Effect of Cardiovascular Risk Factors on 30-Day All-Cause Mortality in Cardiogenic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients, Design and Data Collection
2.2. Inclusion and Exclusion Criteria, Study Endpoints
2.3. Statistical Methods
3. Results
3.1. Study Population
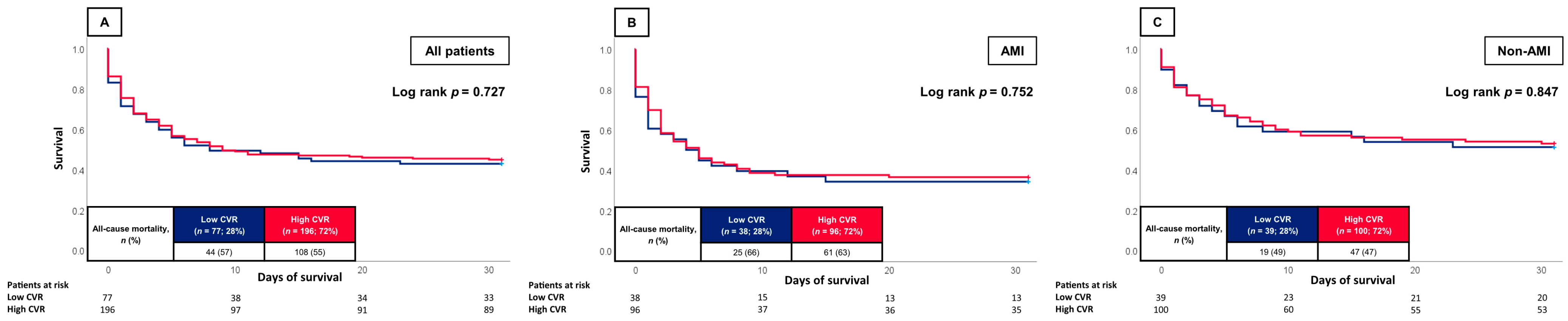
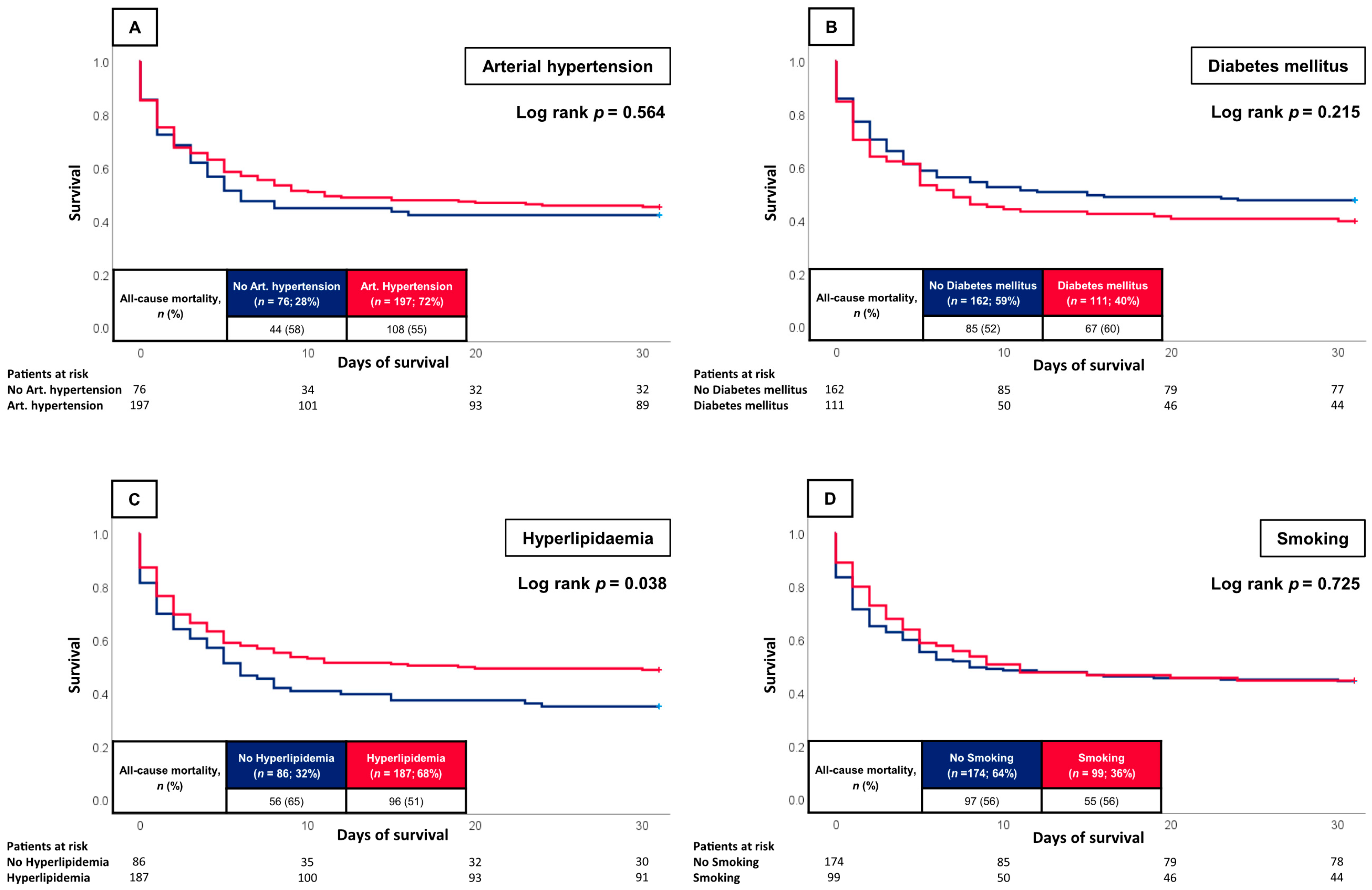
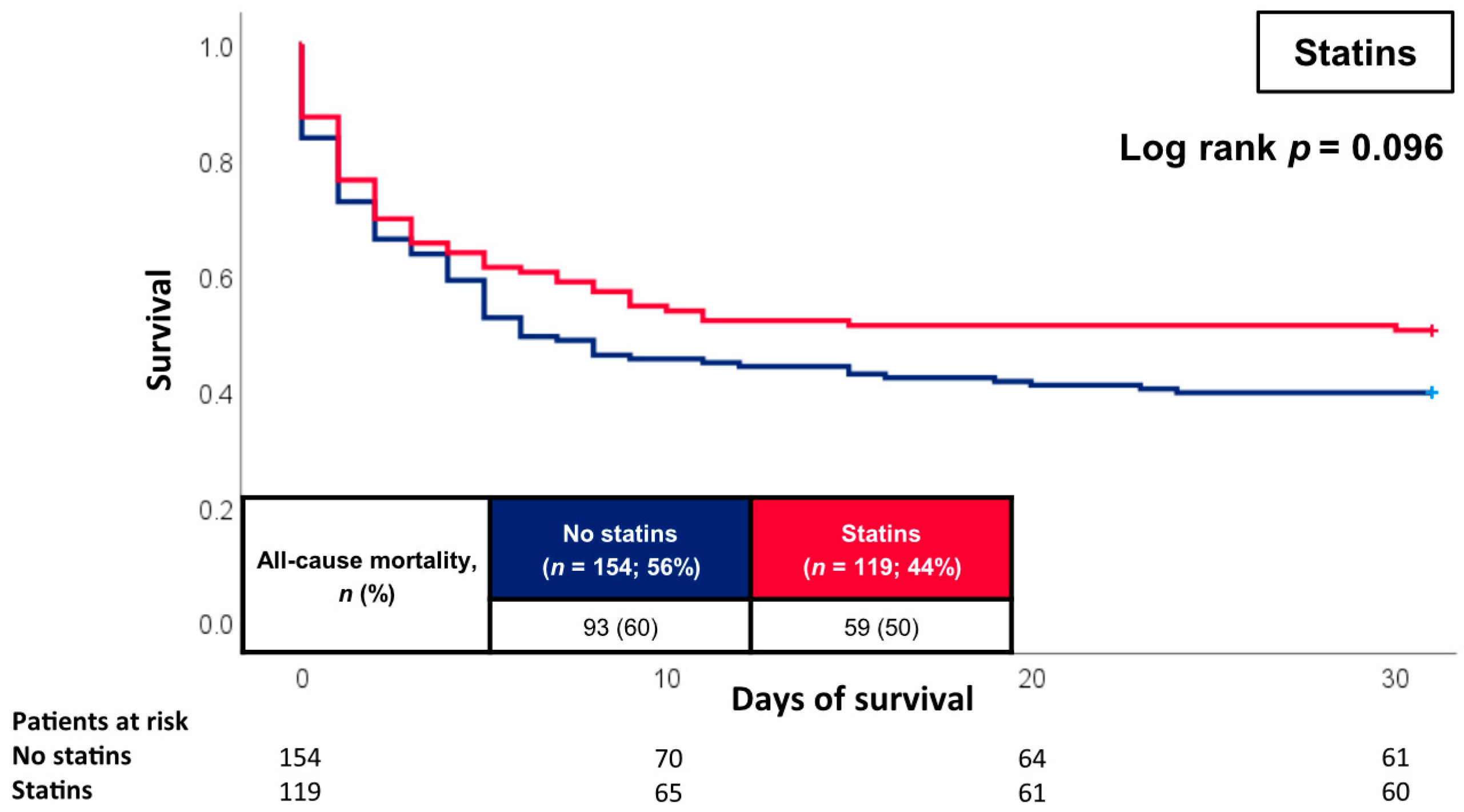
3.2. Prognostic Impact of the CVR
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: A document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 183–197. [Google Scholar] [CrossRef]
- Rathod, K.S.; Koganti, S.; Iqbal, M.B.; Jain, A.K.; Kalra, S.S.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Dalby, M.C.; Lockie, T.; et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 16–27. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Dewaswala, N.; Sundaragiri, P.R.; Bhopalwala, H.M.; Cheungpasitporn, W.; Doshi, R.; Miller, P.E.; Bell, M.R.; Singh, M. Cardiogenic Shock Complicating ST-Segment Elevation Myocardial Infarction: An 18-Year Analysis of Temporal Trends, Epidemiology, Management, and Outcomes. Shock 2022, 57, 360–369. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.A.; Mauri, L.; et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc. Interv. 2016, 9, 341–351. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Weidner, K.; Behnes, M.; Schupp, T.; Rusnak, J.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; Ellguth, D.; Engelke, N.; et al. Type 2 diabetes is independently associated with all-cause mortality secondary to ventricular tachyarrhythmias. Cardiovasc. Diabetol. 2018, 17, 125. [Google Scholar] [CrossRef]
- Saito, Y.; Inohara, T.; Kohsaka, S.; Wada, H.; Takamisawa, I.; Yamaji, K.; Amano, T.; Kobayashi, Y.; Kozuma, K. Characteristics and outcomes of patients with no standard modifiable risk factors undergoing primary revascularization for acute myocardial infarction: Insights from the nationwide Japanese percutaneous coronary intervention registry. Am. Heart J. 2023, 258, 69–76. [Google Scholar] [CrossRef]
- Papazoglou, A.S.; Farmakis, I.T.; Zafeiropoulos, S.; Moysidis, D.V.; Karagiannidis, E.; Stalikas, N.; Kartas, A.; Stamos, K.; Sofidis, G.; Doundoulakis, I.; et al. Angiographic severity in acute coronary syndrome patients with and without standard modifiable risk factors. Front. Cardiovasc. Med. 2022, 9, 934946. [Google Scholar] [CrossRef]
