Thoracic Endometriosis Syndrome (TES) in Martinique, a French West Indies Island
Abstract
:1. Introduction
- -
- Coelomic metaplasia;
- -
- Lymphatic or hematogenous embolization from the uterus or pelvis;
- -
- Retrograde menstruation with subsequent transperitoneal–transdiaphragmatic migration of endometrial tissue,
- -
- Spontaneous rupture of blebs;
- -
- Transdiaphragmatic passage of air from the genital tract;
- -
- Sloughing of endometrial implants from visceral pleura with subsequent air leak;
- -
- Alveolar rupture caused by prostaglandin-induced bronchiolar constriction or by a check-valve mechanism exerted by bronchiolar endometrial implants.
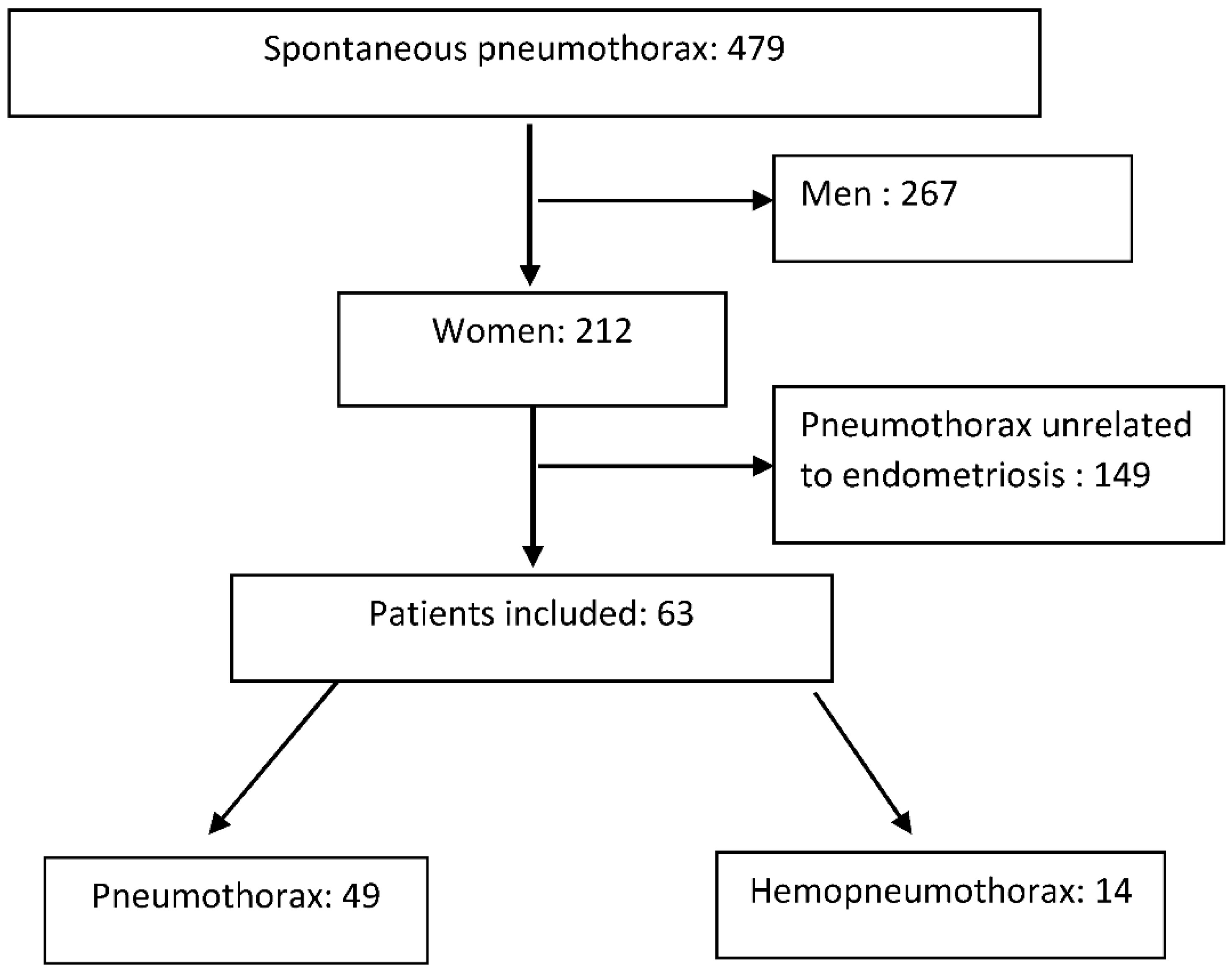
2. Patients and Methods
- -
- Catamenial pneumothorax or endometriosis-related pneumothorax with per-operative findings or histological evidence;
- -
- Catamenial hemothorax or endometriosis-related hemothorax with per-operative findings or histological evidence;
- -
- Catamenial hemoptysis;
- -
- Catamenial pulmonary nodule.
