Benefits of Premaquick® Combined Detection of IL-6/Total IGFBP-1/Native IGFBP-1 to Predict Preterm Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Population
2.3. Study Procedures and Data Collected
2.4. Description of the Tests
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enquête Nationale Périnatale, Rapport 2021. Les Naissances, Le Suivi à 2 Mois et Les Établissements: Situation et Évolution Depuis 2016. Available online: https://www.santepubliquefrance.fr/etudes-et-enquetes/enquete-nationale-perinatale-2021 (accessed on 7 March 2023).
- Langer, B.; Sénat, M.-V.; Sentilhes, L. Guidelines for clinical practice: Prevention of spontaneous preterm birth (excluding preterm premature rupture of membranes). J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 1208–1209. [Google Scholar] [CrossRef]
- Kiefer, D.G.; Vintzileos, A.M. The Utility of Fetal Fibronectin in the Prediction and Prevention of Spontaneous Preterm Birth. Rev. Obstet. Gynecol. 2008, 1, 106. [Google Scholar] [PubMed]
- Räikkönen, K.; Gissler, M.; Kajantie, E. Associations between Maternal Antenatal Corticosteroid Treatment and Mental and Behavioral Disorders in Children. JAMA 2020, 323, 1924–1933. [Google Scholar] [CrossRef]
- Wilson, A.; MacLean, D.; Skeoch, C.H.; Jackson, L. An evaluation of the financial and emotional impact of in utero transfers upon families: A Scotland–wide audit. Inf. Dent. 2010, 6, 38–40. [Google Scholar]
- Sotiriadis, A.; Papatheodorou, S.; Kavvadias, A.; Makrydimas, G. Transvaginal cervical length measurement for prediction of preterm birth in women with threatened preterm labor: A meta-analysis. Ultrasound Obstet. Gynecol. 2010, 35, 54–64. [Google Scholar] [CrossRef] [PubMed]
- van Baaren, G.J.; Vis, J.Y.; Wilms, F.F.; Oudijk, M.A.; Kwee, A.; Porath, M.M.; Scheepers, H.C.; Spaanderman, M.E.; Bloemenkamp, K.W.; Haak, M.C.; et al. Cost effectiveness of diagnostic testing strategies including cervical-length measurement and fibronectin testing in women with symptoms of preterm labor. Ultrasound Obstet. Gynecol. 2018, 51, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Chen, F.K.; Wessel, J.; Buscher, U.; Dudenhausen, J.W. Elevation of interleukin-6 levels in cervical secretions as a predictor of preterm delivery. Acta Obstet. Gynecol. Scand. 2003, 82, 326–329. [Google Scholar] [CrossRef]
- Tsiartas, P.; Holst, R.M.; Wennerholm, U.B.; Hagberg, H.; Hougaard, D.M.; Skogstrand, K.; Pearce, B.D.; Thorsen, P.; Kacerovsky, M.; Jacobsson, B. Prediction of spontaneous preterm delivery in women with threatened preterm labour: A prospective cohort study of multiple proteins in maternal serum. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 866–873. [Google Scholar] [CrossRef]
- Taylor, B.D.; Holzman, C.B.; Fichorova, R.N.; Tian, Y.; Jones, N.M.; Fu, W.; Senagore, P.K. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum. Reprod. Oxf. Engl. 2013, 28, 942–952. [Google Scholar] [CrossRef]
- Jung, E.Y.; Park, J.W.; Ryu, A.; Lee, S.Y.; Cho, S.-H.; Park, K.H. Prediction of impending preterm delivery based on sonographic cervical length and different cytokine levels in cervicovaginal fluid in preterm labor. J. Obstet. Gynaecol. Res. 2016, 42, 158–165. [Google Scholar] [CrossRef]