- Kong, G.; Chin, Y.H.; Chong, B.; Goh, R.S.J.; Lim, O.Z.H.; Ng, C.H.; Muthiah, M.; Foo, R.; Vernon, S.T.; Loh, P.H.; et al. Higher mortality in acute coronary syndrome patients without standard modifiable risk factors: Results from a global meta-analysis of 1,285,722 patients. Int. J. Cardiol. 2023, 371, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Chew, N.W.S.; Ng, C.H.; Chin, Y.H.; Zeng, R.; Foo, R.; Chan, K.H.; Low, A.F.; Lee, C.H.; Chan, M.Y.; et al. Long-term outcomes in acute coronary syndrome patients without standard modifiable risk factors: A multi-ethnic retrospective cohort study Of 5400 asian patients. J. Thromb. Thrombolysis 2022, 54, 569–578. [Google Scholar] [CrossRef]
- Kong, G.; Chew, N.W.S.; Ng, C.H.; Chin, Y.H.; Lim, O.Z.H.; Ambhore, A.; Ng, G.; Kong, W.; Poh, K.K.; Foo, R.; et al. Prognostic Outcomes in Acute Myocardial Infarction Patients Without Standard Modifiable Risk Factors: A Multiethnic Study of 8680 Asian Patients. Front. Cardiovasc. Med. 2022, 9, 869168. [Google Scholar] [CrossRef]
- Iwata, J.; Inohara, T.; Shiraishi, Y.; Nakamaru, R.; Niimi, N.; Ueda, I.; Suzuki, M.; Noma, S.; Numasawa, Y.; Fukuda, K.; et al. Standard modifiable cardiovascular risk factors in patients with acute coronary syndrome: A report from multicenter percutaneous coronary intervention registry. J. Cardiol. 2023, 81, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Schupp, T.; Behnes, M.; Rusnak, J.; Ruka, M.; Dudda, J.; Forner, J.; Egner-Walter, S.; Barre, M.; Abumayyaleh, M.; Bertsch, T.; et al. Does Albumin Predict the Risk of Mortality in Patients with Cardiogenic Shock? Int. J. Mol. Sci. 2023, 24, 7375. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2019, 41, 255–323. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Vernon, S.T.; Coffey, S.; Bhindi, R.; Soo Hoo, S.Y.; Nelson, G.I.; Ward, M.R.; Hansen, P.S.; Asrress, K.N.; Chow, C.K.; Celermajer, D.S.; et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 2017, 24, 1824–1830. [Google Scholar] [CrossRef]