- -
- Surgical management, via a thoracoscopic approach, consisting of exhaustive inspection, partial resection or prosthetic reinforcement of diaphragmatic lesion, resection of lung lesions if present, and systematic pleurodesis (mostly talc pleurodesis);
- -
- Medical management, consisting of 6–12 months of prolonged hormone blockade, usually with Gonadotrophin Releasing Hormone (GnRH) analogues.
Patient and Public Involvement
3. Results
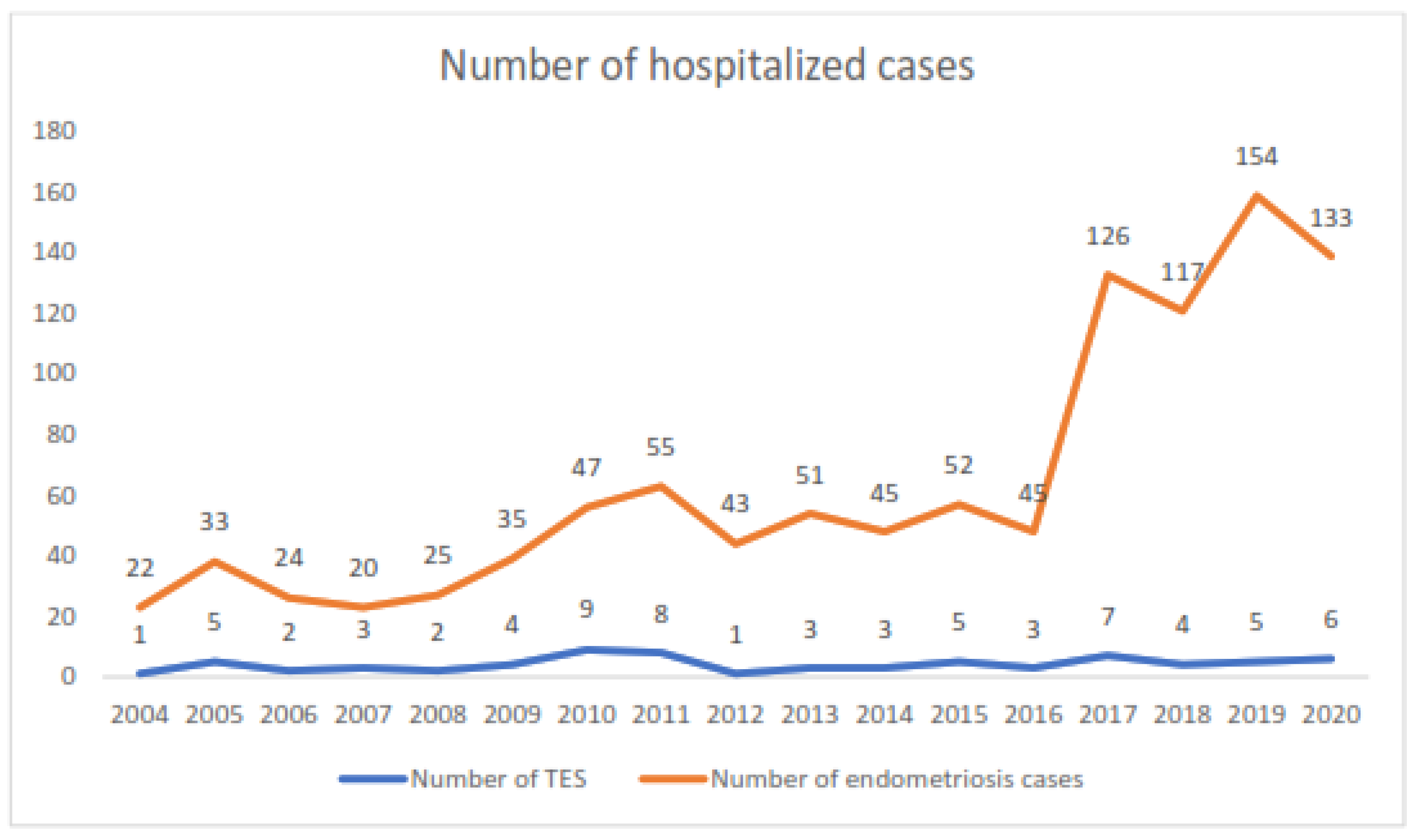
3.1. Epidemiology
3.2. Clinical Presentation
3.3. Management
3.4. Outcomes
- -
- After discontinuation of medical treatment: seventeen cases (38.6%);
- -
- Medical treatment without prior surgery: nine cases (20.5%);
- -
- Surgery alone (without hormonal treatment): six cases (13.6%);
- -
- Pleural evacuation (awaiting diagnostic confirmation): six cases (13.6%);
- -
- Unknown: six cases (13.6%).