- Abo El-Ezz, A.E.; Askar, A.E.A. Predictive value of phosphorylated insulin-like growth factor binding protein-1 (PIGFBP-1) (bedside test) in preterm labor. J. Egypt Soc. Parasitol. 2014, 44, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kwek, K.; Khi, C.; Ting, H.S.; Yeo, G.S. Evaluation of a bedside test for phosphorylated insulin-like growth factor binding protein-1 in preterm labour. Ann. Acad. Med. Singap. 2004, 33, 780–783. [Google Scholar] [PubMed]
- DeFranco, E.A.; Lewis, D.F.; Odibo, A.O. Improving the screening accuracy for preterm labor: Is the combination of fetal fibronectin and cervical length in symptomatic patients a useful predictor of preterm birth? A systematic review. Am. J. Obstet. Gynecol. 2013, 208, 233.e1–233.e6. [Google Scholar] [CrossRef]
- Eleje, G.U.; Ezugwu, E.C.; Eke, A.C.; Eleje, L.I.; Ikechebelu, J.I.; Ezebialu, I.U.; Obiora, C.C.; Nwosu, B.O.; Ezeama, C.O.; Udigwe, G.O.; et al. Accuracy of a combined insulin-like growth factor-binding protein-1/interleukin-6 test (Premaquick) in predicting delivery in women with threatened preterm labor. J. Perinat. Med. 2017, 45, 915–924. [Google Scholar] [CrossRef]
- Asiegbu, A.C.; Eleje, G.U.; Ibeneme, E.M.; Onyegbule, O.A.; Chukwu, L.C.; Egwim, A.V.; Okonko, C.O.; Eze, S.C.; Eke, A.C. Combined insulin-like growth factor binding protein-1/interleukin-6 (Premaquick) versus fetal fibronectin for predicting preterm delivery among women with preterm contractions. Int. J. Gynaecol. Obstet. 2020, 149, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, I.A.; Amer, O.O.; Shikanova, S.; Karimova, B. Diagnostic accuracy of PremaQuick in detection of preterm labor in symptomatic women. Ginekol. Pol. 2021; ahead of print. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Ville, Y.; Rozenberg, P. Predictors of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 23–32. [Google Scholar] [CrossRef]
- Mourgues, C.; Rossi, A.; Favre, N.; Delabaere, A.; Roszyk, L.; Sapin, V.; Debost-Legrand, A.; Gallot, D. Fetal fibronectin test for threatened preterm delivery 48 h after admission: Cost-effectiveness study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Abu-Faza, M.; Abdelazim, I.A.; Svetlana, S.; Nusair, B.; Farag, R.H.; Nair, S.R. Diagnostic accuracy of Premaquick versus ActimPartus in prediction of preterm labour in symptomatic women within 14 days. Open J. Obstet. Gynecol. 2018, 8, 741–755. [Google Scholar] [CrossRef]
- Lembet, A.; Eroglu, D.; Ergin, T.; Kuscu, E.; Zeyneloglu, H.; Batioglu, S.; Haberal, A. New rapid bed-side test to predict preterm delivery: Phosphorylated insulin-like growth factor binding protein-1 in cervical secretions. Acta Obstet. Gynecol. Scand. 2002, 81, 706–712. [Google Scholar] [CrossRef]
- Ting, H.-S.; Chin, P.-S.; Yeo, G.S.H.; Kwek, K. Comparison of bedside test kits for prediction of preterm delivery: Phosphorylated insulin-like growth factor binding protein-1 (pIGFBP-1) test and fetal fibronectin test. Ann. Acad. Med. Singap. 2007, 36, 399–402. [Google Scholar] [CrossRef]
- Cooper, S.; Lange, I.; Wood, S.; Tang, S.; Miller, L.; Ross, S. Diagnostic accuracy of rapid phIGFBP-I assay for predicting preterm labor in symptomatic patients. J. Perinatol. 2012, 32, 460–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mesure de la Longueur du col de l’uterus par Echographie Endovaginale.pdf [Internet]. [Cited 6 June 2019]. Available online: https://www.hassante.fr/portail/upload/docs/application/pdf/2010-10/mesure_de_la_longueur_du_col_de_luterus_par_echographie_endovaginale_-_document_davis.pdf (accessed on 10 January 2023).
