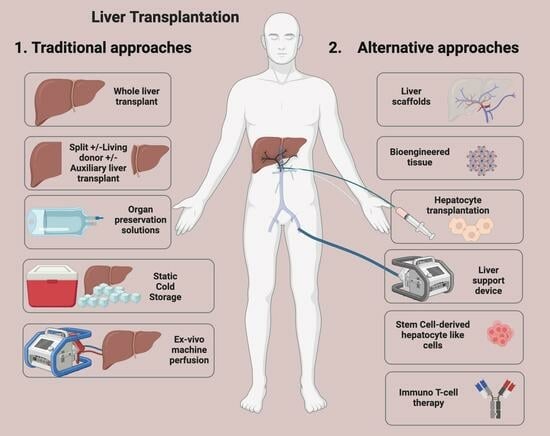
New Developments and Challenges in Liver Transplantation
Abstract
1. Introduction
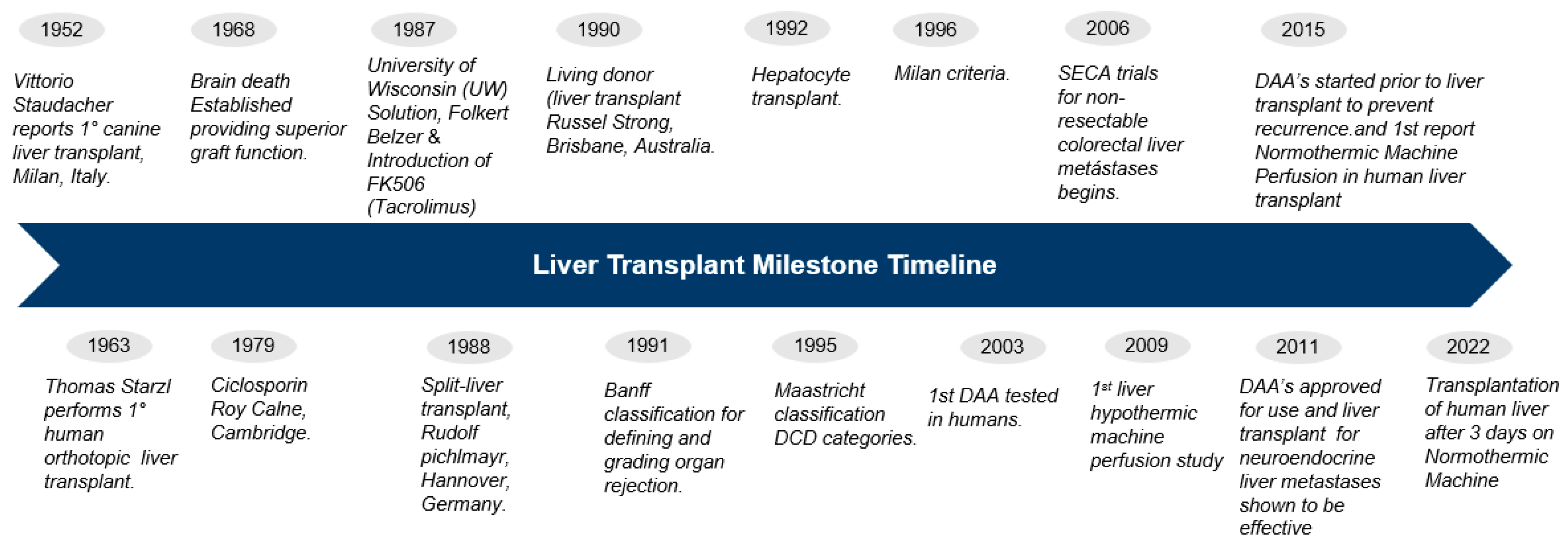
2. Key Developments
2.1. Brain Death Legislation
2.2. Drug Therapies for Immunosuppression
2.3. Organ Preservation Solutions
2.4. Coagulation Control
2.5. Donor after Brain Death (DBD) and Donor after Circulatory Death (DCD)
2.6. Living Donor Liver Transplantation (LDLT)
Auxiliary Liver Transplantation
2.7. Hepatocyte Transplant
2.8. Machine Perfusion
2.8.1. Hypothermic Machine Perfusion (HMP)
2.8.2. Normothermic Machine Perfusion (NMP)
2.9. Liver Transplantation for Viral Hepatitis
2.9.1. Hepatitis C (HCV)
2.9.2. Hepatitis B (HBV)
2.10. Transplant for Cancer
2.11. Improving the Diagnosis of Organ Rejection: The Banff Classification
2.12. Organ Allocation Prioritisation
3. Current Limitations to Liver Transplant
3.1. Limited Availability of Organ Transplantation
3.2. Transplant Organ Shortage
3.3. Use of High-Risk Donor Organs
3.4. Cost of Liver Transplantation
3.5. Obesity and Non-Alcoholic Steatohepatitis (NASH)
3.5.1. Bariatric Surgery Pre-Liver Transplant
3.5.2. Bariatric Surgery during Liver Transplant
3.5.3. Bariatric Surgery Post-Liver Transplant
4. Developing Strategies in Liver Transplant
4.1. Reducing Transplant Organ Requirements: Artificial and Bioartificial Liver Support Devices
4.2. Organ Assessment and Recovery Centres (ARCs)
4.3. Liver Scaffolds
4.4. Cell Therapy for Liver Failure
4.5. Gene Therapy for Metabolic Liver Disease
4.6. Immuno-T-Cell Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zarrinpar, A.; Busuttil, R.W. Liver transplantation: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Iwatsuki, S.; Van Thiel, D.H.; Gartner, J.C.; Zitelli, B.J.; Malatack, J.J.; Schade, R.R.; Shaw, B.W., Jr.; Hakala, T.R.; Rosenthal, J.T.; et al. Evolution of liver transplantation. Hepatology 1982, 2, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Calne, R.Y.; Williams, R. Liver transplantation in man. I. Observations on technique and organization in five cases. Br. Med. J. 1968, 4, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Rampes, S.; Ma, D. Hepatic ischemia-reperfusion injury in liver transplant setting: Mechanisms and protective strategies. J. Biomed. Res. 2019, 33, 221–234. [Google Scholar] [CrossRef]
- Merion, R.M.; Sharma, P.; Mathur, A.K.; Schaubel, D.E. Evidence-based development of liver allocation: A review. Transpl. Int. 2011, 24, 965–972. [Google Scholar] [CrossRef]
