Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. RIDTs
2.3. Diagnostic Methods for Comorbidity
2.4. Statistical Analyses
3. Results
3.1. Participants
3.2. Initial Symptoms
3.3. Clinical Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, U.I.; Wang, J.T.; Ho, Y.C.; Pan, S.C.; Chen, Y.C.; Chang, S.C. Factors associated with development of complications among adults with influenza: A 3-year prospective analysis. J. Formos. Med. Assoc. 2012, 111, 364–369. [Google Scholar] [CrossRef][Green Version]
- Ma, X.; Conrad, T.; Alchikh, M.; Reiche, J.; Schweiger, B.; Rath, B. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev. Med. Virol. 2018, 28, e1997. [Google Scholar] [CrossRef]
- Trilla, A.; Trilla, G.; Daer, C. The 1918 “Spanish flu” in Spain. Clin. Infect. Dis. 2008, 47, 668–673. [Google Scholar] [CrossRef]
- Kwok, K.O.; Riley, S.; Perera, R.; Wei, V.W.I.; Wu, P.; Wei, L.; Chu, D.K.W.; Barr, I.G.; Malik Peiris, J.S.; Cowling, B.J. Relative incidence and individual-level severity of seasonal influenza A H3N2 compared with 2009 pandemic H1N1. BMC Infect. Dis. 2017, 17, 337. [Google Scholar] [CrossRef]
- Choi, W.S.; Cowling, B.J.; Noh, J.Y.; Song, J.Y.; Wie, S.H.; Lee, J.S.; Seo, Y.B.; Lee, J.; Jeong, H.W.; Kim, Y.K.; et al. Disease burden of 2013–2014 seasonal influenza in adults in Korea. PLoS ONE 2017, 12, e0172012. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, H.D.; Cheong, H.M.; Lee, A.; Lee, N.J.; Chu, H.; Lee, J.Y.; Kim, S.S.; Choi, J.H. Nation-wide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J. Med. Virol. 2018, 90, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, H.; Lee, N.J.; Kim, E.J. COVID-19 Impact on Influenza and Respiratory Viruses Surveillance. Public Health Wkly. Rep. 2020, 50, 3537–3548. Available online: www.cdc.go.kr (accessed on 10 December 2020).