- Figtree, G.A.; Vernon, S.T.; Hadziosmanovic, N.; Sundström, J.; Alfredsson, J.; Arnott, C.; Delatour, V.; Leósdóttir, M.; Hagström, E. Mortality in STEMI patients without standard modifiable risk factors: A sex-disaggregated analysis of SWEDEHEART registry data. Lancet 2021, 397, 1085–1094. [Google Scholar] [CrossRef]
- Moledina, S.M.; Rashid, M.; Nolan, J.; Nakao, K.; Sun, L.Y.; Velagapudi, P.; Wilton, S.B.; Volgman, A.S.; Gale, C.P.; Mamas, M.A. Addressing disparities of care in non-ST-segment elevation myocardial infarction patients without standard modifiable risk factors: Insights from a nationwide cohort study. Eur. J. Prev. Cardiol. 2022, 29, 1084–1092. [Google Scholar] [CrossRef]
- Yamamoto, K.; Natsuaki, M.; Morimoto, T.; Shiomi, H.; Takeji, Y.; Yamaji, K.; Matsumura-Nakano, Y.; Yoshikawa, Y.; Yamamoto, E.; Fuki, M.; et al. Coronary Artery Disease Without Standard Cardiovascular Risk Factors. Am. J. Cardiol. 2022, 164, 34–43. [Google Scholar] [CrossRef]
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden cardiac death: Epidemiology and risk factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.; Behnes, M.; Rusnak, J.; Taton, G.; Schupp, T.; Reiser, L.; Bollow, A.; Reichelt, T.; Ellguth, D.; Engelke, N.; et al. Risk factor paradox: No prognostic impact of arterial hypertension and smoking in patients with ventricular tachyarrhythmias. Cardiol. J. 2020, 27, 715–725. [Google Scholar] [CrossRef]
- Avis, S.R.; Vernon, S.T.; Hagström, E.; Figtree, G.A. Coronary artery disease in the absence of traditional risk factors: A call for action. Eur. Heart J. 2021, 42, 3822–3824. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, J.; Behnes, M.; Schupp, T.; Lang, S.; Reiser, L.; Taton, G.; Bollow, A.; Reichelt, T.; Ellguth, D.; Engelke, N.; et al. Statin therapy is associated with improved survival in patients with ventricular tachyarrhythmias. Lipids Health Dis. 2019, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Schupp, T.; Behnes, M.; Ellguth, D.; Müller, J.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; Engelke, N.; Kim, S.H.; et al. Impact of Different Pharmacotherapies on Long-Term Outcomes in Patients with Electrical Storm. Pharmacology 2019, 103, 179–188. [Google Scholar] [CrossRef]