- Calne, R.Y.; Rolles, K.; White, D.J.; Thiru, S.; Evans, D.B.; McMaster, P.; Dunn, D.C.; Craddock, G.N.; Henderson, R.G.; Aziz, S.; et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979, 2, 1033–1036. [Google Scholar] [CrossRef]
- Fung, J.J.; Abu-Elmagd, K.; Todo, S.; Shapiro, R.; Tzakis, A.; Jordan, M.; Armitage, J.; Jain, A.; Alessiani, M.; Martin, M.; et al. Overview of FK506 in transplantation. Clin. Transpl. 1990, 1990, 115–121. [Google Scholar]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Doenecke, A.; Sothmann, J.L.; Loss, M.; Farkas, S.A.; Hartl, J.; Tsui, T.Y.; Baier, L.; Kirchner, G.; Obed, A.; et al. Improved outcome after ‘bottom-up’ immunosuppression in liver transplant recipients with preoperative renal impairment. Eur. Surg. Res. 2010, 45, 356–367. [Google Scholar] [CrossRef]
- Lerut, J.; Iesari, S. Samuele Iesari.Immunosuppression and Liver Transplantation. Engineering 2023, 21, 175–187. [Google Scholar] [CrossRef]
- Kalayoglu, M.; Sollinger, H.W.; Stratta, R.J.; D’Alessandro, A.M.; Hoffmann, R.M.; Pirsch, J.D.; Belzer, F.O. Extended preservation of the liver for clinical transplantation. Lancet 1988, 1, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Todo, S.; Nery, J.; Yanaga, K.; Podesta, L.; Gordon, R.D.; Starzl, T.E. Extended preservation of human liver grafts with UW solution. JAMA 1989, 261, 711–714. [Google Scholar] [CrossRef]
- Cleland, S.; Corredor, C.; Ye, J.J.; Srinivas, C.; McCluskey, S.A. Massive haemorrhage in liver transplantation: Consequences, prediction and management. World J. Transplant. 2016, 6, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Dalmau, A.; Sabate, A.; Lama, C.; Llado, L.; Figueras, J.; Jaurrieta, E. Intraoperative red blood cell transfusion in liver transplantation: Influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003, 9, 1320–1327. [Google Scholar] [CrossRef]
- Croome, K.P.; Taner, C.B. The Changing Landscapes in DCD Liver Transplantation. Curr. Transpl. Rep. 2020, 7, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Widmer, J.; Eden, J.; Carvalho, M.F.; Dutkowski, P.; Schlegel, A. Machine Perfusion for Extended Criteria Donor Livers: What Challenges Remain? J. Clin. Med. 2022, 11, 5218. [Google Scholar] [CrossRef]
- Organ Donation and Transplantation Activity Report 2021/22 (NHS Blood and Transplant, 2022). Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27107/activity-report-2021-2022.pdf (accessed on 10 February 2023).