- Rondy, M.; Kissling, E.; Emborg, H.D.; Gherasim, A.; Pebody, R.; Trebbien, R.; Pozo, F.; Larrauri, A.; McMenamin, J.; Valenciano, M.; et al. Interim 2017/18 influenza seasonal vaccine effectiveness: Combined results from five European studies. Euro Surveill. 2018, 23, 18-00086. [Google Scholar] [CrossRef]
- Irving, S.A.; Patel, D.C.; Kieke, B.A.; Donahue, J.G.; Vandermause, M.F.; Shay, D.K.; Belongia, E.A. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses 2012, 6, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Soldevila, N.; Romero-Tamarit, A.; Torner, N.; Godoy, P.; Rius, C.; Jane, M.; Dominguez, A.; Surveillance of Hospitalized Cases of Severe Influenza in Catalonia Working Group. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. PLoS ONE 2019, 14, e0210353. [Google Scholar] [CrossRef]
- Mohammad, S.; Korn, K.; Schellhaas, B.; Neurath, M.F.; Goertz, R.S. Clinical Characteristics of Influenza in Season 2017/2018 in a German Emergency Department: A Retrospective Analysis. Microbiol. Insights 2019, 12, 1178636119890302. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Falcon-Escobedo, R. Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses. Arch. Med. Res. 2009, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Sreta, D.; Kedkovid, R.; Tuamsang, S.; Kitikoon, P.; Thanawongnuwech, R. Pathogenesis of swine influenza virus (Thai isolates) in weanling pigs: An experimental trial. Virol. J. 2009, 6, 34. [Google Scholar] [CrossRef]
- Kaji, M.; Watanabe, A.; Aizawa, H. Differences in clinical features between influenza A H1N1, A H3N2, and B in adult patients. Respirology 2003, 8, 231–233. [Google Scholar] [CrossRef]
