Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
Participants
3. Materials
3.1. MRI Acquisition
3.2. Cerebrospinal Fluid Biomarkers and APOE Genotype
3.3. Neuropsychological Assessment
3.4. Follow-Up Assessments and Outcomes
4. Statistical Analysis
5. Results
5.1. Participant Characteristics
5.2. Clinical Features and Neuroimaging Markers at Presentation
5.3. Cerebrospinal Fluid Biomarkers and APOE Genotypes
5.4. Predictors of TFNEs and Cognitive Impairment at Presentation
5.5. Follow-Up Data and Predictors of ICH Recurrence
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charidimou, A.; Boulouis, G.; Gurol, M.E.; Ayata, C.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017, 140, 1829–1850. [Google Scholar] [CrossRef]
- Keage, H.A.; Carare, R.O.; Friedland, R.P.; Ince, P.G.; Love, S.; Nicoll, J.A.; Wharton, S.B.; Weller, R.O.; Brayne, C. Population studies of sporadic cerebral amyloid angiopathy and dementia: A systematic review. BMC Neurol. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Alzheimer disease and cerebrovascular pathology: An update. J. Neural Transm. 2002, 109, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.A.; Rosand, J.; Karluk, D.; Greenberg, S.M. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001, 56, 537–539. [Google Scholar] [CrossRef]
- Linn, J.; Halpin, A.; Demaerel, P.; Ruhland, J.; Giese, A.D.; Dichgans, M.; van Buchem, M.A.; Bruckmann, H.; Greenberg, S.M. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010, 74, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Zompola, C.; Theodorou, A.; Katsanos, A.H.; Shoamanesh, A.; Gupta, H.; Beshara, S.; Goyal, N.; Chang, J.; Tayal, A.H.; et al. Prevalence, characteristics, and outcomes of undetermined intracerebral hemorrhage: A systematic review and meta-analysis. Stroke 2021, 52, 3602–3612. [Google Scholar] [CrossRef]
- Wollenweber, F.A.; Opherk, C.; Zedde, M.; Catak, C.; Malik, R.; Duering, M.; Konieczny, M.J.; Pascarella, R.; Samões, R.; Correia, M.; et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2019, 92, e792–e801. [Google Scholar] [CrossRef]
- Malhotra, K.; Theodorou, A.; Katsanos, A.H.; Zompola, C.; Shoamanesh, A.; Boviatsis, E.; Paraskevas, G.P.; Spilioti, M.; Cordonnier, C.; Werring, D.J.; et al. Prevalence of Clinical and Neuroimaging Markers in Cerebral Amyloid Angiopathy: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 1944–1953. [Google Scholar] [CrossRef]