- Kumari, A.; Saini, V.; Jain, P.K.; Gupta, M. Prediction of Delivery in Women with 38 Threatening Preterm Labour using Phosphorylated Insulin-Like Growth Factor Binding Protein-1 and Cervical Length using Transvaginal Ultrasound. J. Clin. Diagn. Res. JCDR 2017, 11, QC01–QC04. [Google Scholar] [PubMed]
- Lockwood, C.J.; Wein, R.; Lapinski, R.; Casal, D.; Berkowitz, R.L. Increased interleukin-6 concentrations in cervical secretions are associated with preterm delivery. Am. J. Obstet. Gynecol. 1994, 171, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Prairie, E.; Côté, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 2021, 59, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Kurkinen-Räty, M.; Ruokonen, A.; Vuopala, S.; Koskela, M.; Rutanen, E.M.; Kärkkäinen, T.; Jouppila, P. Combination of cervical interleukin-6 and -8, phosphorylated insulin-like growth factorbinding protein-1 and transvaginal cervical ultrasonography in assessment of the risk of preterm birth. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Torloni, M.R.; Voltolini, C.; Torricelli, M.; Merialdi, M.; Betrán, A.P.; Widmer, M.; Allen, T.; Davydova, I.; Khodjaeva, Z.; et al. Biomarkers of Spontaneous Preterm Birth: An Overview of The Literature in the Last Four Decades. Reprod. Sci. 2011, 18, 1046–1070. [Google Scholar] [CrossRef]
| All Patients | Premaquick® + | Premaquick® − | p | Quikcheck fFN™ + | Quikcheck fFN™ − | p | |
|---|---|---|---|---|---|---|---|
| n | 193 | 101 | 92 | 121 | 72 | ||
| Age at inclusion (years) | 28.3 ± 5.3 | 28.2 ± 5.5 | 28.4 ± 5.2 | 0.82 | 28.2 ± 5.6 | 28.5 ± 4.8 | 0.68 |
| Gestational age at inclusion (GW) | 30.8 ± 2.7 | 30.6 ± 2.8 | 30.9 ± 2.5 | 0.53 | 30.6 ± 2.9 | 31.1 ± 2.4 | 0.21 |
| BMI 1 (kg/m2) before pregnancy | 23.3 ± 4.8 | 23.1 ± 4.6 | 23.44 ± 5.0 | 0.65 | 23.4 ± 4.8 | 23.05 ± 4.7 | 0.60 |
| Marital status | |||||||
| Single | 14.6 (28) | 11.0 (11) | 18.5 (17) | 0.14 | 14.2 (17) | 15.3 (11) | 0.83 |
| In a relationship | 85.4 (164) | 89.0 (89) | 81.5 (75) | 85.8 (103) | 84.7 (61) | ||
| Gestation | |||||||
