The Role of Multimodality Imaging in Pediatric Cardiomyopathies
Abstract
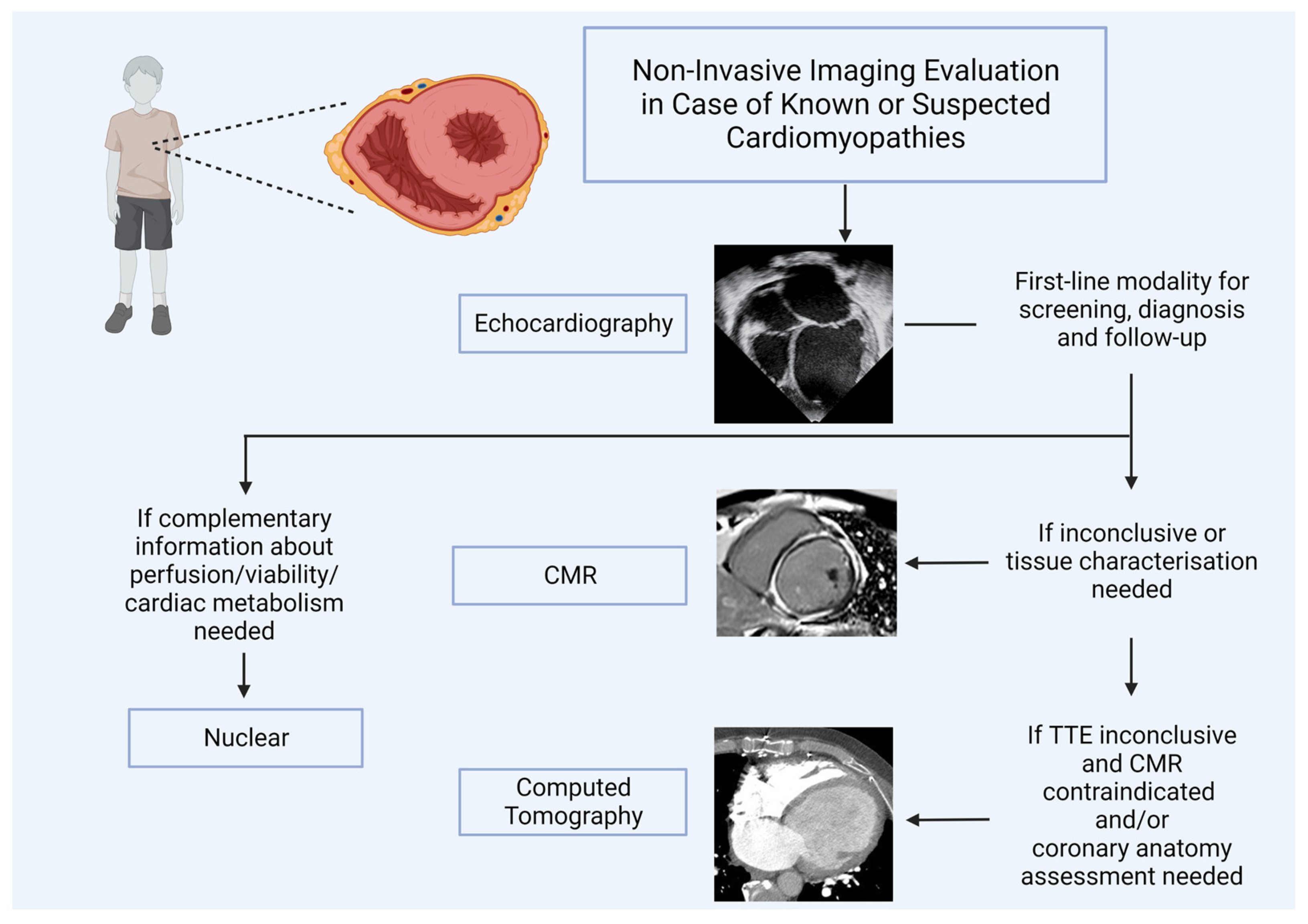
1. Introduction
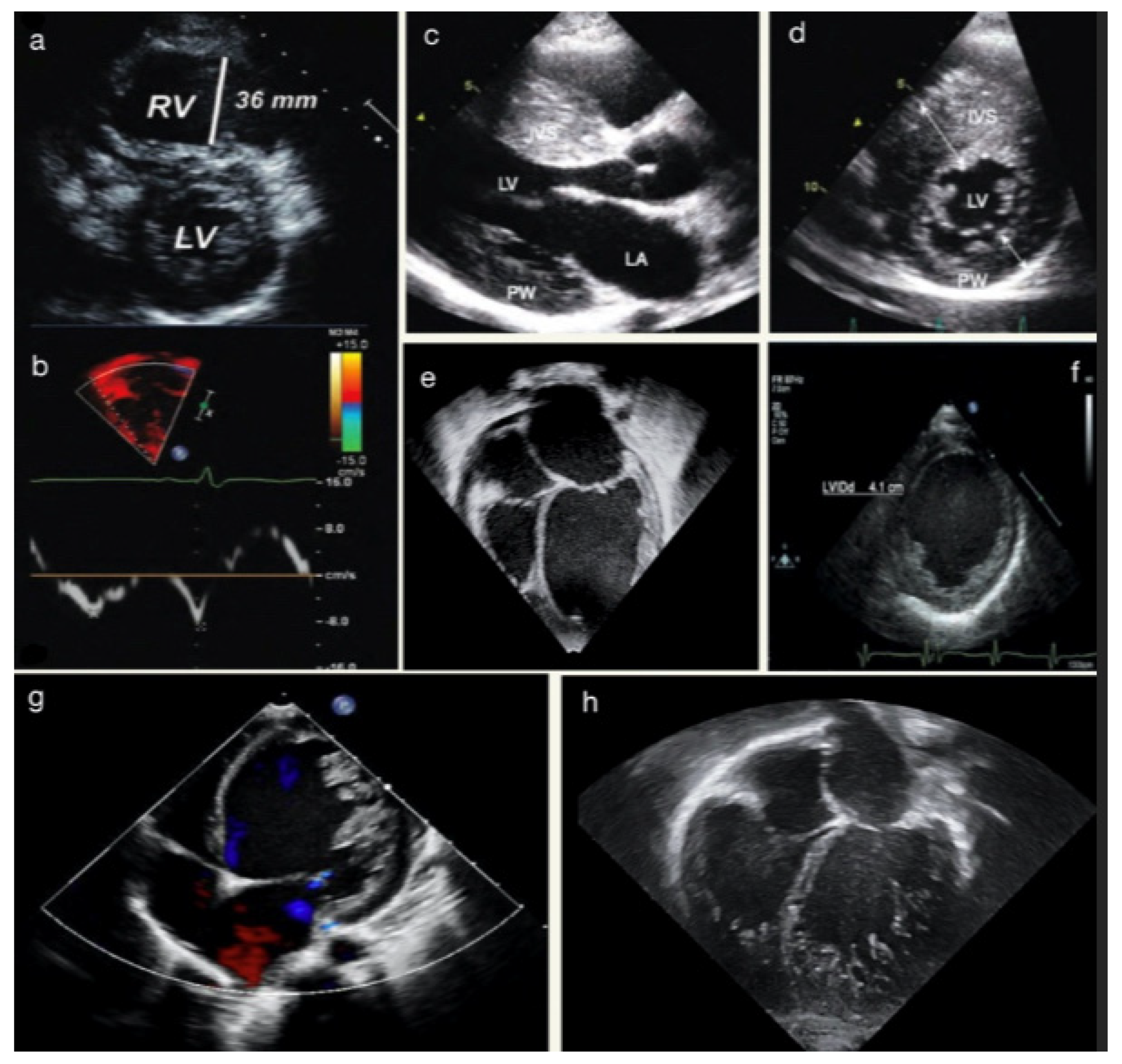
2. Transthoracic Echocardiography in Cardiomyopathy
2.1. Protocols
2.1.1. Two-Dimensional Echocardiography
2.1.2. Three-Dimensional Echocardiography
2.1.3. Spectral and Tissue Doppler Imaging
2.1.4. Speckle Tracking Echocardiography
2.2. Echocardiography Application to Paediatric Cardiomyopathies
- Jenni criteria: the ratio of noncompacted to compacted myocardium in systole;
- Chin criteria: epicardial surface to trabeculation trough divided by epicardial surface to trabeculation peak in end-diastole;