| All Patients (n = 273) | Survivors (n = 121) | Non-Survivors (n = 152) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age, median; (IQR) | 73 | (63–81) | 72 | (62–79) | 74 | (64–81) | 0.152 |
| Male sex, n (%) | 164 | (60.1) | 73 | (60.3) | 91 | (59.9) | 0.938 |
| Body mass index, kg/m2 (median, (IQR)) | 26.3 | (24.2–30.0) | 26.1 | (23.9–28.0) | 26.7 | (24.5–30.5) | 0.111 |
| Entry criteria, (median, (IQR)) | |||||||
| Body temperature (°C) | 36.0 | (35.0–36.6) | 36.1 | (35.3–36.6) | 35.8 | (34.7–36.5) | 0.033 |
| Heart rate (bpm) | 88 | (71–109) | 85 | (69–105) | 94 | (72–111) | 0.049 |
| Systolic blood pressure (mmHg) | 109 | (92–130) | 110 | (94–131) | 106 | (89–127) | 0.228 |
| Respiratory rate (breaths/min) | 20 | (17–24) | 19 | (16–22) | 20 | (18–25) | 0.066 |
| Cardiovascular risk factors, n (%) | |||||||
| Arterial hypertension | 197 | (72.2) | 89 | (73.6) | 108 | (71.1) | 0.647 |
| Diabetes mellitus Type 1 | 2 | (0.7) | 0 | (0.0) | 2 | (1.3) | 0.205 |
| Diabetes mellitus Type 2 | 108 | (39.6) | 44 | (36.4) | 64 | (42.1) | 0.335 |
| Hyperlipidaemia | 187 | (68.5) | 91 | (75.2) | 96 | (63.2) | 0.033 |
| Smoking | 99 | (36.3) | 44 | (36.4) | 55 | (36.2) | 0.976 |
| Prior medical history, n (%) | |||||||
| Coronary artery disease | 101 | (37.0) | 44 | (36.4) | 57 | (37.5) | 0.847 |
| Congestive heart failure | 96 | (35.2) | 43 | (35.5) | 53 | (34.9) | 0.908 |
| Atrial fibrillation | 87 | (31.9) | 39 | (32.2) | 48 | (31.6) | 0.909 |
| Chronic kidney disease | 93 | (34.1) | 41 | (33.9) | 52 | (34.2) | 0.955 |
| Stroke | 37 | (13.6) | 21 | (17.4) | 16 | (10.5) | 0.102 |
| COPD | 52 | (19.0) | 19 | (15.7) | 33 | (21.7) | 0.209 |
| Liver cirrhosis | 9 | (3.3) | 6 | (5.0) | 3 | (2.0) | 0.170 |
| Medication on admission, n (%) | |||||||
| ACE-inhibitor | 92 | (33.7) | 43 | (35.5) | 49 | (32.2) | 0.567 |
| ARB | 48 | (17.6) | 22 | (18.2) | 26 | (17.1) | 0.816 |
| Beta-blocker | 135 | (49.5) | 63 | (52.1) | 72 | (47.4) | 0.441 |
| ARNI | 8 | (2.9) | 5 | (4.1) | 3 | (2.0) | 0.293 |
| Aldosterone antagonist | 41 | (15.0) | 19 | (15.7) | 22 | (14.5) | 0.778 |
| Diuretics | 117 | (42.9) | 48 | (39.7) | 69 | (45.4) | 0.342 |
| ASA | 78 | (28.6) | 33 | (27.3) | 45 | (29.6) | 0.672 |
| P2Y12-inhibitor | 23 | (8.4) | 10 | (8.3) | 13 | (8.6) | 0.932 |
| Statin | 119 | (43.6) | 60 | (49.6) | 59 | (38.8) | 0.075 |