- Hwang, S.; Lee, S.G.; Belghiti, J. Liver transplantation for HCC: Its role: Eastern and Western perspectives. J. Hepatobiliary Pancreat. Sci. 2010, 17, 443–448. [Google Scholar] [CrossRef]
- Bismuth, H.; Houssin, D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery 1984, 95, 367–370. [Google Scholar]
- Pichlmayr, R.; Ringe, B.; Gubernatis, G.; Hauss, J.; Bunzendahl, H. Transplantation of a donor liver to 2 recipients (splitting transplantation)—A new method in the further development of segmental liver transplantation. Langenbecks Arch. Chir. 1988, 373, 127–130. (In German) [Google Scholar] [CrossRef]
- Strong, R.W.; Lynch, S.V.; Ong, T.H.; Matsunami, H.; Koido, Y.; Balderson, G.A. Successful liver transplantation from a living donor to her son. N. Engl. J. Med. 1990, 322, 1505–1507. [Google Scholar] [CrossRef]
- Navarro, J.G.; Choi, G.H.; Kim, M.S.; Jung, Y.B.; Lee, J.G. Right anterior section graft for living-donor liver transplantation: A case report. Medicine 2019, 98, e15212. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, S.; Abe, Y.; Shimata, K.; Narita, Y.; Irie, T.; Yamamoto, H.; Sugawara, Y.; Hibi, T. Living Donor Liver Transplantation With a Left Trisection Plus Caudate Lobe Graft. Liver Transpl. 2019, 25, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R. Living donor liver transplantation: Eliminating the wait for death in end-stage liver disease? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 373–382. [Google Scholar] [CrossRef]
- Lee, K.W.; Choi, Y.; Hong, S.K.; Lee, S.; Hong, S.Y.; Suh, S.; Han, E.S.; Yi, N.J.; Suh, K.S. Laparoscopic donor and recipient hepatectomy followed by robot-assisted liver graft implantation in living donor liver transplantation. Am. J. Transplant. 2022, 22, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Broering, D.; Sturdevant, M.L.; Zidan, A. Robotic donor hepatectomy: A major breakthrough in living donor liver transplantation. Am. J. Transplant. 2022, 22, 14–23. [Google Scholar] [CrossRef]
- Cherqui, D.; Soubrane, O.; Husson, E.; Barshasz, E.; Vignaux, O.; Ghimouz, M.; Branchereau, S.; Chardot, C.; Gauthier, F.; Fagniez, P.L.; et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002, 359, 392–396. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Tzvetanov, I.; Jeon, H.; Bianco, F.; Spaggiari, M.; Oberholzer, J.; Benedetti, E. Robot-assisted right lobe donor hepatectomy. Transpl. Int. 2012, 25, e5–e9. [Google Scholar] [CrossRef]
- Brunner, S.M.; Brennfleck, F.W.; Junger, H.; Grosse, J.; Knoppke, B.; Geissler, E.K.; Melter, M.; Schlitt, H.J. Successful auxiliary two-staged partial resection liver transplantation (ASPIRE-LTx) for end-stage liver disease to avoid small-for-size situations. BMC Surg. 2021, 21, 166. [Google Scholar] [CrossRef]
- Ito, M.; Nagata, H.; Miyakawa, S.; Fox, I.J. Review of hepatocyte transplantation. J. Hepatobiliary Pancreat. Surg. 2009, 16, 97–100. [Google Scholar] [CrossRef]