- Stöllberger criteria: number of trabeculations that move synchronously with myocardium in end-diastole.
2.3. Limitations and Pitfalls
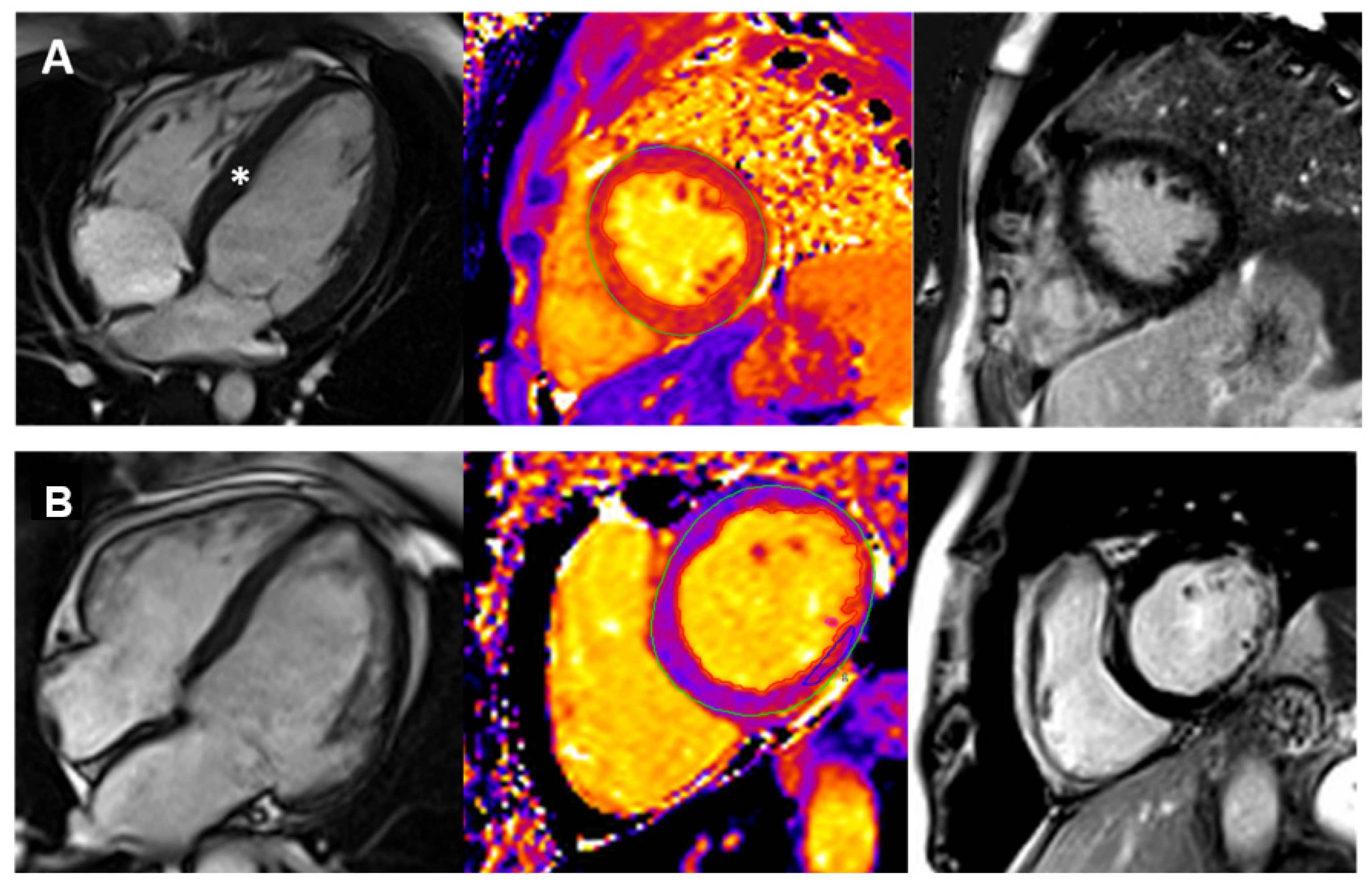
3. Cardiovascular Magnetic Resonance (CMR) in Pediatric Cardiomyopathies
3.1. CMR Sequences for Paediatric Cardiomyopathies
- Initial localizers images;
- Functional and volumetric assessment with long-axis and short-axis cine (balanced steady-state free precession—bSSFP);
- Pre-contrast oedema sequence (if required);
- Pre-contrast parametric mapping (if required);
- Contrast administration;
- Post-contrast early gadolinium enhancement (if required);
- Post-contrast late gadolinium enhancement.