| Metformin | 31 | (11.4) | 15 | (12.4) | 16 | (10.5) | 0.628 |
| Sulfonylureas | 3 | (1.1) | 3 | (2.5) | 0 | (0.0) | 0.051 |
| GLP-1-RA | 4 | (1.5) | 2 | (1.7) | 2 | (1.3) | 0.818 |
| DPP-4-inhibitors | 36 | (13.2) | 16 | (13.2) | 20 | (13.2) | 0.987 |
| SGLT2-inhibitors | 10 | (3.7) | 4 | (3.3) | 6 | (3.9) | 0.779 |
| Insulin | 47 | (17.2) | 20 | (16.5) | 27 | (17.8) | 0.788 |
| All Patients (n = 273) | Survivors (n = 121) | Non-Survivors (n = 152) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Cause of CS, n (%) | |||||||
| Acute myocardial infarction | 134 | (49.1) | 48 | (39.7) | 86 | (56.6) | 0.005 |
| Arrhythmic | 32 | (11.7) | 25 | (20.7) | 7 | (4.6) | 0.001 |
| ADHF | 67 | (24.5) | 27 | (22.3) | 40 | (26.3) | 0.445 |
| Pulmonary embolism | 15 | (5.5) | 4 | (3.3) | 11 | (7.2) | 0.157 |
| Valvular | 12 | (4.4) | 8 | (6.6) | 4 | (2.6) | 0.111 |
| Cardiomyopathy | 7 | (2.6) | 4 | (3.3) | 3 | (2.0) | 0.489 |
| Pericardial tamponade | 5 | (1.8) | 5 | (4.1) | 0 | (0.0) | 0.011 |
| Aortic dissection | 1 | (0.4) | 0 | (0.0) | 1 | (0.7) | 0.371 |
| Classification of CS, n (%) | |||||||
| Stage A | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | - |
| Stage B | 6 | (2.2) | 6 | (5.0) | 0 | (0.0) | 0.006 |
| Stage C | 96 | (35.2) | 56 | (46.3) | 40 | (26.3) | 0.001 |
| Stage D | 20 | (7.3) | 9 | (7.4) | 11 | (7.2) | 0.949 |
| Stage E | 151 | (55.3) | 50 | (41.3) | 101 | (66.4) | 0.001 |
| Transthoracic echocardiography | |||||||
| LVEF > 55%, (n, %) | 28 | (11.2) | 16 | (13.8) | 12 | (9.0) | 0.226 |
| LVEF 54–41%, (n, %) | 32 | (12.8) | 21 | (18.1) | 11 | (8.2) | 0.020 |
| LVEF 40–30%, (n, %) | 61 | (24.4) | 35 | (30.2) | 26 | (19.4) | 0.048 |
| LVEF <30%, (n, %) | 129 | (51.6) | 44 | (37.9) | 85 | (63.4) | 0.001 |
| LVEF not documented, (n, %) | 23 | - | 5 | - | 18 | - | - |
| VCI, cm (median, (IQR)) | 1.8 | (1.5–2.2) | 1.8 | (1.4–2.2) | 1.9 | (1.6–2.2) | 0.237 |
| TAPSE, mm (median, (IQR)) | 15.0 | (11.2–18.3) | 16.0 | (11.4–20.0) | 14.5 | (11.0–17.0) | 0.368 |
| Cardiopulmonary resuscitation | |||||||
| OHCA, n (%) | 104 | (38.1) | 37 | (30.6) | 67 | (44.1) | 0.022 |
| IHCA, n (%) | 47 | (17.2) | 13 | (10.7) | 34 | (22.4) | 0.011 |
| Shockable rhythm, n (%) | 76 | (27.8) | 34 | (28.1) | 42 | (27.6) | 0.932 |