- Fox, I.J.; Chowdhury, J.R. Hepatocyte transplantation. Am. J. Transplant. 2004, 4, 7–13. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, A.; Lehec, S.C.; Hughes, R.D.; Mitry, R.R.; Knisely, A.S.; Devereaux, S.; Richards, J.; Rela, M.; Heaton, N.D.; Portmann, B.C.; et al. Liver after hepatocyte transplantation for liver-based metabolic disorders in children. Cell Transplant. 2008, 17, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Carrel, A.; Lindbergh, C.A. The Culture of Whole Organs. Science 1935, 81, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, D.; Brassil, J. Machine perfusion of the liver: Past, present and future. Curr. Opin. Organ. Transplant. 2010, 15, 160–166. [Google Scholar] [CrossRef]
- Song, A.T.; Avelino-Silva, V.I.; Pecora, R.A.; Pugliese, V.; D’Albuquerque, L.A.; Abdala, E. Liver transplantation: Fifty years of experience. World J. Gastroenterol. 2014, 20, 5363–5374. [Google Scholar] [CrossRef]
- Abbas, S.H.; Friend, P.J. Principles and current status of abdominal organ preservation for transplantation. Surg. Pract. Sci. 2020, 3, 100020. [Google Scholar] [CrossRef]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis C research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- Kao, J.-H.; Chen, D.-S. Universal hepatitis B vaccination: Killing 2 birds with 1 stone. Am. J. Med. 2008, 121, 1029–1031. [Google Scholar] [CrossRef]
- Beasley, R.P. Hepatitis B virus as the etiologic agent in hepatocellular carcinoma—Epidemiologic considerations. Hepatology 1982, 2, 21S–26S. [Google Scholar]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.; Lau, D.T.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18035. [Google Scholar] [CrossRef]
- Flemming, J.A.; Kim, W.R.; Brosgart, C.L.; Terrault, N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2016, 65, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Terrault, N. Management of hepatitis B in special populations. Best. Pract. Res. Clin. Gastroenterol. 2017, 31, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A. Liver Transplantation for Hepatocellular Carcinoma beyond Milan Criteria: Multidisciplinary Approach to Improve Outcome. ISRN Hepatol. 2014, 2014, 706945. [Google Scholar] [CrossRef] [PubMed]
- Ince, V.; Sahin, T.T.; Akbulut, S.; Yilmaz, S. Liver transplantation for hepatocellular carcinoma: Historical evolution of transplantation criteria. World J. Clin. Cases 2022, 10, 10413–10427. [Google Scholar] [CrossRef]
- Farkas, S.; Hackl, C.; Schlitt, H.J. Overview of the indications and contraindications for liver transplantation. Cold Spring Harb. Perspect. Med. 2014, 4, a015602. [Google Scholar] [CrossRef]
- Zanetto, A.; Shalaby, S.; Gambato, M.; Germani, G.; Senzolo, M.; Bizzaro, D.; Russo, F.P.; Burra, P. New Indications for Liver Transplantation. J. Clin. Med. 2021, 10, 3867. [Google Scholar] [CrossRef]
- Ali, J.M.; Bonomo, L.; Brais, R.; Griffiths, W.J.; Lomas, D.J.; Huguet, E.L.; Praseedom, R.K.; Jamieson, N.V.; Jah, A. Outcomes and diagnostic challenges posed by incidental cholangiocarcinoma after liver transplantation. Transplantation 2011, 91, 1392–1397. [Google Scholar] [CrossRef]
- Hagness, M.; Foss, A.; Line, P.D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, O.; Gladhaug, I.P.; Egge, T.S.; et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef]
- Adam, R.; Cailliez, V.; Majno, P.; Karam, V.; McMaster, P.; Calne, R.Y.; O’Grady, J.; Pichlmayr, R.; Neuhaus, P.; Otte, J.B.; et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet 2000, 356, 621–627. [Google Scholar] [CrossRef]