3.1.1. Initial Localizers
3.1.2. Cine (bSSFP)
3.1.3. T2 Weighted Images (Oedema Assessment)
3.1.4. Native T1,T2 Mapping
3.1.5. Late Gadolinium Enhancement (LGE)
3.2. CMR Application in Paediatric Cardiomyopathies
3.3. Limitations and Pitfalls
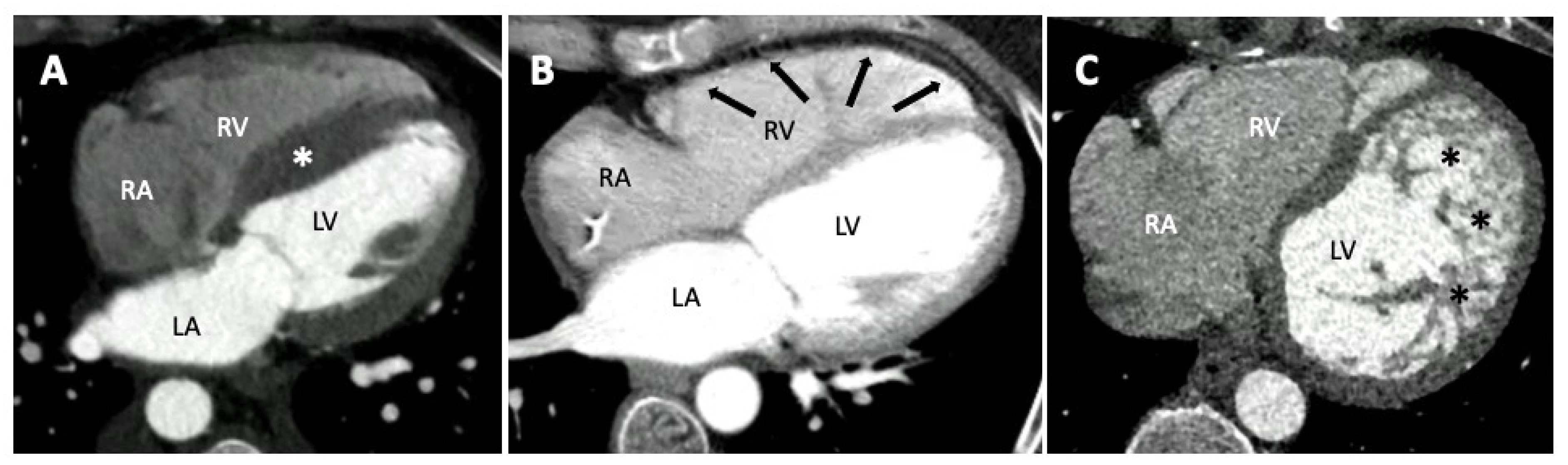
4. Computed Tomography in Pediatric Cardiomyopathies
4.1. Protocols
4.2. Role of CT in Paediatric Cardiomyopathies
4.3. Future Perspectives
5. Role of Nuclear Medicine Imaging in Pediatric Cardiomyopathies
5.1. Protocols
5.2. Nuclear Imaging Application in Pediatric Cardiomyopathies
5.3. Limitations and Technical Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM/ARVC | Arrhythmogenic Cardiomyopathy |
| bSSFP | Balanced Steady-State Free Precession |
| DCM | Dilated Cardiomyopathy |
| DSP | Desmoplakin |
| CA | Coronary Artery |
| CAD | Coronary Artery Disease |
| CT | Computed Tomography |
| CCT | Cardiovascular Computed Tomography |
| CMR | Cardiovascular Magnetic Resonance |
| ECV | Extracellular Volume |
| EGE | Early Gadolinium Enhancement |
| GBCA | Gadolinium-Based Contrast Agent |
| GLS | Global Longitudinal Strain |
| HCM | Hypertrophic Cardiomyopathy |
| HF | Heart Failure |
| LA | Left Atrium |
| LV | Left Ventricle |
| LVEDD | Left Ventricle End-Diastolic Diameter |
| LVEFLVESD | Left Ventricle Ejection FractionLeft Ventricle End-Systolic diameter |
| LVH | Left Ventricle Hypertrophy |
| LVM | Left Ventricle Mass |
| LVNC | Left Ventricular Non-Compaction Cardiomyopathy |
| LVOTO | Left Ventricular Outflow Tract Obstruction |
| NSF | Nephrogenic Systemic Fibrosis |
| RV | Right Ventricle |
| SAX | Short Axis |
| STE | Speckle Tracking Echocardiography |
| STIR | Short-TI Triple-Inversion Recovery Prepared Fast Spin Echo Sequences |
| TDI | Tissue Doppler Imaging |
| TR | Tricuspid Regurgitation |
| TTE | Transthoracic Echocardiography |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
References
- Lee, T.M.; Hsu, D.T.; Kantor, P.; Towbin, J.A.; Ware, S.M.; Colan, S.D.; Chung, W.K.; Jefferies, J.L.; Rossano, J.W.; Castleberry, C.D.; et al. Pediatric Cardiomyopathies. Circ. Res. 2017, 121, 855–873. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Sleeper, L.A.; Towbin, J.A.; Lowe, A.M.; Orav, E.J.; Cox, G.F.; Lurie, P.R.; McCoy, K.L.; McDonald, M.A.; Messere, J.E.; et al. The Incidence of Pediatric Cardiomyopathy in Two Regions of the United States. N. Engl. J. Med. 2003, 348, 1647–1655. [Google Scholar] [CrossRef]
- Webber, S.A. New-Onset Heart Failure in Children in the Absence of Structural Congenital Heart Disease. Circulation 2008, 117, 11–12. [Google Scholar] [CrossRef][Green Version]
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.B.; Cheung, M.; Wilkinson, L.C.; Davis, A.M.; Kahler, S.G.; Chow, C.; Wilkinson, J.L.; et al. The Epidemiology of Childhood Cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639–1646. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Law, Y.M.; Asante-Korang, A.; Austin, E.D.; Dipchand, A.I.; Everitt, M.D.; Hsu, D.T.; Lin, K.Y.; Price, J.F.; Wilkinson, J.D.; et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e9–e68. [Google Scholar] [CrossRef]
- Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672–673. [CrossRef]
- Barry, J.M. Contemporary Definitions and Classification of the Cardiomyopathies. Circulation 2006, 113, 1807–1816. [Google Scholar]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2007, 29, 270–276. [Google Scholar] [CrossRef]
- Jan, M.F.; Tajik, A.J. Modern Imaging Techniques in Cardiomyopathies. Circ. Res. 2017, 121, 874–891. [Google Scholar] [CrossRef]
- Sarnari, R.; Kamal, R.Y.; Friedberg, M.K.; Silverman, N.H. Doppler Assessment of the Ratio of the Systolic to Diastolic Duration in Normal Children: Relation to Heart Rate, Age and Body Surface Area. J. Am. Soc. Echocardiogr. 2009, 22, 928–932. [Google Scholar] [CrossRef]
- Tissot, C.; Singh, Y.; Sekarski, N. Echocardiographic Evaluation of Ventricular Function—For the Neonatologist and Pediatric Intensivist. Front. Pediatr. 2018, 6, 79. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression Equations for Calculation of Z Scores of Cardiac Structures in a Large Cohort of Healthy Infants, Children, and Adolescents: An Echocardiographic Study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef]
- Demir, H.; Tan, Y.Z.; Kozdağ, G.; Işgören, S.; Anik, Y.; Ural, D.; Demirci, A.; Berk, F. Comparison of gated SPECT, echocardiography and cardiac magnetic resonance imaging for the assessment of left ventricular ejection fraction and volumes. Ann. Saudi Med. 2007, 27, 415–420. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Jenkins, C.; Kühl, H.P.; Nesser, H.-J.; Marwick, T.; Franke, A.; Ebner, C.; Freed, B.H.; Steringer-Mascherbauer, R.; Pollard, H.; et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: Multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc. Imaging 2008, 1, 413–423. [Google Scholar] [CrossRef]