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ciriaco, P.; Muriana, P.; Lembo, R.; Carretta, A.; Negri, G. Treatment of Thoracic Endometriosis Syndrome: A Meta-Analysis and Review. Ann. Thorac. Surg. 2022, 113, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.L.; Schwartz, L.B. Endometriosis. N. Engl. J. Med. 1993, 328, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.C.; Goldberg, J.M. Extrapelvic Endometriosis. Semin. Reprod. Med. 2017, 35, 98–101. [Google Scholar]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Clement, P.B. The pathology of endometriosis: A survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Casalechi, M.; Vieira-Lopes, M.; Quessada, M.P.; Arão, T.C.; Reis, F.M. Endometriosis and related pelvic pain: Association with stress, anxiety and depressive symptoms. Minerva Obstet. Gynecol. 2021, 73, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Farina, R.; Palmucci, S.; Vizzini, I.A.A.; Libertini, N.; Coronella, M.; Spadola, S.; Caltabiano, R.; Iraci, M.; Basile, A.; et al. Endometriosis: Clinical features, MR imaging findings and pathologic correlation. Insights Imaging 2018, 9, 149–172. [Google Scholar] [CrossRef]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Lukac, S.; Schmid, M.; Pfister, K.; Janni, W.; Schäffler, H.; Dayan, D. Extragenital Endometriosis in the Differential Diagnosis of Non- Gynecological Diseases. Dtsch. Arztebl. Int. 2022, 119, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Agely, A.; Bolan, C.; Metcalfe, A.; VanBuren, W.; Menias, C. Genitourinary manifestations of endometriosis with emphasis on the urinary tract. Abdom. Radiol. 2020, 45, 1711–1722. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Tiferes, D.A.; de Macedo Neto, A.C.; Serafini, P.C. Atypical Sites of Deeply Infiltrative Endometriosis: Clinical Characteristics and Imaging Findings. Radiographics 2018, 38, 309–328. [Google Scholar] [CrossRef]
- Alifano, M.; Trisolini, R.; Cancellieri, A.; Regnard, J.F. Thoracic endometriosis: Current knowledge. Ann. Thorac. Surg. 2006, 81, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Lindheim, S.R.; Backhus, L.; Vu, M.; Vang, N.; Nezhat, A.; Nezhat, C. Thoracic Endometriosis Syndrome: A Review of Diagnosis and Management. JSLS 2019, 23, e2019.00029. [Google Scholar] [CrossRef]
- Soares, T.; Oliveira, M.A.; Panisset, K.; Habib, N.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. Diaphragmatic endometriosis and thoracic endometriosis syndrome: A review on diagnosis and treatment. Horm. Mol. Biol. Clin. Investig. 2021, 43, 137–143. [Google Scholar] [CrossRef]
- Ciriaco, P.; Muriana, P.; Carretta, A.; Ottolina, J.; Candiani, M.; Negri, G. Catamenial Pneumothorax as the First Expression of Thoracic Endometriosis Syndrome and Pelvic Endometriosis. J. Clin. Med. 2022, 11, 1200. [Google Scholar] [CrossRef]
- Bobbio, A.; Dechartres, A.; Bouam, S.; Damotte, D.; Rabbat, A.; Régnard, J.F.; Roche, N.; Alifano, M. Epidemiology of spontaneous pneumothorax: Gender-related differences. Thorax 2015, 70, 653–658. [Google Scholar] [CrossRef]
- Saito, T.; Saito, Y.; Fukumoto, K.J.; Matsui, H.; Nakano, T.; Taniguchi, Y.; Kaneda, H.; Konobu, T.; Tsuta, K.; Murakawa, T. Clinical and pathological characteristics of spontaneous pneumothorax in women: A 25-year single-institutional experience. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 516–522. [Google Scholar] [CrossRef]
- Alifano, M.; Jablonski, C.; Kadiri, H.; Falcoz, P.; Gompel, A.; Camilleri-Broet, S.; Regnard, J.F. Catamenial and noncatamenial, endometriosis-related or nonendometriosis-related pneumothorax referred for surgery. Am. J. Respir. Crit. Care Med. 2007, 176, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Dossier Complet—Département de la Martinique (972). Insee. Available online: https://www.insee.fr/fr/statistiques/2011101?geo=DEP-972 (accessed on 7 July 2022).