| 1 | 39.9 (77) | 44.6 (45) | 34.8 (32) | 0.26 | 39.7 (48) | 40.3 (29) | 0.49 |
| 2 | 26.4 (51) | 26.7 (27) | 26.1 (24) | 24.0 (29) | 30.6 (22) | ||
| ≥3 | 33.7 (65) | 28.7 (29) | 39.1 (36) | 36.3 (44) | 29.2 (21) | ||
| Parity | |||||||
| 0 | 50.8 (98) | 54.5 (55) | 46.7 (43) | 0.24 | 47.9 (58) | 55.6 (40) | 0.50 |
| 1 | 15.0 (29) | 16.8 (17) | 13.0 (12) | 14.9 (18) | 15.3 (11) | ||
| ≥2 | 34.2 (66) | 28.7 (29) | 40.2 (37) | 37.2 (45) | 29.2 (21) | ||
| Active smoking | |||||||
| Before pregnancy | 32.1 (62) | 32.7 (33) | 31.5 (29) | 0.86 | 36.4 (44) | 25.0 (18) | 0.10 |
| During pregnancy | 21.2 (41) | 21.8 (22) | 20.6 (19) | 0.85 | 24.0 (29) | 16.7 (12) | 0.23 |
| Gestational diabetes | 10.9 (21) | 8.0 (8) | 14.1 (13) | 0.17 | 11.7 (14) | 9.7 (7) | 0.68 |
| Sexual intercourse < 24 h at inclusion | 12.1 (21) | 14.6 (13) | 9.4 (8) | 0.29 | 12.5 (14) | 11.3 (7) | 0.81 |
| History (previous pregnacies) | |||||||
| TPL | 18.7 (36) | 20.8 (21) | 16.3 (15) | 0.42 | 19.8 (24) | 16.7 (12) | 0.58 |
| Preterm birth | 15.5 (30) | 13.9 (14) | 17.4 (16) | 0.50 | 16.3 (20) | 13.9 (10) | 0.62 |
| ROM 2 | 4.2 (8) | 5.0 (5) | 3.3 (3) | 0.56 | 4.1 (5) | 4.2 (3) | 0.99 |
| Cervical surgery | 2.1 (4) | 2.0% (2) | 2.2% (2) | 0.92 | 2.5 (3) | 1.4 (1) | 0.607 |
| All Patients | Premaquick® + | Premaquick® − | p | Quikcheck fFN™ + | Quikcheck fFN™ − | p | |
|---|---|---|---|---|---|---|---|
| n | 193 | 101 | 92 | 121 | 72 | ||
| Number of UC/10 min | 1.70± 1.50 | 1.88 ± 1.60 | 1.44 ± 1.42 | 0.04 | 1.86 ± 1.58 | 1.36 ± 1.40 | 0.03 |
| Cervical length (mm) | 18.9 ± 5.9 | 18.1 ± 6.2 | 19.8 ± 5.5 | 0.05 | 18.7 ± 6.2 | 19.3 ± 5.5 | 0.47 |
| Orientation | |||||||
| Hospitalization * | 82.9 (160) | 85.2 (86) | 80.4 (74) | 0.38 | 81.0 (98) | 86.1 (62) | 0.36 |
| Use of antenatal betamethasone | 81.2 (155) | 84.9 (84) | 77.2 (71) | 0.17 | 79.8 (95) | 83.3 (60) | 0.55 |
| Tocolysis ** | 28.6 (40) | 34.2 (26) | 21.9 (14) | 0.11 | 33.3 (30) | 20.0 (10) | 0.09 |
| IL-6 | IGFBP-1 Total | IGFBP-1 Native | n (+) | Delivery in 7 Days (n = 9) | Delivery in 14 Days (n = 14) | Delivery before 37 GW (n = 34) |