- Paul Glezen, W.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The burden of influenza B: A structured literature review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Zhan, Y.Q.; Chen, R.C.; Zhou, R.; Wang, Y.T.; Luo, Y.; Jiang, M.; Li, J.Q.; Qin, S.; Guan, W.D.; et al. A prospective comparison of the epidemiological and clinical characteristics of pandemic (H1N1) 2009 influenza A virus and seasonal influenza A viruses in Guangzhou, South China in 2009. JPN J. Infect. Dis. 2012, 65, 208–214. [Google Scholar] [CrossRef][Green Version]
- Ebell, M.H.; White, L.L.; Casault, T. A systematic review of the history and physical examination to diagnose influenza. J. Am. Board Fam. Pract. 2004, 17, 1–5. [Google Scholar] [CrossRef]
- Wong, S.S.; Yuen, K.Y. Antiviral therapy for respiratory tract infections. Respirology 2008, 13, 950–971. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tang, B.S.; Wu, A.K.; Chu, C.M.; Yuen, K.Y. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J. Infect. 2004, 49, 262–273. [Google Scholar] [CrossRef]
- Young, S.; Illescas, P.; Nicasio, J.; Sickler, J.J. Diagnostic accuracy of the real-time PCR cobas((R)) Liat((R)) Influenza A/B assay and the Alere i Influenza A&B NEAR isothermal nucleic acid amplification assay for the detection of influenza using adult nasopharyngeal specimens. J. Clin. Virol. 2017, 94, 86–90. [Google Scholar] [CrossRef]