| Non-shockable rhythm, n (%) | 197 | (72.2) | 87 | (71.9) | 110 | (72.4) | 0.932 |
| ROSC, min (median, IQR) | 15 | (10–27) | 12 | (5–20) | 17 | (11–30) | 0.001 |
| Respiratory status | |||||||
| Mechanical ventilation, n (%) | 156 | (57.1) | 60 | (49.6) | 96 | (63.2) | 0.024 |
| Duration of mechanical ventilation, days, (mean, (IQR)) | 2 | (1–5) | 2 | (0–7) | 2 | (1–5) | 0.250 |
| PaO2/FiO2 ratio, (median, (IQR)) | 217 | (133–353) | 238 | (146–367) | 214 | (120–336) | 0.289 |
| PaO2, mmHg (median, (IQR)) | 103 | (77–165) | 103 | (78–161) | 106 | (77–169) | 0.987 |
| Multiple organ support during ICU | |||||||
| Dosis norepinephrine on admission, µg/kg/min (median, (IQR)) | 0.1 | (0.0–0.3) | 0.1 | (0.0–0.1) | 0.2 | (0.1–0.6) | 0.001 |
| Mechanical circulatory assist device, n (%) | 25 | (9.2) | 5 | (4.1) | 20 | (13.2) | 0.010 |
| Baseline laboratory values, (median, (IQR)) | |||||||
| pH | 7.29 | (7.19–7.37) | 7.32 | (7.24–7.37) | 7.26 | (7.15–7.36) | 0.002 |
| Lactate (mmol/L) | 3.3 | (1.7–7.2) | 2.6 | (1.6–4.0) | 4.7 | (2.4–10.3) | 0.001 |
| Serum sodium (mmol/L) | 138 | (136–141) | 138 | (136–140) | 138 | (136–141) | 0.314 |
| Serum potassium (mmol/L) | 4.3 | (3.8–4.9) | 4.2 | (3.7–4.8) | 4.4 | (3.9–5.0) | 0.250 |
| Serum creatinine (mg/dL) | 1.48 | (1.13–2.17) | 1.31 | (1.06–1.86) | 1.59 | (1.22–2.31) | 0.006 |
| Hemoglobin (g/dL) | 12.4 | (10.3–14.0) | 12.4 | (10.1–14.2) | 12.4 | (10.8–13.9) | 0.913 |
| WBC (106/mL) | 14.71 | (10.47–18.88) | 13.10 | (9.68–17.64) | 15.61 | (12.20–19.73) | 0.002 |
| Platelets (106/mL) | 224 | (171–274) | 223 | (163–285) | 226 | (177–266) | 0.968 |
| Cholesterol (mg/dL) | 130 | (98–170) | 127 | (95–170) | 135 | (102–166) | 0.488 |
| Triglycerides (mg/dL) | 102 | (71–143) | 94 | (65–136) | 108 | (82–153) | 0.026 |
| LDL (mg/dL) | 87 | (54–119) | 87 | (49–119) | 88 | (65–115) | 0.786 |
| HDL (mg/dL) | 38 | (29–49) | 40 | (32–49) | 36 | (28–49) | 0.195 |
| HbA1c (%) | 5.8 | (5.4–6.9) | 5.8 | (5.3–6.8) | 6.0 | (5.6–7.6) | 0.233 |
| INR | 1.17 | (1.08–1.39) | 1.13 | (1.05–1.33) | 1.20 | (1.10–1.46) | 0.002 |
| D-dimer (mg/L) | 9.69 | (2.46–32.00) | 5.49 | (1.94–15.08) | 17.76 | (3.83–32.00) | 0.003 |
| AST (U/L) | 129 | (43–324) | 109 | (38–214) | 167 | (57–490) | 0.022 |
