Clinical Features, Biochemical Parameters, and Treatment Adherence of Individuals Who Started the Treatment for Active Pulmonary Tuberculosis during the Pandemic Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Statements
2.3. Study Population and Data Collection
2.4. Blood Collection and Serum Analysis
2.5. Assessment of Adherence to Anti-Tuberculosis Treatment
2.6. Statistical Analysis
3. Results
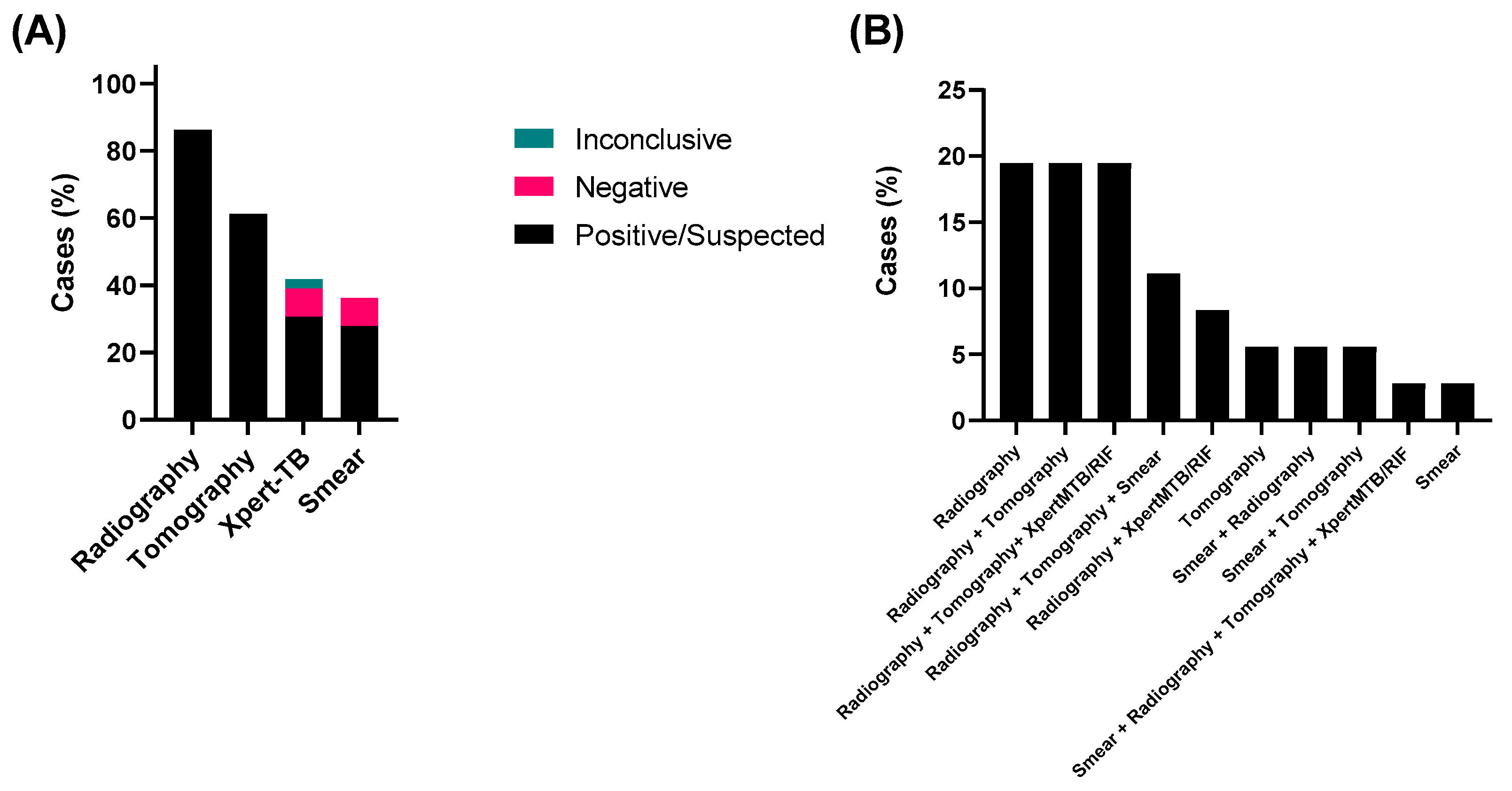
3.1. Analysis of Methods Used for Diagnostic of TB and Sociodemographic Characteristics of the Study Groups
3.2. Prevalence of Anti-SARS-CoV-2 Antibodies in Patients Recently Diagnosed with Active TB
3.3. Clinical Manifestation of Pulmonary Tuberculosis
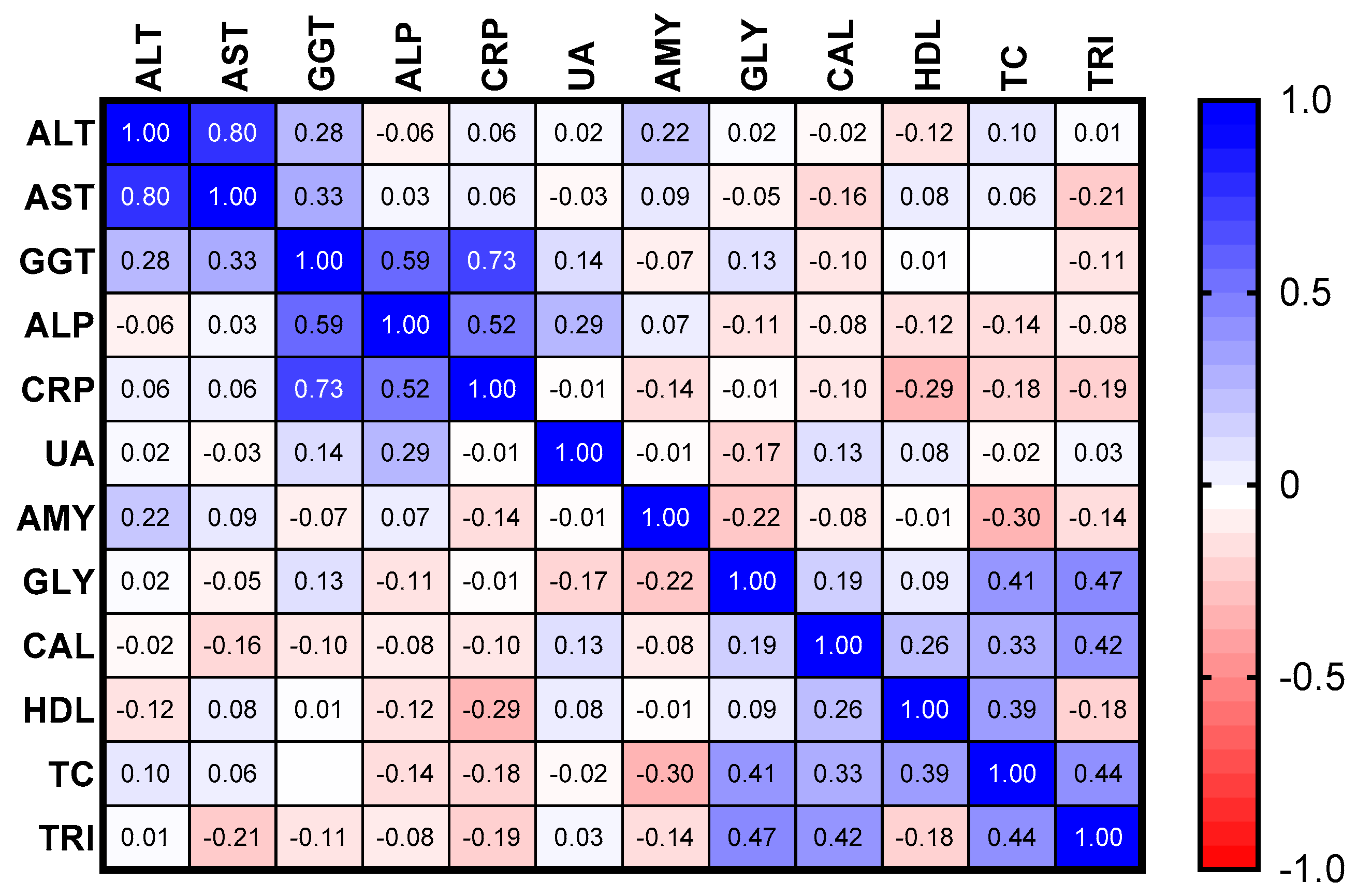
3.4. Measurement of Pre-Therapy Serum Biomarkers in Patients Recently Diagnosed with Active Pulmonary Tuberculosis