|---|---|---|---|---|---|---|
| − | − | − | 67 | 11.1/64.1/1.5/93.7 | 21.4/64.2/4.5/91.3 | 28.6/63.9/14.9/80.2 |
| − | + | − | 45 | 33.3/77.2/6.7/95.9 | 21.4/76.5/6.7/92.9 | 17.1/75.3/13.3/80.4 |
| + | − | − | 22 | 0.0/88.0/0.0/94.7 | 0.0/87.7/0.0/91.8 | 8.6/88.0/13.6/81.3 |
| + | + | − | 42 | 11.1/77.7/2.4/94.7 | 21.4/78.2/7.1/92.7 | 28.6/79.7/23.8/83.4 |
| − | + | + | 3 | 11.1/98.9/33.3/95.8 | 7.1/98.9/33.3/93.2 | 2.9/98.7/33.3/82.2 |
| + | + | + | 13 | 33.3/94.6/23.1/96.7 | 28.6/95.0/30.8/94.4 | 11.4/94.3/30.8/82.8 |
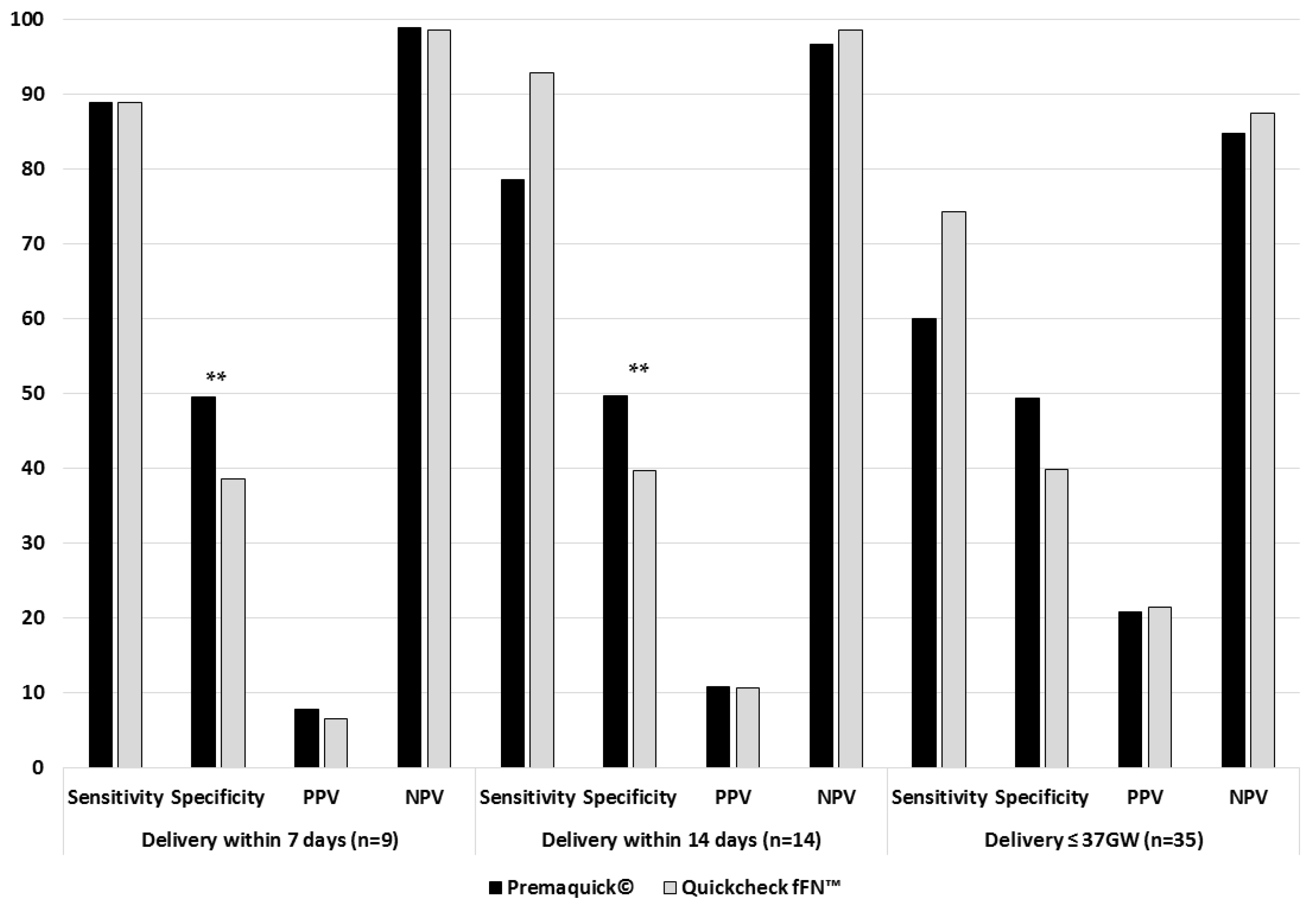
| Positive Premaquick® * | 88.9/49.5/7.9/98.9 | 78.6/49.7/10.9/96.7 | 60.0/49.4/20.8/84.8 | |||
| Positive QuikCheck™ * | 88.9/38.6/6.6/98.6 | 92.9/39.7/10.7/98.6 | 74.3/39.9/21.5/87.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pambet, M.; Sirodot, F.; Pereira, B.; Cahierc, R.; Delabaere, A.; Comptour, A.; Rouzaire, M.; Sapin, V.; Gallot, D. Benefits of Premaquick® Combined Detection of IL-6/Total IGFBP-1/Native IGFBP-1 to Predict Preterm Delivery. J. Clin. Med. 2023, 12, 5707. https://doi.org/10.3390/jcm12175707
Pambet M, Sirodot F, Pereira B, Cahierc R, Delabaere A, Comptour A, Rouzaire M, Sapin V, Gallot D. Benefits of Premaquick® Combined Detection of IL-6/Total IGFBP-1/Native IGFBP-1 to Predict Preterm Delivery. Journal of Clinical Medicine. 2023; 12(17):5707. https://doi.org/10.3390/jcm12175707
Chicago/Turabian StylePambet, Mathilde, Fanny Sirodot, Bruno Pereira, Romain Cahierc, Amélie Delabaere, Aurélie Comptour, Marion Rouzaire, Vincent Sapin, and Denis Gallot. 2023. "Benefits of Premaquick® Combined Detection of IL-6/Total IGFBP-1/Native IGFBP-1 to Predict Preterm Delivery" Journal of Clinical Medicine 12, no. 17: 5707. https://doi.org/10.3390/jcm12175707
APA StylePambet, M., Sirodot, F., Pereira, B., Cahierc, R., Delabaere, A., Comptour, A., Rouzaire, M., Sapin, V., & Gallot, D. (2023). Benefits of Premaquick® Combined Detection of IL-6/Total IGFBP-1/Native IGFBP-1 to Predict Preterm Delivery. Journal of Clinical Medicine, 12(17), 5707. https://doi.org/10.3390/jcm12175707