- Puy, L.; Pasi, M.; Rodrigues, M.; van Veluw, S.J.; Tsivgoulis, G.; Shoamanesh, A.; Cordonnier, C. Cerebral microbleeds: From depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 598–607. [Google Scholar] [CrossRef]
- Lauer, A.; van Veluw, S.J.; William, C.M.; Charidimou, A.; Roongpiboonsopit, D.; Vashkevich, A.; Ayres, A.; Martinez-Ramirez, S.; Gurol, E.M.; Biessels, G.J.; et al. Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology 2016, 87, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Haley, K.; Auriel, E.; van Etten, E.S.; Fotiadis, P.; Reijmer, Y.; Ayres, A.; Vashkevich, A.; Dipucchio, Z.Y.; et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016, 86, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, P.; Reijmer, Y.D.; Van Veluw, S.J.; Martinez-Ramirez, S.; Karahanoglu, F.I.; Gokcal, E.; Schwab, K.M.; Alzheimer’s Disease Neuroimaging Initiative Study Group; Goldstein, J.N.; Rosand, J.; et al. White matter atrophy in cerebral amyloid angiopathy. Neurology 2020, 95, e554–e562. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.M.; Kremer, B.P.H.; Rikkert, M.O.; Van Domburg, P.H.M.F.; Skehan, M.E.; Greenberg, S.M. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann. Neurol. 2009, 66, 245–249. [Google Scholar] [CrossRef]
- Charidimou, A.; Friedrich, J.O.; Greenberg, S.M.; Viswanathan, A. Core cerebrospinal fluid biomarker profile in cerebral amyloid angiopathy: A meta-analysis. Neurology 2018, 90, e754–e762. [Google Scholar] [CrossRef]
- De Kort, A.M.; Kuiperij, H.B.; Marques, T.M.; Jäkel, L.; Berg, E.v.D.; Kersten, I.; van Berckel-Smit, H.E.P.; Duering, M.; Stoops, E.; Abdo, W.F.; et al. Decreased Cerebrospinal Fluid Amyloid β 38, 40, 42, and 43 Levels in Sporadic and Hereditary Cerebral Amyloid Angiopathy. Ann. Neurol. 2023, 93, 1173–1186. [Google Scholar] [CrossRef]
- Charidimou, A.; Zonneveld, H.I.; Shams, S.; Kantarci, K.; Shoamanesh, A.; Hilal, S.; Yates, P.A.; Boulouis, G.; Na, H.K.; Pasi, M.; et al. APOE and cortical superficial siderosis in CAA: Meta-analysis and potential mechanisms. Neurology 2019, 93, e358–e371. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Standards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Pasi, M.; Auriel, E.; van Etten, E.S.; Haley, K.