| ALT (U/L) | 77 | (32–186) | 53 | (30–130) | 96 | (35–295) | 0.013 |
| Bilirubin (mg/dL) | 0.63 | (0.43–0.99) | 0.61 | (0.41–0.95) | 0.63 | (0.46–1.00) | 0.440 |
| Albumin (g/L) | 30.0 | (25.5–33.9) | 30.7 | (27.6–34.4) | 28.7 | (23.8–33.0) | 0.005 |
| Troponin I (µg/L) | 0.763 | (0.164–6.154) | 0.332 | (0.087–2.494) | 1.850 | (0.344–12.431) | 0.001 |
| NT-pro BNP (pg/mL) | 4387 | (729–13,595) | 3689 | (454–12,343) | 4462 | (1110–13,946) | 0.225 |
| Procalcitonin (ng/mL) | 0.30 | (0.11–0.94) | 0.31 | (0.07–0.67) | 0.28 | (0.17–1.38) | 0.529 |
| CRP (mg/L) | 13 | (4–45) | 12 | (4–51) | 15 | (4–42) | 0.665 |
| Primary endpoint | |||||||
| All-cause mortality at 30 days, n (%) | 152 | (55.7) | 0 | (0.0) | 152 | (100.0) | - |
| Follow up data, n (%) | |||||||
| ICU time, days (median, (IQR)) | 4 | (2–8) | 4 | (3–10) | 3 | (1–6) | 0.001 |
| Death ICU, n (%) | 151 | (55.3) | 4 | (3.3) | 147 | (96.7) | 0.001 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (years) | 1.009 | 0.997–1.022 | 0.135 | 1.008 | 0.992–1.025 | 0.326 |
| Sex | 1.046 | 0.756–1.447 | 0.785 | 1.241 | 0.823–1.871 | 0.304 |
| BMI (kg/m2) | 1.017 | 0.987–1.048 | 0.265 | 0.999 | 0.962–1.037 | 0.951 |
| Coronary artery disease | 1.033 | 0.744–1.434 | 0.848 | 0.811 | 0.500–1.317 | 0.397 |
| Congestive heart failure | 0.937 | 0.671–1.309 | 0.703 | 0.998 | 0.574–1.738 | 0.995 |
| Atrial fibrillation | 0.976 | 0.693–1.374 | 0.890 | 0.802 | 0.494–1.302 | 0.372 |
| Chronic kidney disease | 1.015 | 0.726–1.420 | 0.929 | 0.895 | 0.504–1.589 | 0.705 |
| Stroke | 0.655 | 0.390–1.100 | 0.110 | 0.716 | 0.367–1.396 | 0.327 |
| COPD | 1.124 | 0.764–1.653 | 0.553 | 1.422 | 0.890–2.272 | 0.141 |
| Acute myocardial infarction | 1.653 | 1.199–2.281 | 0.002 | 2.089 | 1.205–3.620 | 0.009 |
| ADHF | 1.036 | 0.722–1.486 | 0.849 | 1.711 | 0.903–3.243 | 0.100 |
| Acute kidney injury | 1.798 | 1.216–2.658 | 0.003 | 1.721 | 0.985–3.004 | 0.056 |
| Acute liver failure | 0.995 | 0.608–1.627 | 0.983 | 0.666 | 0.342–1.298 | 0.232 |
| Lactate (mmol/L) | 1.127 | 1.093–1.161 | 0.001 | 1.134 | 1.087–1.183 | 0.001 |
| Creatinine (mg/dL) | 1.113 | 1.015–1.221 | 0.023 | 1.160 | 0.991–1.357 | 0.065 |
| cTNI (µg/L) | 1.002 | 1.001–1.003 | 0.001 | 1.001 | 1.000–1.003 | 0.040 |