- Stanton, T.; Jenkins, C.; Haluska, B.A.; Marwick, T.H. Association of outcome with left ventricular parameters measured by two-dimensional and three-dimensional echocardiography in patients at high cardiovascular risk. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 65–73. [Google Scholar] [CrossRef]
- Wu, V.C.-C.; Takeuchi, M.; Kuwaki, H.; Iwataki, M.; Nagata, Y.; Otani, K.; Haruki, N.; Yoshitani, H.; Tamura, M.; Abe, H.; et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: Comparison with 2D echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1025–1035. [Google Scholar] [CrossRef]
- Montserrat, S.; Gabrielli, L.; Borrás, R.; Poyatos, S.; Berruezo, A.; Bijnens, B.; Brugada, J.; Mont, L.; Sitges, M. Left atrial size and function by three-dimensional echocardiography to predict arrhythmia recurrence after first and repeated ablation of atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 515–522. [Google Scholar] [CrossRef]
- De Potter, T.; Weytjens, C.; Motoc, A.; Luchian, M.L.; Scheirlynck, E.; Roosens, B.; Tanaka, K.; Houard, L.; Droogmans, S.; Cosyns, B. Feasibility, Reproducibility and Validation of Right Ventricular Volume and Function Assessment Using Three-Dimensional Echocardiography. Diagnostics 2021, 11, 699. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A. A clinical approach to the assessment of left ventricular diastolic function by Doppler echocardiography: Update 2003. Heart Br. Card. Soc. 2003, 89 (Suppl. S3), iii18–iii23. [Google Scholar] [CrossRef]
- Rohde, L.E.; Palombini, D.V.; Polanczyk, C.A.; Goldraich, L.A.; Clausell, N. A hemodynamically oriented echocardiography-based strategy in the treatment of congestive heart failure. J. Card. Fail. 2007, 13, 618–625. [Google Scholar] [CrossRef]
- Koestenberger, M.; Nagel, B.; Ravekes, W.; Avian, A.; Cvirn, G.; Rehak, T.; Gamillscheg, A. Reference values of the mitral annular peak systolic velocity (Sm) in 690 healthy pediatric patients, calculation of Z-score values, and comparison to the mitral annular Plane systolic excursion (MAPSE). Echocardiography 2014, 31, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Bachinski, L.L.; Meyer, D.; Hill, R.; Zoghbi, W.A.; Tam, J.W.; Quiñones, M.A.; Roberts, R.; Marian, A.J. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 2001, 104, 128–130. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; van Zwet, E.W.; Delgado, V.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Marsan, N.A. Global Longitudinal Strain and Left Atrial Volume Index Provide Incremental Prognostic Value in Patients with Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005706. [Google Scholar] [CrossRef]
- Sabatino, J.; Di Salvo, G.; Prota, C.; Bucciarelli, V.; Josen, M.; Paredes, J.; Borrelli, N.; Sirico, D.; Prasad, S.; Indolfi, C.; et al. Left Atrial Strain to Identify Diastolic Dysfunction in Children with Cardiomyopathies. J. Clin. Med. 2019, 8, 1243. [Google Scholar] [CrossRef]
- Teixeira, K.L.M.; Correia, E.B.; Tressino, C.G.; Peçanha, M.M.; Melchior, W.A.; Barretto, R.B.M.; Medeiros, B.G.; Le Bihan, D. Echocardiographic assessment of atrial function in patients with hypertrophic cardiomyopathy with and without paroxysmal atrial fibrillation. Rev. Port. Cardiol. 2022, 41, 771–779. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.K.; Rhee, T.M.; Choi, Y.J.; Hwang, I.C.; Yoon, Y.E.; Park, J.B.; Lee, S.P.; Kim, Y.J.; Cho, G.Y. Left Atrial Reservoir Strain-Based Left Ventricular Diastolic Function Grading and Incident Heart Failure in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2022, 15, e013556. [Google Scholar] [CrossRef]
- Rady, M.; Ulbrich, S.; Heidrich, F.; Jellinghaus, S.; Ibrahim, K.; Linke, A.; Sveric, K.M. Left Ventricular Torsion—A New Echocardiographic Prognosticator in Patients with Non-Ischemic Dilated Cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 595–603. [Google Scholar] [CrossRef]
- Sabatino, J.; Di Salvo, G.; Krupickova, S.; Fraisse, A.; Prota, C.; Bucciarelli, V.; Josen, M.; Paredes, J.; Sirico, D.; Voges, I.; et al. Left Ventricular Twist Mechanics to Identify Left Ventricular Noncompaction in Childhood. Circ. Cardiovasc. Imaging 2019, 12, e007805. [Google Scholar] [CrossRef]
- Borrelli, N.; Di Salvo, G.; Ciriello, G.D.; Sabatino, J.; Avesani, M.; Leo, I.; Barracano, R.; Scognamiglio, G.; Russo, M.G.; Sarubbi, B. Myocardial work in children with Wolff-Parkinson-White syndrome. Int. J. Cardiovasc. Imaging 2023, 1–9. [Google Scholar] [CrossRef]