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Goldstein, J.N.; et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017, 88, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Nandigam, R.N.K.; Delgado, P.; Betensky, R.A.; Rosand, J.; Viswanathan, A.; Frosch, M.P.; Smith, E.E. Microbleeds versus macrobleeds: Evidence for distinct entities. Stroke 2009, 40, 2382–2386. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Rosand, J.; Muzikansky, A.; Kumar, A.; Wisco, J.J.; Smith, E.E.; Betensky, R.A.; Greenberg, S.M. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann. Neurol. 2005, 58, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Charidimou, A.; Ayata, C.; Werring, D.J.; Greenberg, S.M. Cerebral Amyloid Angiopathy–Related Transient Focal Neurologic Episodes. Neurology 2021, 97, 231–238. [Google Scholar] [CrossRef]
- Theodorou, A.; Chondrogianni, M.; Bakola, E.; Kaloudi, G.; Foska, A.; Michalakakou, S.; Melanis, K.; Paraskevas, G.P.; Tsivgoulis, G. Cortical Superficial Siderosis and Transient Focal Neurological Episode Preceding Lobar Hemorrhage in Cerebral Amyloid Angiopathy. Stroke 2023, 54, e48–e51. [Google Scholar] [CrossRef]
- Sanchez-Caro, J.M.; Ubago, I.d.L.M.d.; Ruiz, E.d.C.; Arribas, A.B.; Calviere, L.; Raposo, N.; Blancart, R.G.; Fuentes, B.; Diez-Tejedor, E.; Rodriguez-Pardo, J. Transient focal neurological events in cerebral amyloid angiopathy and the longterm risk of intracerebral hemorrhage and death: A systematic review and metaanalysis. JAMA Neurol. 2022, 79, 38–47. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Greenberg, S.M.; Viswanathan, A. Cortical superficial siderosis and bleeding risk in cerebral amyloid angiopathy: A meta-analysis. Neurology 2019, 93, e2192–e2202. [Google Scholar] [CrossRef]
- Case, N.F.; Charlton, A.; Zwiers, A.; Batool, S.; McCreary, C.R.; Hogan, D.B.; Ismail, Z.; Zerna, C.; Coutts, S.B.; Frayne, R.; et al. Cerebral Amyloid Angiopathy Is Associated with Executive Dysfunction and Mild Cognitive Impairment. Stroke 2016, 47, 2010–2016. [Google Scholar] [CrossRef]