- Akashi, Y.; Suzuki, H.; Ueda, A.; Hirose, Y.; Hayashi, D.; Imai, H.; Ishikawa, H. Analytical and clinical evaluation of a point-of-care molecular diagnostic system and its influenza A/B assay for rapid molecular detection of the influenza virus. J. Infect. Chemother. 2019, 25, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Daley, P.; Castriciano, S.; Chernesky, M.; Smieja, M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J. Clin. Microbiol. 2006, 44, 2265–2267. [Google Scholar] [CrossRef]
- Kissling, E.; Nunes, B.; Robertson, C.; Valenciano, M.; Reuss, A.; Larrauri, A.; Cohen, J.M.; Oroszi, B.; Rizzo, C.; Machado, A.; et al. I-MOVE multicentre case-control study 2010/11 to 2014/15: Is there within-season waning of influenza type/subtype vaccine effectiveness with increasing time since vaccination? Euro Surveill. 2016, 21, 30201. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Schmedders, K.; Wolfensberger, A.; Schreiber, P.W.; Kuster, S.P. The economic and public health impact of influenza vaccinations: Contributions of Swiss pharmacies in the 2016/17 and 2017/18 influenza seasons and implications for vaccination policy. Swiss Med. Wkly. 2019, 149, w20161. [Google Scholar] [CrossRef] [PubMed]
- Valenciano, M.; Kissling, E.; Larrauri, A.; Nunes, B.; Pitigoi, D.; O’Donnell, J.; Reuss, A.; Horvath, J.K.; Paradowska-Stankiewicz, I.; Rizzo, C.; et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: Results of the European I-MOVE multicentre test-negative case-control study, 2011/2012-2016/2017. Influenza Other Respir Viruses 2018, 12, 567–581. [Google Scholar] [CrossRef]
- Nicholson, K.G.; Aoki, F.Y.; Osterhaus, A.D.; Trottier, S.; Carewicz, O.; Mercier, C.H.; Rode, A.; Kinnersley, N.; Ward, P. Efficacy and safety of oseltamivir in treatment of acute influenza: A randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000, 355, 1845–1850. [Google Scholar] [CrossRef]