- Sabatino, J.; Leo, I.; Strangio, A.; Bella, S.; Borrelli, N.; Avesani, M.; Josen, M.; Paredes, J.; Piccinelli, E.; Sirico, D.; et al. Echocardiographic Normal Reference Ranges for Non-invasive Myocardial Work Parameters in Pediatric Age: Results from an International Multi-Center Study. Front. Cardiovasc. Med. 2022, 25, 792622. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Mital, S.; Mertens, L.; Jeewa, A.; Friedberg, M.K.; Aguet, J.; Adler, A.; Lam, C.Z.; Dragulescu, A.; Rakowski, H.; et al. Pediatric Hypertrophic Cardiomyopathy: Exploring the Genotype-Phenotype Association. J. Am. Heart Assoc. 2022, 11, e024220. [Google Scholar] [CrossRef]
- El Assaad, I.; Gauvreau, K.; Rizwan, R.; Margossian, R.; Colan, S.; Chen, M.H. Value of Exercise Stress Echocardiography in Children with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020, 33, 888–894.e2. [Google Scholar] [CrossRef]
- Beltrami, M.; Bartolini, S.; Pastore, M.C.; Milli, M.; Cameli, M. Relationship between measures of left ventricular systolic and diastolic dysfunction and clinical and biomarker status in patients with hypertrophic cardiomyopathy. Arch. Cardiovasc. Dis. 2022, 115, 598–609. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Pateras, K.; Nicoli, F.; Bampatsias, D.; Beltrami, M.; Finocchiaro, G.; Chiribiri, A.; Masci, P.G.; Olivotto, I. Comparison of Demographic, Clinical, Biochemical, and Imaging Findings in Hypertrophic Cardiomyopathy Prognosis. JACC Heart Fail. 2023, 11, 30–41. [Google Scholar] [CrossRef]
- Dorobantu, D.M.; Wadey, C.A.; Amir, N.H.; Stuart, A.G.; Williams, C.A.; Pieles, G.E. The Role of Speckle Tracking Echocardiography in the Evaluation of Common Inherited Cardiomyopathies in Children and Adolescents: A Systematic Review. Diagnostics 2021, 11, 635. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Wang, H.; Wang, Y. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: A systematic review and meta-analysis. Clin. Cardiol. 2022, 45, 1184–1191. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Phelan, D.; Abraham, T.; Armour, A.; Desai, M.Y.; Dragulescu, A.; Yvonne, G.; Steven, L.; Yasdet, M.; Saidi, M.; et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2022, 35, 533–569. [Google Scholar]
- Halliday, B.P. State of the art: Multimodality imaging in dilated cardiomyopathy. Heart 2022, 108, 1910–1917. [Google Scholar] [CrossRef]
- Jhaveri, S.; Komarlu, R.; Worley, S.; Shahbah, D.; Gurumoorthi, M.; Zahka, K. Left Atrial Strain and Function in Pediatric Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 996–1006. [Google Scholar] [CrossRef]
- Molina, K.M.; Shrader, P.; Colan, S.D.; Mital, S.; Margossian, R.; Sleeper, L.A.; Shirali, G.; Barker, P.; Canter, C.E.; Altmann, K.; et al. Predictors of disease progression in pediatric dilated cardiomyopathy. Circ. Heart Fail. 2013, 6, 1214–1222. [Google Scholar] [CrossRef]
- Protonotarios, A.; Savvatis, K. Myocardial strain analysis in family screening for genetic dilated cardiomyopathy: Testing the boundaries of normality? Int. J. Cardiol. 2021, 323, 201–202. [Google Scholar] [CrossRef]
- Wright, L.K.; McGaughy, F.; Kellerman, M.; Border, W.L.; Sachdeva, R. Prognostic significance of tissue Doppler imaging-derived myocardial performance index in pediatric patients with dilated cardiomyopathy. Pediatr. Transplant. 2020, 24, e13613. [Google Scholar] [CrossRef]
- Joong, A.; Hayes, D.A.; Anderson, B.R.; Zuckerman, W.A.; Carroll, S.J.; Lai, W.W. Comparison of Echocardiographic Diagnostic Criteria of Left Ventricular Noncompaction in a Pediatric Population. Pediatr. Cardiol. 2017, 38, 1493–1504. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A.; Cipriani, A.; Bauce, B.; Bariani, R.; Beffagna, G.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; et al. Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021987. [Google Scholar] [CrossRef]
- Cicenia, M.; Drago, F. Arrhythmogenic Cardiomyopathy: Diagnosis, Evolution, Risk Stratification and Pediatric Population-Where Are We? J. Cardiovasc. Dev. Dis. 2022, 9, 98. [Google Scholar] [CrossRef]
- Pietrzak, R.; Werner, B. Right ventricular function assessment using tissue Doppler imaging and speckle tracking echocardiography. J. Ultrason. 2014, 14, 328–338. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J. Cardiovasc. Magn. Reson. 2016, 18, 1–18. [Google Scholar] [CrossRef]
- Valsangiacomo Buechel, E.R.; Grosse-Wortmann, L.; Fratz, S.; Eichhorn, J.; Sarikouch, S.; Greil, G.F.; Beerbaum, P.; Bucciarelli-Ducci, C.; Bonello, B.; Sieverding, L.; et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: An expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the use of cardiovascular magnetic resonance in pediatric congenital and acquired heart disease: Endorsed by The American Heart Association. J. Cardiovasc. Magn. Reson. 2022, 15, e014415. [Google Scholar]