- Lemaire, G.; Mnif, W.; Mauvais, P.; Balaguer, P.; Rahmani, R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006, 79, 1160–1169. [Google Scholar] [CrossRef]
- Coiplet, E.; Courbiere, B.; Agostini, A.; Boubli, L.; Bretelle, F.; Netter, A. Endometriosis and environmental factors: A critical review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102418. [Google Scholar] [CrossRef] [PubMed]
- Legras, A.; Mansuet-Lupo, A.; Rousset-Jablonski, C.; Bobbio, A.; Magdeleinat, P.; Roche, N.; Regnard, J.-F.; Gompel, A.; Damotte, D.; Alifano, M. Pneumothorax in women of child-bearing age: An update classification based on clinical and pathologic findings. Chest 2014, 145, 354–360. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Goldacre, R.; Landray, M.J.; Rahman, N.M.; Goldacre, M.J. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968–2016. JAMA 2018, 320, 1471–1480. [Google Scholar] [CrossRef]
- Hiyama, N.; Sasabuchi, Y.; Jo, T.; Hirata, T.; Osuga, Y.; Nakajima, J.; Yasunaga, H. The three peaks in age distribution of females with pneumothorax: A nationwide database study in Japan. Eur. J. Cardio-Thorac. Surg. 2018, 54, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Kurihara, M.; Tsuboshima, K.; Nonaka, Y.; Kumasaka, T. Dynamics of thoracic endometriosis in the pleural cavity. PLoS ONE 2022, 17, e0268299. [Google Scholar] [CrossRef]
- Gil, Y.; Tulandi, T. Diagnosis and Treatment of Catamenial Pneumothorax: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 48–53. [Google Scholar] [CrossRef]
- Joseph, J.; Sahn, S.A. Thoracic endometriosis syndrome: New observations from an analysis of 110 cases. Am. J. Med. 1996, 100, 164–170. [Google Scholar] [CrossRef]
- Nezhat, C.; Main, J.; Paka, C.; Nezhat, A.; Beygui, R.E. Multidisciplinary treatment for thoracic and abdominopelvic endometriosis. JSLS 2014, 18, e2014.00312. [Google Scholar] [CrossRef]
- Wetzel, A.; Philip, C.A.; Golfier, F.; Bonnot, P.E.; Cotte, E.; Brichon, P.Y.; Darnis, B.; Chene, G.; Michy, T.; Hoffmann, P.; et al. Surgical management of diaphragmatic and thoracic endometriosis’: A French multicentric descriptive study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102147. [Google Scholar] [CrossRef]
- Fukuda, S.; Hirata, T.; Neriishi, K.; Nakazawa, A.; Takamura, M.; Izumi, G.; Harada, M.; Hirota, Y.; Koga, K.; Wada-Hiraike, O.; et al. Thoracic endometriosis syndrome: Comparison between catamenial pneumothorax or endometriosis-related pneumothorax and catamenial hemoptysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 118–123. [Google Scholar] [CrossRef]
- Alifano, M.; Legras, A.; Rousset-Jablonski, C.; Bobbio, A.; Magdeleinat, P.; Damotte, D.; Roche, N.; Regnard, J.-F. Pneumothorax recurrence after surgery in women: Clinicopathologic characteristics and management. Ann. Thorac. Surg. 2011, 92, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG 2019, 126, 1104–1115. [Google Scholar] [CrossRef]
- Sirohi, D.; Al Ramadhani, R.; Knibbs, L.D. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: A systematic literature review. Rev. Environ. Health 2021, 36, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Stephens, V.R.; Rumph, J.T.; Ameli, S.; Bruner-Tran, K.L.; Osteen, K.G. The Potential Relationship Between Environmental Endocrine Disruptor Exposure and the Development of Endometriosis and Adenomyosis. Front. Physiol. 2021, 12, 807685. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean | Standard Deviation | Missing Data |
