Google Scholar] [CrossRef]
- Davies, K.; Maharani, A.; Chandola, T.; Todd, C.; Pendleton, N. The longitudinal relationship between loneliness, social isolation, and frailty in older adults in England: A prospective analysis. Lancet Healthy Longev. 2021, 2, e70–e77. [Google Scholar] [CrossRef]
- Ge, L.; Yap, C.W.; Heng, B.H. Associations of social isolation, social participation, and loneliness with frailty in older adults in Singapore: A panel data analysis. BMC Geriatr. 2022, 22, 26. [Google Scholar] [CrossRef]
- Sha, S.; Chan, S.; Chen, L.; Xu, Y.; Pan, Y. The Association between Trajectories of Loneliness and Physical Frailty in Chinese Older Adults: Does Age Matter? Int. J. Environ. Res. Public Health 2022, 19, 5105. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.D.; Allaire, J.C. Cardiovascular Intraindividual Variability in Later Life: The Influence of Social Connectedness and Positive Emotions. Psychol. Aging 2005, 20, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.D.; Uchino, B.N.; Wethington, E. Loneliness and health in older adults: A minireview and synthesis. Gerontology 2016, 62, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Herrera−Badilla, A.; Navarrete−Reyes, A.P.; Amieva, H.; Avila−Funes, J.A. Loneliness is associated with frailty in community−dwelling elderly adults. J. Am. Geriatr. Soc. 2015, 63, 607–609. [Google Scholar] [CrossRef]
- Chu, W.; Chang, S.; Ho, H.; Lin, H. The relationship between depression and frailty in community-dwelling older people: A systematic review and meta-analysis of 84,351 older adults. J. Nurs. Scholarsh. 2019, 51, 547–559. [Google Scholar] [CrossRef]
- Veronese, N.; Noale, M.; Cella, A.; Custodero, C.; Smith, L.; Barbagelata, M.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; et al. Multidimensional frailty and quality of life: Data from the English Longitudinal Study of Ageing. Qual Life Res 2022, 31, 0123456789. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Richardson, S.; Shaffer, J.A.; Falzon, L.; Krupka, D.; Davidson, K.W.; Edmondson, D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am. J. Cardiol. 2012, 110, 1711–1716. [Google Scholar] [CrossRef]
- Rosengren, A.; Hawken, S.; Ounpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthi-amorn, C.; Sato, H.; Yusuf, S.; et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Parnicka, A.; Mielimąka, M.; Walczewska, J.; Falisz, K.; Skalska, A.; Rutkowski, K.; Grodzicki, T. Are all the former Siberian deportees with posttraumatic stress disorder patients at risk for unsuccessful aging? Int. J. Geriatr. Psychiatry 2018, 33, 671–672. [Google Scholar] [CrossRef]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Bethmann, A.; Bergmann, M.; Scherpenzeel, A. SHARE Sampling Guide—Wave 8; Working Paper Series; SHARE-ERIC: Munich, Germany, 2019; p. 33. [Google Scholar]
- Börsch-Supan, A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 8, Release version: 8.0.0; SHARE-ERIC: Munich, Germany, 2022. [Google Scholar] [CrossRef]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Cuenoud, P.; Spagnoli, J.; Junod, J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 675–681. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Walsh, C.D.; Lawlor, B.A.; Kenny, R.A. A Frailty Instrument for primary care: Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010, 10, 1–12. [Google Scholar] [CrossRef]
- Prince, M.J.; Reischies, F.; Beekman, A.T.F.; Fuhrer, R.; Jonker, C.; Kivela, S.L.; Lawlor, B.; Lobo, A.; Magnusson, H.; Fichter, M.M.; et al. Development of the EURO-D scale—A European Union initiative to compare symptoms of depression in 14 European centres. Br. J. Psychiatry 1999, 174, 330–338. [Google Scholar] [CrossRef]
- Portellano-Ortiz, C.; Garre-Olmo, J.; Calv´o-Perxas, L.; Conde-Sala, J.L. Factor structure of depressive symptoms using the EURO-D scale in the over-50s in Europe. Findings from the SHARE project. Aging Ment. Health 2018, 22, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Tomás, J.M.; Torres, Z.; Oliver, A.; Enrique, S.; Fernández, I. Psychometric properties of the EURO-D scale of depressive symptomatology: Evidence from SHARE wave 8. J. Affect. Disord. 2022, 313, 49–55. [Google Scholar] [CrossRef]
- Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Cacioppo, J.T. A short scale for measuring loneliness in large surveys results from two population-based studies. Res. Aging 2004, 26, 655–672. [Google Scholar] [CrossRef]
- Velarde-Mayol, C.; Fragua-Gil, S.; García-de-Cecilia, J.M. Validation of the UCLA loneliness scale in an elderly population that live alone. Semergen 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Von dem Knesebeck, O.; Hyde, M.; Higgs, P.; Kupfer, A.; Siegrist, J. Quality of Life and Wellbeing. In Health, Ageing and Retirement in Europe—First Results from the Survey of Health, Ageing and Retirement in Europe; Börsch-Supan, A., Brugiavini, A., Jürges, H., Mackenbach, J., Siegrist, J., Weber, G., Eds.; Mannheim Research Institute for the Economics of Aging (MEA): Mannheim, Germany, 2005. [Google Scholar]
- Borrat-Besson, C.; Ryser, V.A.; Gonçalves, J. An Evaluation of the CASP-12 Scale Used in the Survey of Health, Ageing and Retirement in Europe (SHARE) to Measure Quality of Life among People Aged 50; FORS: Lausanne, Switzerland, 2015. [Google Scholar]
- Oliver, A.; Sentandreu-Mañó, T.; Tomás, J.M.; Fernández, I.; Sancho, P. Quality of Life in European Older Adults of SHARE Wave 7: Comparing the Old and the Oldest-Old. J. Clin. Med. 2021, 10, 2850. [Google Scholar] [CrossRef]
- Litwin, H.; Stoeckel, K.; Roll, A.; Shiovitz-EzraIn, S.; Kotte, M. Social Network Measurement in SHARE Wave Four. In SHARE Wave 4: Innovations & Methodology; Malter, F., Börsch-Supan, A., Eds.; MEA, Max Planck Institute for Social Law and Social Policy: Munich, Germany, 2013. [Google Scholar]
- Litwin, H.; Stoeckel, K.J. Engagement and social capital as elements of active aging: An analysis of older Europeans. Sociol. E Politiche Soc. 2014, 17, 9–29. [Google Scholar]
- Litwin, H.; Levinson, M. The association of mobility limitation and social networks in relation to late-life activity. Ageing Soc. 2018, 38, 1771–1790. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Finney, S.J.; DiStefano, C. Nonnormal and Categorical Data in Structural Equation Modeling. In Structural Equation Modeling: A Second Course; Hancock, G.R., Mueller, R.O., Eds.; IAP Information Age Publishing: Charlotte, NC, USA, 2013; pp. 439–492. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- Marsh, H.W.; Hau, K.T.; Wen, Z. In search of golden rules: Comment on hypothesis- testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing hu and bentler’s (1999) findings. Struct. Equ. Model. A Multidiscip. J. 2004, 11, 320–341. [Google Scholar] [CrossRef]
- Acock, A.C. A Gentle Introduction to Stata, 4th ed.; Stata Press: Lakeway Drive College Station, TX, USA, 2014. [Google Scholar]
- Bremmer, M.; Beekman, A.; Deeg, D.; Penninx, B.; Dik, M.; Hack, C.; Hoogendijk, W. Inflammatory markers in latelife depression: Results from a population-based study. J. Affect Disord. 2008, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Corsi, A.M.; Penninx, B.W.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: The InCHIANTI study. Biol. Psychiatry 2009, 65, 973–978. [Google Scholar] [CrossRef]
- Veltman, E.M.; Lamers, F.; Comijs, H.C.; Stek, M.L.; van der Mast, R.C.; Rhebergen, D. Inflammatory markers and cortisol parameters across depressive subtypes in an older cohort. J. Affect. Disord. 2018, 234, 54–58. [Google Scholar] [CrossRef]
- Engel, G.L. The clinical application of the biopsychosocial model. Am. J. Psychiatry 1980, 137, 535–544. [Google Scholar] [CrossRef]
- Borrell-Carrió, F.; Suchman, A.L.; Epstein, R.M. The biopsychosocial model 25 years later: Principles, practice, and scientific inquiry. Ann. Fam. Med. 2004, 2, 576–582. [Google Scholar] [CrossRef]
- Subramaniapillai, M.; Chin-Hung Chen, V.; McIntyre, R.S.; Yang, Y.; Chen, Y. Added burden of major depressive disorder on cardiovascular morbidity and mortality among patients with cardiovascular disease and the modifying effects of antidepressants: A national retrospective cohort study. J. Affect. Disord. 2021, 294, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American heart association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef]