- Xiong, L.; Davidsdottir, S.; Reijmer, Y.D.; Shoamanesh, A.; Roongpiboonsopit, D.; Thanprasertsuk, S.; Martinez-Ramirez, S.; Charidimou, A.; Ayres, A.M.; Fotiadis, P.; et al. Cognitive Profile and its Association with Neuroimaging Markers of Non-Demented Cerebral Amyloid Angiopathy Patients in a Stroke Unit. J. Alzheimer’s Dis. 2016, 52, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; van Veluw, S.J.; Bounemia, N.; Charidimou, A.; Pasi, M.; Boulouis, G.; Reijmer, Y.D.; Giese, A.-K.; Davidsdottir, S.; Fotiadis, P.; et al. Cerebral Cortical Microinfarcts on Magnetic Resonance Imaging and Their Association With Cognition in Cerebral Amyloid Angiopathy. Stroke 2018, 49, 2330–2336. [Google Scholar] [CrossRef]
- Xiong, L.; Charidimou, A.; Pasi, M.; Boulouis, G.; Pongpitakmetha, T.; Schirmer, M.D.; Singh, S.; Benson, E.; Gurol, E.M.; Rosand, J.; et al. Predictors for Late Post-Intracerebral Hemorrhage Dementia in Patients with Probable Cerebral Amyloid Angiopathy. J. Alzheimer’s Dis. 2019, 71, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Margraf, N.G.; Jensen-Kondering, U.; Weiler, C.; Leypoldt, F.; Maetzler, W.; Philippen, S.; Bartsch, T.; Flüh, C.; Röcken, C.; Möller, B.; et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy: New Data and Quantitative Meta-Analysis. Front. Aging Neurosci. 2022, 14, 783996. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature 2023, 613, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat. Rev. Neurol. 2019, 16, 30–42. [Google Scholar] [CrossRef]
- Blitstein, M.K.; Tung, G.A. MRI of cerebral microhemorrhages. Am. J. Roentgenol. 2007, 189, 720–725. [Google Scholar] [CrossRef]
- Kuroedov, D.; Cunha, B.; Pamplona, J.; Castillo, M.; Ramalho, J. Cerebral cavernous malformations: Typical and atypical imaging characteristics. J. Neuroimaging 2023, 33, 202–217. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.-W.; Xu, W.-H. High-resolution MRI of radiation-induced intracranial vasculopathy. Neurology 2015, 84, 631. [Google Scholar] [CrossRef]
- Van der Eerden, A.W.; van den Heuvel, T.L.A.; Maas, M.C.; Vart, P.; Vos, P.E.; Platel, B.; Góraj, B.M.; Manniesing, R. The radiological interpretation of possible microbleeds after moderate or severe traumatic brain injury: A longitudinal study. Neuroradiology 2022, 64, 1145–1156. [Google Scholar] [CrossRef]
- Di Donato, I.; Bianchi, S.; De Stefano, N.; Dichgans, M.; Dotti, M.T.; Duering, M.; Jouvent, E.; Korczyn, A.D.; Lesnik-Oberstein, S.A.J.; Malandrini, A.; et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: Update on clinical, diagnostic, and management aspects. BMC Med. 2017, 15, 41. [Google Scholar] [CrossRef]