|---|---|---|---|
| Age at diagnosis of TES | 36 | 6 | 1 |
| Age at diagnosis of pelvic endometriosis | 29 | 6 | 9 |
| Number of pregnancies | 1 | 1 | 28 |
| Number of births | 1 | 1 | 25 |
| Time from symptom onset to diagnosis (weeks) | 24 | 50 | 17 |
| Time to recurrence (months) | 20 | 33 | 0 |
| Medical history | |||
| n | % | ||
| Tobacco/cannabis use | 6 | 12.0 | 7 |
| History of pelvic endometriosis | 57 | 82.6 | 2 |
| Previous contraception | 25 | 38.5 | 6 |
| History of abdominopelvic surgery | 32 | 47.1 | 3 |
| Other localization | 17 | 24.0 | 0 |
| Main presenting symptom | |||
| Dyspnea | 32 | 45.1 | 0 |
| Chest pain | 38 | 53.5 | |
| Cough | 1 | 1.4 | |
| Sidedness | |||
| Right | 67 | 94.4 | 0 |
| Left | 4 | 5.6 | 0 |
| Initial treatment | |||
| Medical treatment | 20 | 28.2 | 0 |
| Surgical treatment | 18 | 25.4 | 0 |
| Combined treatment | 33 | 46.5 | 0 |
| Localizations n = 17 | n | % |
|---|---|---|
| Peritoneum | 8 | 47.1 |
| Umbilical | 3 | 17.6 |
| Colon | 2 | 11.8 |
| Disseminated | 2 | 11.8 |
| Bladder | 1 | 5.9 |
| Liver | 1 | 5.9 |
| Recurrence | n | % | p |
|---|---|---|---|
| Medical treatment n = 19 | 16 | 84.2 | 0.03 |
| Surgery n = 17 | 11 | 64.7 | |
| Combined n = 31 | 17 | 51.8 | |
| Pneumothorax n = 49 | 27 | 55.0 | 0.3 |
| Hemothorax/hemopneumothorax n = 22 | 16 | 72.2 |
| SP Annual Incidence/100,000 | Women SP Annual Incidence/100,000 | Sex Ratio (M/W) | TES Annual Incidence/100,000 | |
|---|---|---|---|---|
| France | 22.7 | 5.3 | 3.3/1 | 0.42 * |
| England | 14.0 | 7.6 | 2.7/1 | 0.16 * |
| Japan | 37.2 | 6.6 | 4.7/1 | 0.42 * |
| Martinique | 7.7 | 3.4 | 1.3/1 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agossou, M.; Sanchez, B.-G.; Alauzen, P.-H.; Olivier, M.; Cécilia-Joseph, E.; Chevallier, L.; Jean-Laurent, M.; Aline-Fardin, A.; Dramé, M.; Venissac, N. Thoracic Endometriosis Syndrome (TES) in Martinique, a French West Indies Island. J. Clin. Med. 2023, 12, 5578. https://doi.org/10.3390/jcm12175578
Agossou M, Sanchez B-G, Alauzen P-H, Olivier M, Cécilia-Joseph E, Chevallier L, Jean-Laurent M, Aline-Fardin A, Dramé M, Venissac N. Thoracic Endometriosis Syndrome (TES) in Martinique, a French West Indies Island. Journal of Clinical Medicine. 2023; 12(17):5578. https://doi.org/10.3390/jcm12175578
Chicago/Turabian StyleAgossou, Moustapha, Bruno-Gilbert Sanchez, Paul-Henri Alauzen, Maud Olivier, Elsa Cécilia-Joseph, Ludivine Chevallier, Mehdi Jean-Laurent, Aude Aline-Fardin, Moustapha Dramé, and Nicolas Venissac. 2023. "Thoracic Endometriosis Syndrome (TES) in Martinique, a French West Indies Island" Journal of Clinical Medicine 12, no. 17: 5578. https://doi.org/10.3390/jcm12175578
APA StyleAgossou, M., Sanchez, B.-G., Alauzen, P.-H., Olivier, M., Cécilia-Joseph, E., Chevallier, L., Jean-Laurent, M., Aline-Fardin, A., Dramé, M., & Venissac, N. (2023). Thoracic Endometriosis Syndrome (TES) in Martinique, a French West Indies Island. Journal of Clinical Medicine, 12(17), 5578. https://doi.org/10.3390/jcm12175578