- Tsien, C.; Tan, H.; Sharma, S.; Palaniyappan, N.; Wijayasiri, P.; Leung, K.; Hayre, J.; Mowlem, E.; Kang, R.; Eddowes, P.J.; et al. Long-term outcomes of liver transplant recipients followed up in non-transplant centres: Care closer to home. Clin. Med. 2021, 21, e32–e38. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Saigal, S.; Bansal, R.K.; Saraf, N.; Gautam, D.; Soin, A.S. Acute and Chronic Rejection After Liver Transplantation: What A Clinician Needs to Know. J. Clin. Exp. Hepatol. 2017, 7, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J. Diagnosis of renal transplant rejection: Banff classification and beyond. Kidney Res. Clin. Pract. 2020, 39, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Ormonde, D.G.; de Boer, W.B.; Kierath, A.; Bell, R.; Shilkin, K.B.; House, A.K.; Jeffrey, G.P.; Reed, W.D. Banff schema for grading liver allograft rejection: Utility in clinical practice. Liver Transpl. Surg. 1999, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Demetris, A.J.; Batts, K.P.; Dhillon, A.P.; Ferrell, L.; Fung, J.; Geller, S.A.; Hart, J.; Hayry, P.; Hofmann, W.J.; Hubscher, S.; et al. Banff schema for grading liver allograft rejection: An international consensus document. Hepatology 1997, 25, 658–663. [Google Scholar] [CrossRef]
- Demetris, A.; Adams, D.; Bellamy, C.; Blakolmer, K.; Clouston, A.; Dhillon, A.P.; Fung, J.; Gouw, A.; Gustafsson, B.; Haga, H.; et al. Update of the International Banff Schema for Liver Allograft Rejection: Working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Heparology 2000, 31, 792–799. [Google Scholar] [CrossRef]
- Banff Working Group; Demetris, A.J.; Adeyi, O.; Bellamy, C.O.; Clouston, A.; Charlotte, F.; Czaja, A.; Daskal, I.; El-Monayeri, M.S.; Fontes, P.; et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology 2006, 44, 489–501. [Google Scholar] [CrossRef]
- Banff Working Group on Liver Allograft Pathology; Demetris, A. Importance of liver biopsy findings in immunosuppression management: Biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl. 2012, 18, 1154–1170. [Google Scholar] [CrossRef]
- Haas, M.; Sis, B.; Racusen, L.C.; Solez, K.; Glotz, D.; Colvin, R.B.; Castro, M.C.; David, D.S.; David-Neto, E.; Bagnasco, S.M.; et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 2014, 14, 272–283. [Google Scholar] [CrossRef]
- Demetris, A.J.; Bellamy, C.; Hübscher, S.G.; O’Leary, J.; Randhawa, P.S.; Feng, S.; Neil, D.; Colvin, R.B.; McCaughan, G.; Fung, J.J.; et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Neil, D.A.H.; Minervini, M.; Smith, M.L.; Hubscher, S.G.; Brunt, E.M.; Demetris, A.J. Banff consensus recommendations for steatosis assessment in donor livers. Hepatology 2022, 75, 1014–1025. [Google Scholar] [CrossRef]