- Lanfranconi, S.; Markus, H.S. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: A systematic review. Stroke 2010, 41, e513–e518. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Palaiodimou, L.; Malhotra, K.; Zompola, C.; Katsanos, A.H.; Shoamanesh, A.; Boviatsis, E.; Dardiotis, E.; Spilioti, M.; Sacco, S.; et al. Clinical, Neuroimaging, and Genetic Markers in Cerebral Amyloid Angiopathy-Related Inflammation: A Systematic Review and Meta-Analysis. Stroke 2023, 54, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Antolini, L.; DiFrancesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef] [PubMed]
- Rannikmäe, K.; Samarasekera, N.; Martînez-Gonzâlez, N.A.; Al-Shahi Salman, R.; Sudlow, C.L. Genetics of cerebral amyloid angiopathy: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 901–908. [Google Scholar] [CrossRef] [PubMed]
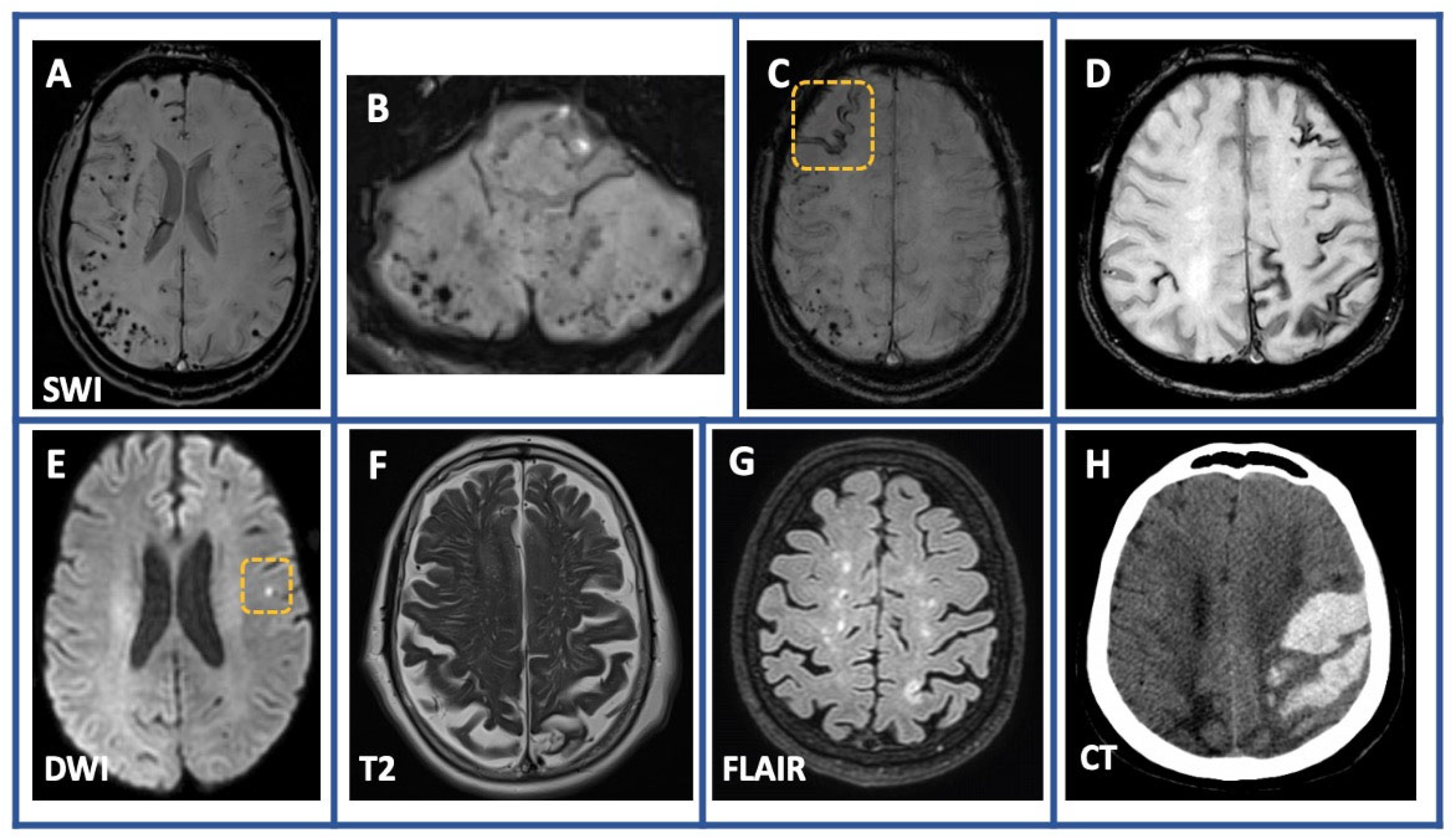
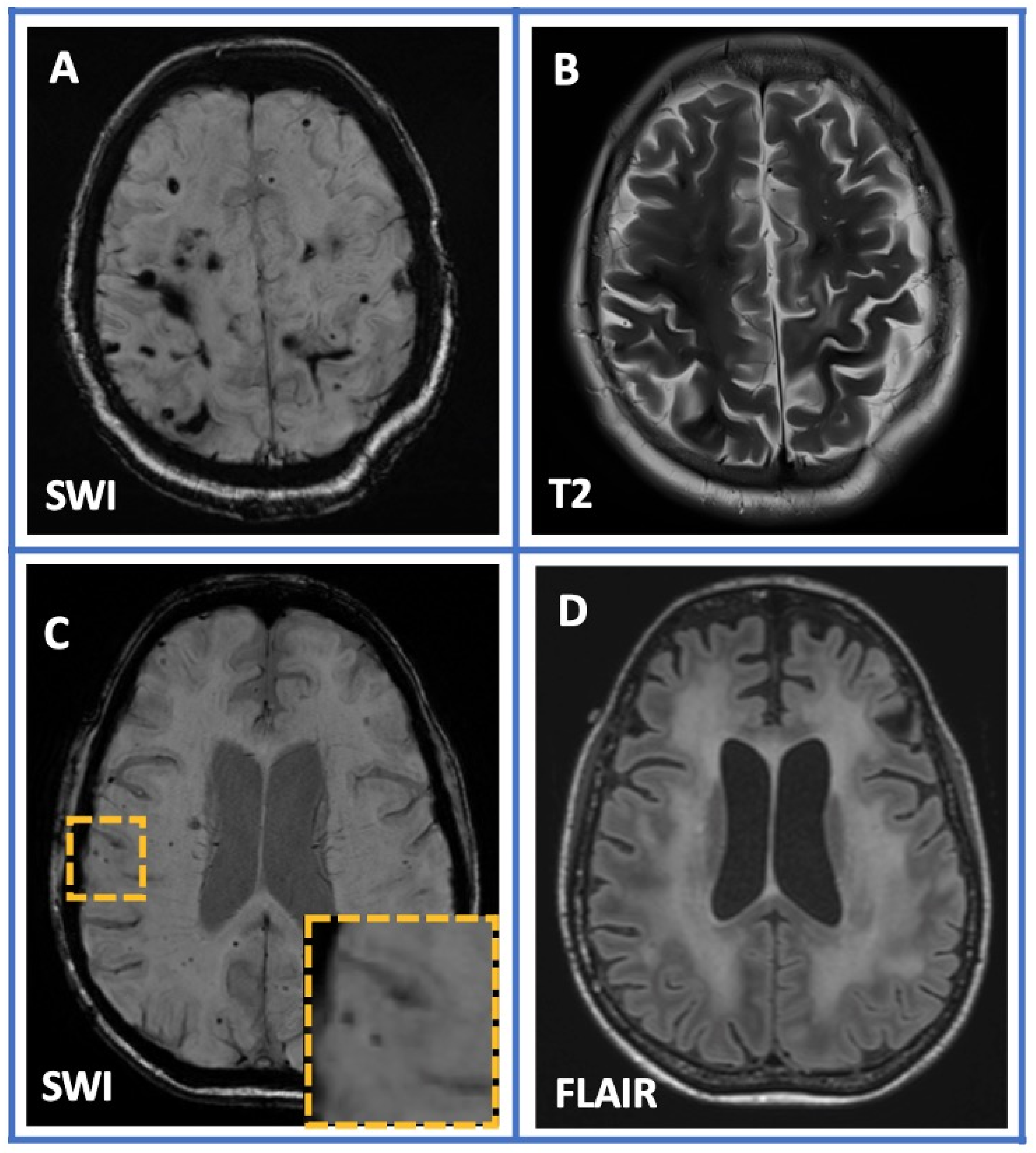
| Variable | Overall (n = 68) |
|---|---|
| Age at presentation (years), mean (SD*) | 70.9 (8.3) |
| Sex—male, n (%) | 36 (52.9) |
| Diagnosis based on biopsy/autopsy, n (%) | 0 (0.0) |
| Diagnosis based on Boston Criteria v.1.5 Definite—probable—possible, n (%) | 0 (0.0)–53 (77.9)–15 (22.1) |
| History of brain surgery, n (%) | 0 (0.0) |
| 3 Tesla brain MRI SWI vs. GRE-T2* sequence | 52 (76.5%) 57 (80.9%) |
| Follow-up (months), median (IQR*) | 20 (12–40) |
| Vascular risk factors | |
| Arterial hypertension, n (%) | 48 (70.6) |
| Hyperlipidemia, n (%) | 38 (55.9) |
| Diabetes mellitus, n (%) | 14 (20.6) |
| History of atrial fibrillation, n (%) Left atrial appendance occlusion, n (%) | 8 (11.8) 3 (37.5) |
| Antiplatelet use at presentation | 18 (26.5) |
| Anticoagulant use at presentation | 6 (8.8) |
| Treatment with rtPA at presentation | 1 (0.01) |
| Clinical signs at presentation | |
| Signs of intracerebral hemorrhage, n (%) | 29 (42.6) |
| Focal neurological signs, n (%) | 51 (75.0) |
| TFNEs, n (%) | 14 (20.6) |
| Cognitive impairment, n (%) | 39 (57.4) |
| MMSE—score, median (IQR*) | 24 (21–27) |
| Headache, n (%) | 17 (25.0) |
| Seizures, n (%) | 11 (16.2) |
| Behavioral changes/ psychiatric signs, n (%) | 19 (27.9) |