| High CVR | 0.942 | 0.663–1.338 | 0.738 | 1.039 | 0.648–1.667 | 0.873 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (years) | 1.009 | 0.997–1.022 | 0.135 | 1.016 | 1.000–1.032 | 0.048 |
| Sex | 1.046 | 0.756–1.447 | 0.785 | 1.157 | 0.797–1.680 | 0.444 |
| BMI (kg/m2) | 1.017 | 0.987–1.048 | 0.265 | 1.013 | 0.979–1.048 | 0.453 |
| Lactate (mmol/L) | 1.127 | 1.093–1.161 | 0.001 | 1.119 | 1.079–1.162 | 0.001 |
| Creatinine (mg/dL) | 1.113 | 1.015–1.221 | 0.023 | 1.101 | 0.985–1.231 | 0.090 |
| Acute myocardial infarction | 1.653 | 1.199–2.281 | 0.002 | 1.863 | 1.286–2.697 | 0.001 |
| Arterial hypertension | 0.906 | 0.638–1.287 | 0.582 | 0.679 | 0.433–1.065 | 0.092 |
| Diabetes mellitus | 1.213 | 0.881–1.671 | 0.237 | 1.112 | 0.751–1.646 | 0.596 |
| Hyperlipidemia | 0.718 | 0.516–0.998 | 0.049 | 0.801 | 0.536–1.195 | 0.277 |
| Smoking | 0.945 | 0.679–1.315 | 0.737 | 1.292 | 0.872–1.913 | 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forner, J.; Schupp, T.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Behnes, M.; Akin, M.; Ayoub, M.; Mashayekhi, K.; Akin, I.; et al. Effect of Cardiovascular Risk Factors on 30-Day All-Cause Mortality in Cardiogenic Shock. J. Clin. Med. 2023, 12, 4870. https://doi.org/10.3390/jcm12144870
Forner J, Schupp T, Weidner K, Ruka M, Egner-Walter S, Behnes M, Akin M, Ayoub M, Mashayekhi K, Akin I, et al. Effect of Cardiovascular Risk Factors on 30-Day All-Cause Mortality in Cardiogenic Shock. Journal of Clinical Medicine. 2023; 12(14):4870. https://doi.org/10.3390/jcm12144870
Chicago/Turabian StyleForner, Jan, Tobias Schupp, Kathrin Weidner, Marinela Ruka, Sascha Egner-Walter, Michael Behnes, Muharrem Akin, Mohamed Ayoub, Kambis Mashayekhi, Ibrahim Akin, and et al. 2023. "Effect of Cardiovascular Risk Factors on 30-Day All-Cause Mortality in Cardiogenic Shock" Journal of Clinical Medicine 12, no. 14: 4870. https://doi.org/10.3390/jcm12144870
APA StyleForner, J., Schupp, T., Weidner, K., Ruka, M., Egner-Walter, S., Behnes, M., Akin, M., Ayoub, M., Mashayekhi, K., Akin, I., & Rusnak, J. (2023). Effect of Cardiovascular Risk Factors on 30-Day All-Cause Mortality in Cardiogenic Shock. Journal of Clinical Medicine, 12(14), 4870. https://doi.org/10.3390/jcm12144870