| Variables | 2016–2017 Season (A/H3N2 Dominance) | 2018–2019 Season (A/H1N1 Dominance) | p-Value |
|---|---|---|---|
| Total number of ILI | 1897 | 2191 | |
| Number of RIDT positive | 1424 | 1568 | |
| Age, years | 17 (10–37) | 27 (14–43) | <0.001 |
| Male, n (%) | 847 (44.7) | 945 (43.1) | 0.343 |
| Contact history | 89 (4.7) | 178 (8.1) | <0.001 |
| Swab site | <0.001 | ||
| Nasopharynx via nose, n (%) | 1735 (91.5) | 1645 (79.1) | |
| Oropharynx via mouth, n (%) | 0 | 104 (5) | |
| Nasopharynx via both nose, n (%) | 0 | 332 (16.0) | |
| Nasopharynx and pharynx, n (%) | 162 (8.5) | 0 | |
| RIDT results | <0.001 | ||
| Positive, n (%) | 1362 (71.8) | 1425 (65.0) | |
| Weak positive, n (%) | 62 (3.3) | 143 (6.5) | |
| Negative, n (%) | 473 (24.9) | 623 (28.4) | |
| Vaccination | <0.001 | ||
| Before 2 weeks, n (%) | 384 (22.1) | 526 (26.2) | |
| Within 2 weeks, n (%) | 32 (1.8) | 5 (0.3) | |
| No vaccination, n (%) | 1320 (76.0) | 1475 (73.5) |
| Symptoms | 2016–2017 Season (A/H3N2 Dominance) n = 1424 | 2018–2019 Season (A/H1N1 Dominance) n = 1568 | p-Value |
|---|---|---|---|
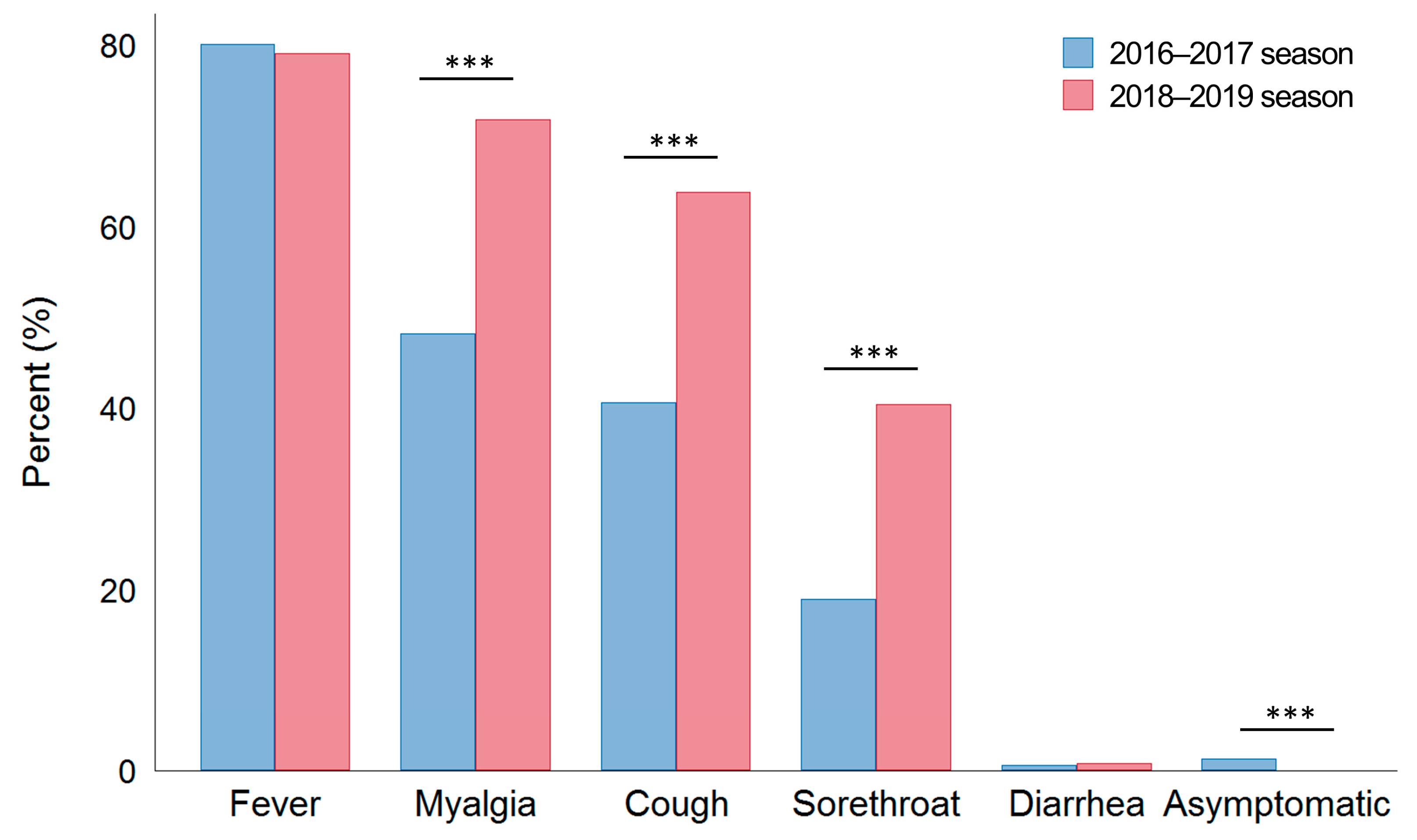
| Fever, n (%) | 1141 (80.1) | 1240 (79.1) | 0.496 |
| Average BT, °C | 38.1 (37.7–38.6) | 37.9 (37.4–38.4) | <0.001 |
| BT ≥ 38 °C, n (%) | 861 (60.5) | 740 (47.2) | <0.001 |
| Myalgia, n (%) | 686 (48.2) | 1126 (71.8) | <0.001 |
| Cough, n (%) | 580 (40.7) | 1001 (63.8) | <0.001 |
| Sore throat, n (%) | 271 (19.0) | 634 (40.4) | <0.001 |
| Diarrhea, n (%) | 8 (0.6) | 12 (0.8) | 0.654 |
| Antiviral Drugs | 2016–2017 Season (A/H3N2 Dominance) n = 1424 | 2018–2019 Season (A/H1N1 Dominance) n = 1568 | p-Value |
|---|---|---|---|
| Prescription, n (%) | 1171 (82.2) | 1275 (81.3) | 0.516 |