- Hu, J.; Fitzgerald, S.M.; Owen, A.J.; Ryan, J.; Joyce, J.; Chowdhury, E.; Reid, C.M.; Britt, C.; Woods, L.; McNeil, J.J.; et al. Social isolation, social support, loneliness, and cardiovascular disease risk factors: A cross-sectional study among older adults. Int. J. Geriatr. Psychiatry 2021, 36, 1795–1809. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Hawkley, L.C.; Thisted, R.A.; Cacioppo, J.T. A marginal structural model analysis for loneliness: Implications for intervention trials and clinical practice. J. Consult. Clin. Psychol. 2011, 79, 225–235. [Google Scholar] [CrossRef]
- Griffin, S.C.; Blakey, S.M.; Brant, T.R.; Eshera, Y.M.; Calhoun, P.S. Disentangling the Longitudinal Relationship between Loneliness and Depressive Symptoms in U.S. Adults Over 50. Clin. Gerontol. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Browne, M.W.; Cacioppo, J.T. How Can I Connect With Thee?: Let Me Count the Ways. Psychol. Sci. 2005, 16, 798–804. [Google Scholar] [CrossRef]
- Dussault, M.; Fernet, C.; Austin, S.; Leroux, M. Revisiting the Factorial Validity of the Revised UCLA Loneliness Scale: A Test of Competing Models in a Sample of Teachers. Psychol. Rep. 2009, 105, 849–856. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Su, S.-C.; Chen, C.-W.; Kang, Y.-W.; Hu, M.-H.; Hsu, L.-L.; Wu, S.-Y.; Chen, L.; Chang, H.-Y.; Chuang, S.-Y.; et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. IJBNPA 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J. Risk Factors for Frailty and Cardiovascular Diseases: Are They the Same? Adv. Exp. Med. Biol. 2020, 1216, 39–50. [Google Scholar] [CrossRef]
- Vaughan, M.; Lavalley, M.P.; Alheresh, R.; Keysor, J.J. Which features of the environment impact community participation of older adults? A systematic review and meta-analysis. J. Aging Health 2016, 28, 957–978. [Google Scholar] [CrossRef]
- Bergmann, M.; Scherpenzeel, A.; Börsch-Supan, A. SHARE Wave 7 Methodology: Panel Innovations and Life Histories; Max Plank Institute for Social Law and Social Policy, Munich Center for the Economics of Aging: Munich, Germany, 2019. [Google Scholar]
- Aryafard, H.; Dehvan, F.; Albatineh, A.N.; Dalvand, S.; Gheshlagh, R.G. Spiritual Health in Iranian Patients with Cardiovascular Diseases: A Systematic Review and Meta-analysis. Omega 2022, 12, 00302228221108293. [Google Scholar] [CrossRef]
- Behlke, L.M.; Lenze, E.J.; Carney, R.M. The Cardiovascular Effects of Newer Antidepressants in Older Adults and Those with or At High Risk for Cardiovascular Diseases. CNS Drugs 2020, 34, 1133–1147. [Google Scholar] [CrossRef]
- Yazawa, A.; Inoue, Y.; Fujiwara, T.; Stickley, A.; Shirai, K.; Amemiya, A.; Kondo, N.; Watanabe, C.; Kondo, K. Association between social participation and hypertension among older people in Japan: The JAGES Study. Hypertens. Res. 2016, 39, 818–824. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean or Percentage (Min–Max) | SD |
|---|---|---|
| Age | 70.40 (50–103) | 9.33 |
| Mobility | 1.74 (1–4) | 1.10 |
| Handgrip strength | 31.92 (1–85) | 11.28 |
| Loneliness | 3.99 (3–9) | 1.44 |
| Social connectedness | 1.98 (0–4) | 0.90 |
| Economic status | 2.77 (1–4) | 1.01 |
| Quality of life | 37.29 (12–48) | 6.32 |
| Depressive symptomatology | 2.01 (0–10) | 1.92 |
| Gender | Male = 42.6% Female = 57.4% | |
| Myocardial infarction | No= 86.9% Yes = 13.1% | |
| Hypertension | No = 54% Yes = 46% | |
| High cholesterol | No = 74.8% Yes = 25.2% | |
| Diabetes | No = 85.2% Yes = 14.8% | |
| Slowness | No = 87.2% Yes = 12.8% | |
| Appetite | No = 90.4% Yes = 9.6% | |
| Fatigue | No = 63.9% Yes = 36.1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomás, J.M.; Oliver, A.; Torres, Z.; Parker, J.; Marques-Sule, E.; Sentandreu-Mañó, T. A Biopsychosocial Model Predicting Myocardial Infarction. J. Clin. Med. 2023, 12, 5715. https://doi.org/10.3390/jcm12175715
Tomás JM, Oliver A, Torres Z, Parker J, Marques-Sule E, Sentandreu-Mañó T. A Biopsychosocial Model Predicting Myocardial Infarction. Journal of Clinical Medicine. 2023; 12(17):5715. https://doi.org/10.3390/jcm12175715
Chicago/Turabian StyleTomás, José M., Amparo Oliver, Zaira Torres, Janhavi Parker, Elena Marques-Sule, and Trinidad Sentandreu-Mañó. 2023. "A Biopsychosocial Model Predicting Myocardial Infarction" Journal of Clinical Medicine 12, no. 17: 5715. https://doi.org/10.3390/jcm12175715
APA StyleTomás, J. M., Oliver, A., Torres, Z., Parker, J., Marques-Sule, E., & Sentandreu-Mañó, T. (2023). A Biopsychosocial Model Predicting Myocardial Infarction. Journal of Clinical Medicine, 12(17), 5715. https://doi.org/10.3390/jcm12175715