| Neuroimaging findings at presentation | |
| Lobar hemorrhage, n (%) | 29 (42.6) |
| Lobar cerebral microbleeds, n (%) | 62 (92.5) |
| Cerebellar microbleeds, n (%) | 26 (38.8) |
| cSAH, n (%) | 9 (13.2) |
| cSS, n (%) Disseminated cSS vs. focal cSS, n (%) | 32 (47.8) 21 (30.9) |
| Cerebellar cSS, n (%) | 0 (0.0) |
| Enlarged perivascular spaces in centrum semiovale, n (%) | 34 (50.0) |
| Multispot white matter hyperintensities pattern, n (%) | 34 (50.0) |
| Cortical microinfarcts, n (%) | 13 (19.4) |
| Gd+ enhancement, n (%) | 4 (0.06) |
| CSF biomarkers and APOE-genotype | |
| APOE 3/3–2/+–4/+ | 11 (61.1)–4 (22.2)–3 (16.7) |
| Total tau | 528.9 (347.8) |
| Phospho–tau | 67.8 (36.9) |
| Amyloid β40 | 6697.0 (1684.5) |
| Amyloid β42 | 452.4 (204.9) |
| Aβ42/ Aβ40 | 0.076 (0.038) |
| Clinical Phenotypes and Neuroimaging Markers | Present Cohort | Recent Meta-Analysis |
|---|---|---|
| Lobar cerebral microbleeds | 93% | 52% |
| cSS | 48% | 49% |
| ICH | 43% | 44% |
| Microinfarcts | 19% | 30% |
| High grades of perivascular spaces located in centrum semiovale | 50% | 56% |
| White matter hyperintensities | 50% | 53% |
| MCI/dementia | 57% | 50% |
| TFNEs | 21% | 48% |
| Variable | Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value * | Odds Ratio (95% CI) | p-Value * | |
| Age | 1.055 (0.979–1.141) | 0.169 | ||
| Age (per 10-year increase) | 1.634 (0.780–3.579) | 0.200 | ||
| Male (sex) | 0.207 (0.043–0.756) | 0.027 | 0.628 (0.169–2.263) | 0.475 |
| Arterial hypertension | 1.676 (0.448–8.155) | 0.472 | ||
| Radiological Findings at Presentation | ||||
| ICH | - | 0.993 | ||
| Lobar CMBs | 0.153 (0.018–1.024) | 0.053 | 0.276 (0.024–2.504) | 0.256 |
| Number of lobar CMBs (>10) | 0.299 (0.069–1.377) | 0.106 | ||
| Cerebellar CMBs | 0.509 (0.126–1.750) | 0.304 | ||
| cSAH | 2.046 (0.385–9.143) | 0.361 | ||
| cSS | 3.750 (1.091–15.229) | 0.045 | 4.504 (1.258–19.088) | 0.027 |
| Disseminated vs. focal cSS | 2.154 (0.395–16.938) | 0.404 | ||
| Multispot white matter Hyperintensities pattern | 2.604 (0.758–10.540) | 0.145 | ||
| EPVS in CSO | 0.960 (0.288–3.203) | 0.946 | ||
| Cortical microinfarcts | 4.607 (1.21–17.861) | 0.024 | 1.209 (0.244–5.631) | 0.808 |
| Variable | Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value * | Odds Ratio (95% CI) | p-value * | |
| Age | 1.024 (0.965–1.090) | 0.441 | ||