- Puricelli, F.; Voges, I.; Gatehouse, P.; Rigby, M.; Izgi, C.; Pennell, D.J.; Krupickova, S. Performance of Cardiac MRI in Pediatric and Adult Patients with Fontan Circulation. Radiol. Cardiothorac. Imaging 2022, 4, e210235. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Follow-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 1–11. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; Simonetti, O.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance imaging. J. Magn. Reson. Imaging 2007, 26, 452–459. [Google Scholar] [CrossRef]
- Edwards, N.C.; Routledge, H.; Steeds, R.P. T2-weighted magnetic resonance imaging to assess myocardial oedema in ischaemic heart disease. Heart 2009, 95, 1357–1361. [Google Scholar] [CrossRef]
- Abbara, S.; Migrino, R.Q.; Sosnovik, D.E.; Leichter, J.A.; Brady, T.J.; Holmvang, G. Value of Fat Suppression in the MRI Evaluation of Suspected Arrhythmogenic Right Ventricular Dysplasia. Am. J. Roentgenol. 2004, 182, 587–591. [Google Scholar] [CrossRef]
- Stirrat, J.; White, J.A. The Prognostic Role of Late Gadolinium Enhancement Magnetic Resonance Imaging in Patients with Cardiomyopathy. Can. J. Cardiol. 2013, 29, 329–336. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of Techniques for the Quantification of Myocardial Scar of Differing Etiology Using Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Robson, M.D.; Neubauer, S.; Karamitsos, T.D. Myocardial tissue characterization by magnetic resonance imaging: Novel applications of T1 and T2 mapping. J. Thorac. Imaging 2014, 29, 147–154. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Sprinkart, A.M.; Doerner, J.; Dabir, D.; Kuetting, D.L.; Block, W.; Andrie, R.P.; Stehning, C.; Fimmers, R.; et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 154–161. [Google Scholar] [CrossRef]
- Axelsson Raja, A.; Farhad, H.; Valente, A.M.; Couce, J.-P.; Jefferies, J.L.; Bundgaard, H.; Zahka, K.; Lever, H.; Murphy, A.M.; Ashley, E.; et al. Prevalence and Progression of Late Gadolinium Enhancement in Children and Adolescents with Hypertrophic Cardiomyopathy. Circulation 2018, 138, 782–792. [Google Scholar] [CrossRef]
- Windram, J.D.; Benson, L.N.; Dragelescu, A.; Yoo, S.J.; Mertens, L.; Wong, D.; Grosse-Wortmann, L. Distribution of Hypertrophy and Late Gadolinium Enhancement in Children and Adolescents with Hypertrophic Cardiomyopathy. Congenit. Heart Dis. 2015, 10, E258–E267. [Google Scholar] [CrossRef]
- Ali, L.A.; Marrone, C.; Martins, D.S.; Khraiche, D.; Festa, P.; Martini, N.; Giuseppe, S.; Giancarlo, T.; Elena, P.; Damien, B.; et al. Prognostic factors in hypertrophic cardiomyopathy in children: An MRI based study. Int. J. Cardiol. 2022, 364, 141–147. [Google Scholar] [CrossRef]
- Pieroni, M.; Ciabatti, M.; Saletti, E.; Tavanti, V.; Santangeli, P.; Martinese, L.; Liistro, F.; Olivotto, I.; Bolognese, L. Beyond Sarcomeric Hypertrophic Cardiomyopathy: How to Diagnose and Manage Phenocopies. Curr. Cardiol. Rep. 2022, 24, 1567–1585. [Google Scholar] [CrossRef] [PubMed]
- Rigolli, M.; Kahn, A.M.; Brambatti, M.; Contijoch, F.J.; Adler, E.D. Cardiac Magnetic Resonance Imaging in Danon Disease Cardiomyopathy. JACC Cardiovasc. Imaging 2021, 14, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.; Secinaro, A.; Perrone, M.A.; Curione, D.; Napolitano, C.; Gagliardi, M.G. Role of cardiovascular magnetic resonance end-systolic 3D-SSFP sequence in repaired tetralogy of Fallot patients eligible for transcatheter pulmonary valve implantation. Int. J. Cardiovasc. Imaging 2019, 35, 1525–1533. [Google Scholar] [CrossRef]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [PubMed]
- Baessato, F.; Romeo, C.; Rabbat, M.G.; Pontone, G.; Meierhofer, C. A Comprehensive Assessment of Cardiomyopathies through Cardiovascular Magnetic Resonance: Focus on the Pediatric Population. Diagnostics 2022, 12, 1022. [Google Scholar] [CrossRef]
- Yoo, S.J.; Grosse-Wortmann, L.; Hamilton, R.M. Magnetic resonance imaging assessment of arrhythmogenic right ventricular cardiomyopathy/dysplasia in children. Korean Circ. J. 2010, 40, 357–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smedsrud, M.K.; Chivulescu, M.; Forså, M.I.; Castrini, I.; Aabel, E.W.; Rootwelt-Norberg, C.; Bogsrud, M.P.; Edvardsen, T.; Hasselberg, N.E.; Früh, A.; et al. Highly malignant disease in childhood-onset arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2022, 43, 4694–4703. [Google Scholar] [CrossRef]
- DeWitt, E.S.; Chandler, S.F.; Hylind, R.J.; Beausejour Ladouceur, V.; Blume, E.D.; VanderPluym, C.; Powell, A.J.; Fynn-Thompson, F.; Roberts, A.E.; Sanders, S.P.; et al. Phenotypic Manifestations of Arrhythmogenic Cardiomyopathy in Children and Adolescents. J. Am. Coll. Cardiol. 2019, 74, 346–358. [Google Scholar] [CrossRef]
- Petersen Steffen, E.; Jensen, B.; Aung, N.; Friedrich Matthias, G.; McMahon Colin, J.; Mohiddin Saidi, A.; Pignatelli, R.H.; Ricci, F.; Anderson, R.H.; Bluemke, D.A. Excessive Trabeculation of the Left Ventricle. JACC Cardiovasc. Imaging 2023, 16, 408–425. [Google Scholar] [CrossRef]