- Krishnan, A.; Woreta, T.A.; Vaidya, D.; Liu, Y.; Hamilton, J.P.; Hong, K.; Dadabhai, A.; Ma, M. MELD or MELD-Na as a Predictive Model for Mortality Following Transjugular Intrahepatic Portosystemic Shunt Placement. J. Clin. Transl. Hepatol. 2023, 11, 38–44. [Google Scholar] [CrossRef]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Julapalli, V.R.; Kramer, J.R.; El-Serag, H.B.; American Association for the Study of Liver Diseases. Evaluation for liver transplantation: Adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005, 11, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Yan, Y.; Rosenkranz, S.J. The power of the liver transplant waiting list: A case presentation. Am. J. Crit. Care 2014, 23, 510–515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tullius, S.G.; Garcia-Cardena, G. Organ procurement and perfusion before transplantation. N. Engl. J. Med. 2009, 360, 78–80. [Google Scholar] [CrossRef]
- Liew, B.; Nasralla, D.; Iype, S.; Pollok, J.M.; Davidson, B.; Raptis, D.A. Liver transplant outcomes after ex vivo machine perfusion: A meta-analysis. Br. J. Surg. 2021, 108, 1409–1416. [Google Scholar] [CrossRef]
- Risbey, C.W.G.; Pulitano, C. Normothermic Ex Vivo Machine Perfusion for Liver Transplantation: A Systematic Review of Progress in Humans. J. Clin. Med. 2023, 12, 3718. [Google Scholar] [CrossRef]
- Webb, A.N.; Izquierdo, D.L.; Eurich, D.T.; Shapiro, A.M.J.; Bigam, D.L. The Actual Operative Costs of Liver Transplantation and Normothermic Machine Perfusion in a Canadian Setting. Pharmacoecon. Open 2021, 5, 311–318. [Google Scholar] [CrossRef]
- Battistella, S.; D’Arcangelo, F.; Grasso, M.; Zanetto, A.; Gambato, M.; Germani, G.; Senzolo, M.; Russo, F.P.; Burra, P. Liver transplantation for non-alcoholic fatty liver disease: Indications and post-transplant management. Clin. Mol. Hepatol. 2023, 29 (Suppl.), S286–S301. [Google Scholar] [CrossRef]
- Samuel, S.; Abulawi, A.; Malik, R. Hepatitis C and Nonalcoholic Steatohepatitis in the 21st Century: Impact on Liver Disease and Liver Transplantation. Gastroenterol. Insights 2023, 14, 249–270. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Márquez-Guillén, E.; Torre, A. Obesity in the Liver Transplant Setting. Nutrients 2019, 11, 2552. [Google Scholar] [CrossRef]
- Khoraki, J.; Katz, M.G.; Funk, L.M.; Greenberg, J.A.; Fernandez, L.A.; Campos, G.M. Feasibility and outcomes of laparoscopic sleeve gastrectomy after solid organ transplantation. Surg. Obes. Relat. Dis. 2016, 12, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tsamalaidze, L.; Stauffer, J.A.; Arasi, L.C.; Villacreses, D.E.; Franco, J.S.S.; Bowers, S.; Elli, E.F. Laparoscopic Sleeve Gastrectomy for Morbid Obesity in Patients After Orthotopic Liver Transplant: A Matched Case-Control Study. Obes. Surg. 2018, 28, 444–450. [Google Scholar] [CrossRef]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef]
- Shah, N.J.; Mousa, O.Y.; Syed, K.; John, S. Acute On Chronic Liver Failure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499902/ (accessed on 2 January 2023).
- Keshavjee, S. Human organ repair centers: Fact or fiction? JTCVS Open 2020, 3, 164–168. [Google Scholar] [CrossRef]
- Izamis, M.L.; Calhoun, C.; Uygun, B.E.; Guzzardi, M.A.; Price, G.; Luitje, M.; Saeidi, N.; Yarmush, M.L.; Uygun, K. Simple machine perfusion significantly enhances hepatocyte yields of ischemic and fresh rat livers. Cell Med. 2013, 4, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Organ Donation and Transplantation 2030: Meeting the Need (NHS Blood and Transplant, 2022). Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/23463/meeting-the-need-2030.pdf (accessed on 23 February 2023).