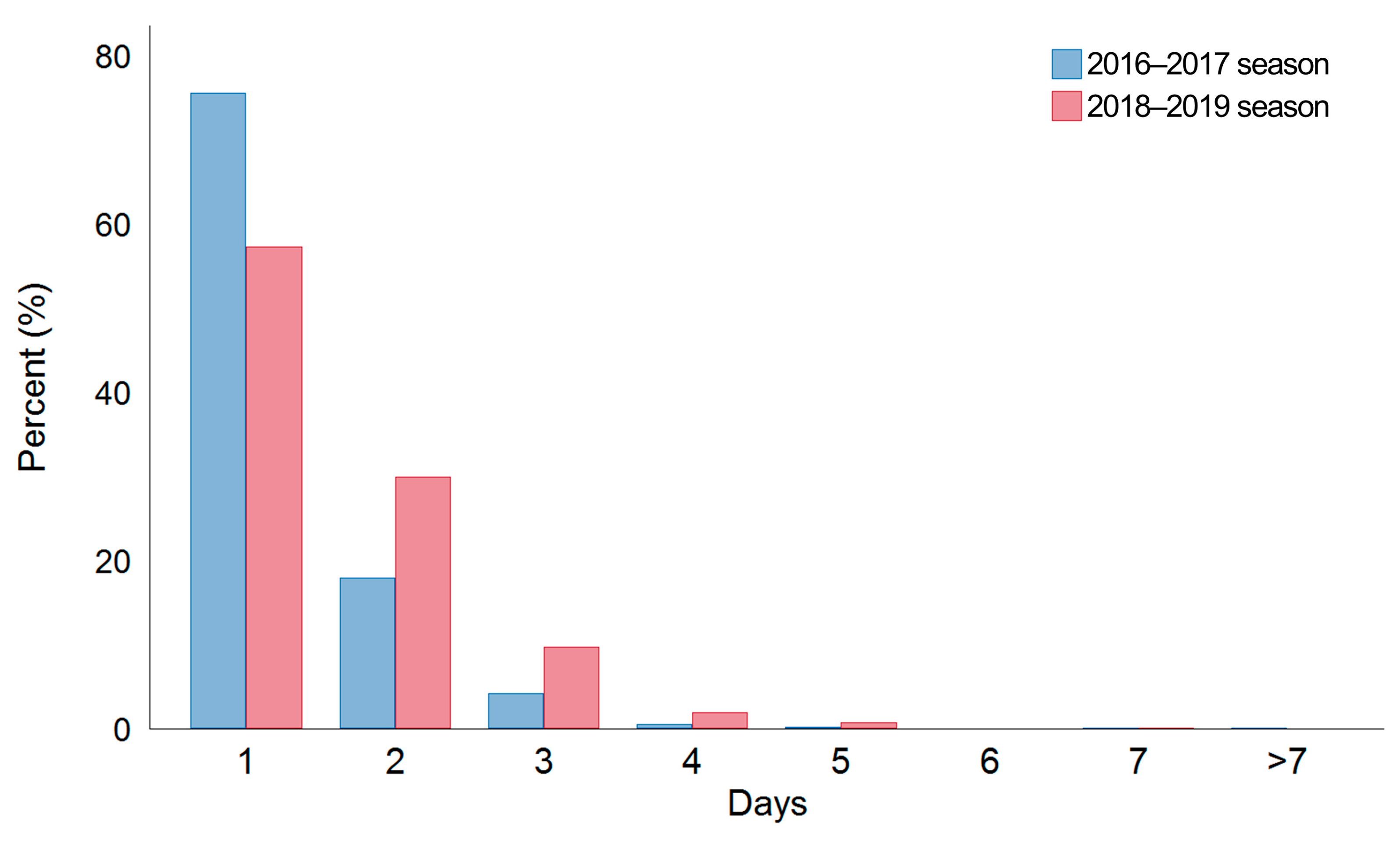
| Resolution of fever | |||
| Within 24 h, n (%) | 840/1171 (71.7) | 379/1250 (30.3) | <0.001 |
| More than 24 h, n (%) | 331/1171 (28.3) | 871/1250 (69.7) | <0.001 |
| Vomiting, n (%) | 26/1171 (2.2) | 31/1275 (2.4) | 0.763 |
| Diseases | 2016–2017 Season (A/H3N2 Dominance) n = 1424 | 2018–2019 Season (A/H1N1 Dominance) n = 1568 | p-Value |
|---|---|---|---|
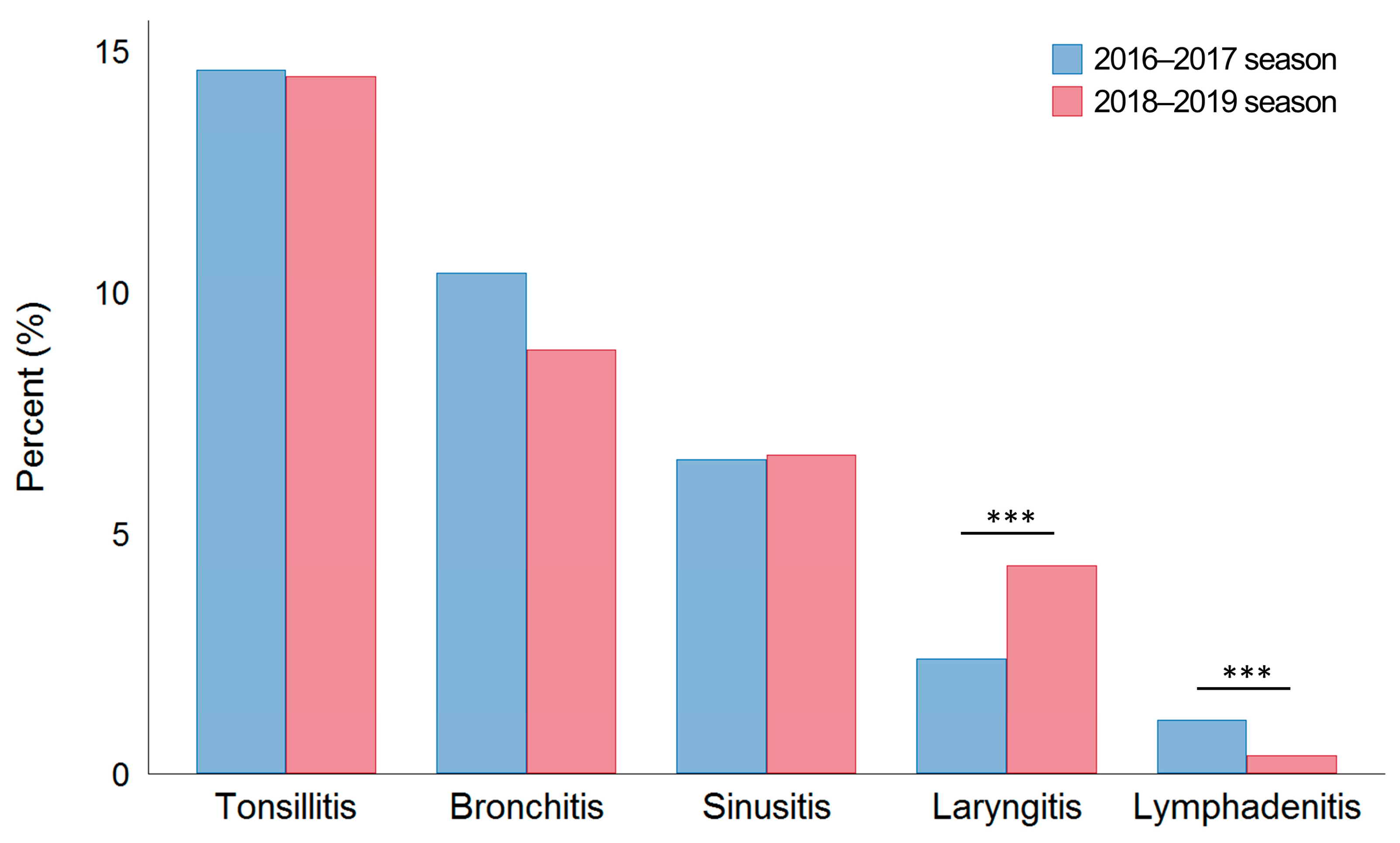
| Upper airway comorbidities | |||
| Tonsillitis, n (%) | 208 (14.6) | 227 (14.5) | 0.092 |
| Bronchitis, n (%) | 148 (10.4) | 138 (8.8) | 0.139 |
| Sinusitis, n (%) | 93 (6.5) | 104 (6.6) | 0.911 |
| Laryngitis, n (%) | 34 (2.4) | 68 (4.3) | 0.003 |
| Lymphadenitis, n (%) | 16 (1.1) | 6 (0.4) | 0.018 |
| Otitis media, n (%) | 2 (0.1) | 0 (0) | 0.226 |
| Systemic complication | |||
| Pneumonia, n (%) | 1 (0.0) | 3 (0.2) | 0.626 |
| Hospitalization, n (%) | 1 (0.0) | 4 (0.3) | 0.377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Ryu, G.; Lee, K.-I. Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea. J. Clin. Med. 2023, 12, 5651. https://doi.org/10.3390/jcm12175651
Lee H-J, Ryu G, Lee K-I. Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea. Journal of Clinical Medicine. 2023; 12(17):5651. https://doi.org/10.3390/jcm12175651
Chicago/Turabian StyleLee, Hyun-Jong, Gwanghui Ryu, and Ki-Il Lee. 2023. "Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea" Journal of Clinical Medicine 12, no. 17: 5651. https://doi.org/10.3390/jcm12175651
APA StyleLee, H.-J., Ryu, G., & Lee, K.-I. (2023). Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea. Journal of Clinical Medicine, 12(17), 5651. https://doi.org/10.3390/jcm12175651