- Shellock, F.G.; Woods, T.O.; Crues, J.V., 3rd. MR Labeling Information for Implants and Devices: Explanation of Terminology. Radiology 2009, 253, 26–30. [Google Scholar] [CrossRef]
- Nazarian, S.; Hansford, R.; Roguin, A.; Goldsher, D.; Zviman, M.M.; Lardo, A.C.; Caffo, B.S.; Frick, K.D.; Kraut, M.A.; Kamel, I.R.; et al. A Prospective Evaluation of a Protocol for Magnetic Resonance Imaging of Patients with Implanted Cardiac Devices. Ann. Intern. Med. 2011, 155, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Ellis, J.H.; Cohan, R.H.; Strouse, P.J.; Jan, S.C. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am. J. Roentgenol. 2007, 189, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Do, C.; De Aguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Secinaro, A.; Ait-Ali, L.; Curione, D.; Clemente, A.; Gaeta, A.; Giovagnoni, A.; Alaimo, A.; Esposito, A.; Tchana, B.; Sandrini, C.; et al. Recommendations for cardiovascular magnetic resonance and computed tomography in congenital heart disease: A consensus paper from the CMR/CCT working group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. Radiol. Med. 2022, 127, 788–802. [Google Scholar]
- Ko, S.M.; Hwang, S.H.; Lee, H.J. Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases. J. Cardiovasc. Imaging 2019, 27, 73–92. [Google Scholar] [CrossRef]
- Clayton, B.; Roobottom, C.; Morgan-Hughes, G. Assessment of the myocardium with cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 603–609. [Google Scholar] [CrossRef]
- Conte, E.; Mushtaq, S.; Muscogiuri, G.; Formenti, A.; Annoni, A.; Mancini, E.; Ricci, F.; Melotti, E.; Gigante, C.; Lorenza, Z.; et al. The Potential Role of Cardiac CT in the Evaluation of Patients with Known or Suspected Cardiomyopathy: From Traditional Indications to Novel Clinical Applications. Front. Cardiovasc. Med. 2021, 8, 709124. [Google Scholar] [CrossRef]
- Kang, E.J. Clinical Applications of Wide-Detector CT Scanners for Cardiothoracic Imaging: An Update. Korean J. Radiol. 2019, 20, 1583–1596. [Google Scholar] [CrossRef]
- Tansey, D.K.; Aly, Z.; Sheppard, M.N. Fat in the right ventricle of the normal heart. Histopathology 2005, 46, 98–104. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy. Just a matter of fat? Cardiovasc. Pathol. 2005, 14, 37–41. [Google Scholar] [CrossRef]
- Kimura, F.; Sakai, F.; Sakomura, Y.; Fujimura, M.; Ueno, E.; Matsuda, N.; Kasanuki, H.; Mitsuhashi, N. Helical CT Features of Arrhythmogenic Right Ventricular Cardiomyopathy. RadioGraphics 2002, 22, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Shimizu, W.; Ohe, T.; Hamada, S.; Kurita, T.; Aihara, N.; Kamakura, S.; Takamiya, M.; Shimomura, K. Usefulness of Electron-Beam Computed Tomography in Arrhythmogenic Right Ventricular Dysplasia. Circulation 1996, 94, 437–444. [Google Scholar] [CrossRef]
- Williams, T.J.; Manghat, N.E.; McKay-Ferguson, A.; Ring, N.J.; Morgan-Hughes, G.J.; Roobottom, C.A. Cardiomyopathy: Appearances on ECG-gated 64-detector row computed tomography. Clin. Radiol. 2008, 63, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Zenooz, N.A.; Zahka, K.G.; Siwik, E.S.; Gilkeson, R.C. Noncompaction Syndrome of the Myocardium. J. Thorac. Imaging 2010, 25, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Aaberge, L.; Abildgaard, A.; Ragnarsson, A.; Edvardsen, T.; Jakobsen, J.; Andersen, R. Coronary computed tomography in heart transplant patients: Detection of significant stenosis and cardiac allograft vasculopathy, image quality, and radiation dose. Acta Radiol. 2018, 59, 1066–1073. [Google Scholar] [CrossRef]
- Yuan, S.-M. Cardiomyopathy in the pediatric patients. Pediatr. Neonatol. 2018, 59, 120–128. [Google Scholar] [CrossRef]
- De Amorim Fernandes, F.; Peix, A.; Giubbini, R.; Karthikeyan, G.; Massardo, T.; Patel, C.; Pabon, L.M.; Jimenez-Heffernan, A.; Alexanderson, E.; Butt, S.; et al. Reproducibility of global LV function and dyssynchrony parameters derived from phase analysis of gated myocardial perfusion SPECT: A multicenter comparison with core laboratory setting. J. Nucl. Cardiol. 2020, 29, 952–961. [Google Scholar] [CrossRef]
- Milanesi, O.; Stellin, G.; Zucchetta, P. Nuclear Medicine in Pediatric Cardiology. Semin. Nucl. Med. 2017, 47, 158–169. [Google Scholar] [CrossRef]
- Malik, N.; Mukherjee, M.; Wu, K.C.; Zimmerman, S.L.; Zhan, J.; Calkins, H.; James, C.A.; Gilotra, N.A.; Sheikh, F.H.; Tandri, H.; et al. Multimodality Imaging in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Imaging 2022, 15, e013725. [Google Scholar] [CrossRef]
- Ziolkowska, L.; Boruc, A.; Sobielarska-Lysiak, D.; Grzyb, A.; Petryka-Mazurkiewicz, J.; Mazurkiewicz, Ł.; Brzezinska-Rajszys, G. Prognostic Significance of Myocardial Ischemia Detected by Single-Photon Emission Computed Tomography in Children with Hypertrophic Cardiomyopathy. Pediatr. Cardiol. 2021, 42, 960–968. [Google Scholar] [CrossRef]