- Dai, Q.; Jiang, W.; Huang, F.; Song, F.; Zhang, J.; Zhao, H. Recent Advances in Liver Engineering With Decellularized Scaffold. Front. Bioeng. Biotechnol. 2022, 10, 831477. [Google Scholar] [CrossRef]
- Stevens, K.R.; Scull, M.A.; Ramanan, V.; Fortin, C.L.; Chaturvedi, R.R.; Knouse, K.A.; Xiao, J.W.; Fung, C.; Mirabella, T.; Chen, A.X.; et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci. Transl. Med. 2017, 9, eaah5505. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Luu, N.T.; Alwahsh, S.M.; Ginai, M.; Alhaque, S.; Dong, H.; Tomaz, R.A.; Vernay, B.; Vigneswara, V.; Hallett, J.M.; et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 2018, 92, 3117–3129. [Google Scholar] [CrossRef]
- Zabaleta, N.; Unzu, C.; Weber, N.D.; Gonzalez-Aseguinolaza, G. Gene therapy for liver diseases—Progress and challenges. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 288–305. [Google Scholar] [CrossRef]
- Maestro, S.; Weber, N.D.; Zabaleta, N.; Aldabe, R.; Gonzalez-Aseguinolaza, G. Novel vectors and approaches for gene therapy in liver diseases. JHEP Rep. 2021, 3, 100300. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzad, M.; Hashemi, M.; Lavasani, Z.M.; Hossein-khannazer, N.; Bakhshandeh, H.; Gramignoli, R.; Keshavarz Alikhani, H.; Najimi, M.; Nikeghbalian, S.; Vosough, M. Novel Gene-Correction-Based Therapeutic Modalities for Monogenic Liver Disorders. Bioengineering 2022, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Oo, Y.H.; Perera, M.T.P.R. Regulatory T-Cell Therapy in Liver Transplantation and Chronic Liver Disease. Front. Immunol. 2021, 12, 719954. [Google Scholar] [CrossRef] [PubMed]
| Developing Strategies | (Bio)Artificial Liver Device | Organ Reconditioning Centres | Decellularised Scaffolds for Cell Transplant | Synthetic Scaffolds | Pluripotent Stem Cell-Derived Hepatocytes | Gene Therapy | Immunotherapy/Treg Expansion |
|---|---|---|---|---|---|---|---|
| Stage of development | Clinical | Clinical | Pre-clinical | Pre-clinical | Pre-clinical | Clinical | Clinical |
| Potential benefits | Prevent patients dying in ALF prior to transplant. Support liver function until native recovery. | Addresses scarcity of organs. Cost-saving. Potential for pharmacological and biological interventions. | Addresses scarcity of donor organs. Rapid and organised development of new livers. | Addresses organ scarcity. Easy to synthesise. Not pathogenic. | Address scarcity of donor organs. Unlimited self-renewal capacity. | Prevent the need for transplantation and lifelong immunosuppression and associated morbidity. Addresses scarcity of organs. | Prevent lifelong immunosuppression and associated morbidity. |
| Main limitations | Failed to demonstrate survival benefits. | Dependant on the clinical translation of complimentary bioengineering developments. | Requires donor organ. Unable to completely decellularise without damaging ECM, hindering engraftment of cells, lack of micro-re-vascularisation, thrombogenicity and unfavourable host immune response. | Difficult to produce liver microstructure. Poor biocompatibility. | Reprogramming efficiency low. Concern regarding tumorgenicity. Viability | Tumorgenicity concerns. Transient gene expression. High costs. Immune response to viral vectors. | Tumorgenicity concern. Despite success in autoimmune and animal models, it has not translated with success in transplanted organs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.; Quaglia, A.; Gélat, P.; Saffari, N.; Rashidi, H.; Davidson, B. New Developments and Challenges in Liver Transplantation. J. Clin. Med. 2023, 12, 5586. https://doi.org/10.3390/jcm12175586
Khalil A, Quaglia A, Gélat P, Saffari N, Rashidi H, Davidson B. New Developments and Challenges in Liver Transplantation. Journal of Clinical Medicine. 2023; 12(17):5586. https://doi.org/10.3390/jcm12175586
Chicago/Turabian StyleKhalil, Amjad, Alberto Quaglia, Pierre Gélat, Nader Saffari, Hassan Rashidi, and Brian Davidson. 2023. "New Developments and Challenges in Liver Transplantation" Journal of Clinical Medicine 12, no. 17: 5586. https://doi.org/10.3390/jcm12175586
APA StyleKhalil, A., Quaglia, A., Gélat, P., Saffari, N., Rashidi, H., & Davidson, B. (2023). New Developments and Challenges in Liver Transplantation. Journal of Clinical Medicine, 12(17), 5586. https://doi.org/10.3390/jcm12175586