| Age (per 10-year increase) | 1.353 (0.732–2.581) | 0.341 | ||
| Male (sex) | 1.569 (0.586–4.284) | 0.372 | ||
| Arterial hypertension | 0.694 (0.223–2.051) | 0.515 | ||
| Radiological Findings at Presentation | ||||
| ICH | 2.639 (0.951–7.789) | 0.068 | 2.616 (0.843–8.873) | 0.105 |
| Lobar CMBs | 2.188 (0.338–17.568) | 0.41 | ||
| Number of lobar CMBs (>10) | 5.333 (1.343–26.967) | 0.024 | 5.418 (1.316–28.497) | 0.027 |
| Cerebellar CMBs | 1.295 (0.471–3.647) | 0.618 | ||
| cSAH | 1.613 (0.384–8.248) | 0.527 | ||
| cSS | 2.625 (0.954–7.606) | 0.067 | 1.613 (0.384–8.248) | 0.527 |
| Disseminated vs. focal cSS | 1.250 (0.210–6.602) | 0.794 | ||
| Multispot white matter Hyperintensities pattern | 0.688 (0.247–1.875) | 0.466 | ||
| EPVS in CSO | 0.929 (0.339–2.529) | 0.884 | ||
| Cortical microinfarcts | 0.553 (0.157–1.895) | 0.344 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorou, A.; Palaiodimou, L.; Papagiannopoulou, G.; Kargiotis, O.; Psychogios, K.; Safouris, A.; Bakola, E.; Chondrogianni, M.; Kotsali-Peteinelli, V.; Melanis, K.; et al. Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5591. https://doi.org/10.3390/jcm12175591
Theodorou A, Palaiodimou L, Papagiannopoulou G, Kargiotis O, Psychogios K, Safouris A, Bakola E, Chondrogianni M, Kotsali-Peteinelli V, Melanis K, et al. Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(17):5591. https://doi.org/10.3390/jcm12175591
Chicago/Turabian StyleTheodorou, Aikaterini, Lina Palaiodimou, Georgia Papagiannopoulou, Odysseas Kargiotis, Klearchos Psychogios, Apostolos Safouris, Eleni Bakola, Maria Chondrogianni, Vasiliki Kotsali-Peteinelli, Konstantinos Melanis, and et al. 2023. "Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study" Journal of Clinical Medicine 12, no. 17: 5591. https://doi.org/10.3390/jcm12175591
APA StyleTheodorou, A., Palaiodimou, L., Papagiannopoulou, G., Kargiotis, O., Psychogios, K., Safouris, A., Bakola, E., Chondrogianni, M., Kotsali-Peteinelli, V., Melanis, K., Tsibonakis, A., Andreadou, E., Vasilopoulou, S., Lachanis, S., Velonakis, G., Tzavellas, E., Tzartos, J. S., Voumvourakis, K., Paraskevas, G. P., & Tsivgoulis, G. (2023). Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. Journal of Clinical Medicine, 12(17), 5591. https://doi.org/10.3390/jcm12175591