- Van der Bijl, P.; Delgado, V.; Bootsma, M.; Bax, J.J. Risk Stratification of Genetic, Dilated Cardiomyopathies Associated With Neuromuscular Disorders. Circulation 2018, 137, 2514–2527. [Google Scholar] [CrossRef] [PubMed]
- Tadamura, E.; Yoshibayashi, M.; Yonemura, T.; Kudoh, T.; Kubo, S.; Motooka, M.; Nohara, R.; Matsumori, A.; Sasayama, S.; Matsuda, T.; et al. Significant regional heterogeneity of coronary flow reserve in paediatric hypertrophic cardiomyopathy. Eur. J. Nucl. Med. 2000, 27, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Pinheiro, A.; Higuchi, T.; Rischpler, C.; Merrill, J.; Santaularia-Tomas, M.; Abraham, M.R.; Wahl, R.L.; Abraham, T.P.; Bengel, F.M. PET/CT Assessment of Symptomatic Individuals with Obstructive and Nonobstructive Hypertrophic Cardiomyopathy. J. Nucl. Med. 2012, 53, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Takahashi, N.; Iswkawa, T.; Uchino, K.; Mochida, Y.; Ebina, T.; Kobayashi, T.; Matsushita, K.; Matsumoto, K.; Kawasaki, N.; et al. Clinical usefulness of ECG-gated18F-FDG PET combined with99mTc-MIBI gated SPECT for evaluating myocardial viability and function. Ann. Nucl. Med. 2004, 18, 375–383. [Google Scholar] [CrossRef]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI Guideline for 18F-FDG Use in Inflammation and Infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef]
- Absi, M.; Bocchini, C.; Price, J.F.; Adachi, I. F-fluorodeoxyglucose-positive emission tomography/CT imaging for left ventricular assist device-associated infections in children. Cardiol. Young 2018, 28, 1157–1159. [Google Scholar] [CrossRef]
- Porcari, A.; De Angelis, G.; Romani, S.; Paldino, A.; Artico, J.; Cannatà, A.; Gentile, P.; Pinamonti, B.; Merlo, M.; Sinagra, G. Current diagnostic strategies for dilated cardiomyopathy: A comparison of imaging techniques. Expert Rev. Cardiovasc. Ther. 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Karasawa, K.; Ayusawa, M.; Noto, N.; Sumitomo, N.; Okada, T.; Harada, K. Assessment of cardiac sympathetic nerve activity in children with chronic heart failure using quantitative iodine-123 metaiodobenzylguanidine imaging. J. Cardiol. 2000, 36, 387–395. [Google Scholar]
- Possner, M.; Buechel, R.R.; Vontobel, J.; Mikulicic, F.; Gräni, C.; Benz, D.C.; Clerc, O.F.; Fuchs, T.A.; Tobler, D.; Stambach, D.; et al. Myocardial blood flow and cardiac sympathetic innervation in young adults late after arterial switch operation for transposition of the great arteries. Int. J. Cardiol. 2020, 299, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Biassoni, L.; Monsieurs, M.; Franzius, C.; Jacobs, F.; EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card. Eur. J. Nucl. Med. Mol. Imaging. 2007, 34, 796–798. [Google Scholar] [CrossRef]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; la Fougère, C.; Mariani, G.; Massalha, S.; et al. Two decades of SPECT/CT—The coming of age of a technology: An updated review of literature evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef] [PubMed]
- Mogi, A. Effect of Scatter, Attenuation and Resolution Correction on a Pediatric Myocardial Perfusion SPECT Image. J. Cardiovasc. Med. Cardiol. 2014, 1, 26–29. [Google Scholar] [CrossRef]

| Advantages | Limitations | |
|---|---|---|
| Echocardiography |
|
|
| Cardiovascular Magnetic Resonance |
|
|
| Nuclear Imaging |
|
|
| Computed Tomography |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatelli, S.; Leo, I.; Bianco, F.; Borrelli, N.; Beltrami, M.; Garofalo, M.; Milano, E.G.; Bisaccia, G.; Iellamo, F.; Bassareo, P.P.; et al. The Role of Multimodality Imaging in Pediatric Cardiomyopathies. J. Clin. Med. 2023, 12, 4866. https://doi.org/10.3390/jcm12144866
Moscatelli S, Leo I, Bianco F, Borrelli N, Beltrami M, Garofalo M, Milano EG, Bisaccia G, Iellamo F, Bassareo PP, et al. The Role of Multimodality Imaging in Pediatric Cardiomyopathies. Journal of Clinical Medicine. 2023; 12(14):4866. https://doi.org/10.3390/jcm12144866
Chicago/Turabian StyleMoscatelli, Sara, Isabella Leo, Francesco Bianco, Nunzia Borrelli, Matteo Beltrami, Manuel Garofalo, Elena Giulia Milano, Giandomenico Bisaccia, Ferdinando Iellamo, Pier Paolo Bassareo, and et al. 2023. "The Role of Multimodality Imaging in Pediatric Cardiomyopathies" Journal of Clinical Medicine 12, no. 14: 4866. https://doi.org/10.3390/jcm12144866
APA StyleMoscatelli, S., Leo, I., Bianco, F., Borrelli, N., Beltrami, M., Garofalo, M., Milano, E. G., Bisaccia, G., Iellamo, F., Bassareo, P. P., Pradhan, A., Cimini, A., & Perrone, M. A. (2023). The Role of Multimodality Imaging in Pediatric Cardiomyopathies. Journal of Clinical Medicine, 12(14), 4866. https://doi.org/10.3390/jcm12144866