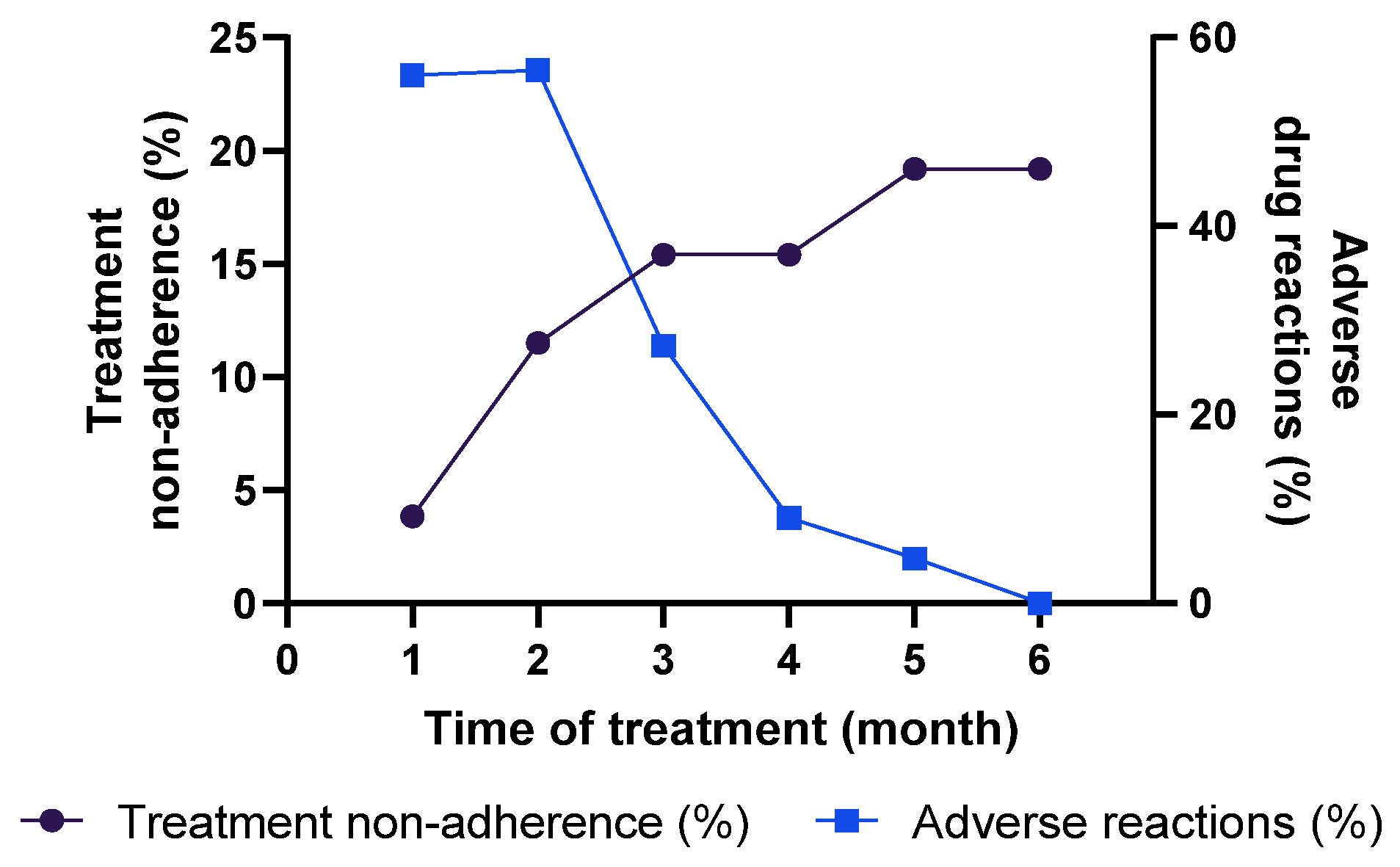
3.5. Assessment of Adherence for Tuberculosis Treatment and Adverse Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azadi, D.; Motallebirad, T.; Ghaffari, K.; Shojaei, H. Mycobacteriosis and Tuberculosis: Laboratory Diagnosis. Open Microbiol. J. 2018, 12, 41. [Google Scholar] [CrossRef]
- Seki, M.; Choi, H.; Kim, K.; Whang, J.; Sung, J.; Mitarai, S. Tuberculosis: A Persistent Unpleasant Neighbour of Humans. J. Infect. Public Health 2021, 14, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zagmignan, A.; da Costa, A.C.; Viana, J.L.; Lima Neto, L.G.; Cristina de Andrade, M.; Gaioso Neto, A.G.; Junqueira-Kipnis, A.P.; de Sousa, E.M. Identification of Specific Antibodies against the Ag85C-MPT51-HspX Fusion Protein (CMX) for Serological Screening of Tuberculosis in Endemic Area. Expert. Rev. Clin. Immunol. 2017, 13, 837–843. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Date, A.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets—Worldwide, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020—Reflections on the Global TB Burden, Treatment and Prevention Efforts. Int. J. Infect. Dis. 2021, 113, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, C.; Petersen, E.; Nantanda, R.; Mungai, B.N.; Migliori, G.B.; Amanullah, F.; Lungu, P.; Ntoumi, F.; Kumarasamy, N.; Maeurer, M.; et al. The WHO Global Tuberculosis 2021 Report—Not so Good News and Turning the Tide Back to End TB. Int. J. Infect. Dis. 2022, 124, 51. [Google Scholar] [CrossRef]
- Hogan, A.B.; Jewell, B.L.; Sherrard-Smith, E.; Vesga, J.F.; Watson, O.J.; Whittaker, C.; Hamlet, A.; Smith, J.A.; Winskill, P.; Verity, R.; et al. Potential Impact of the COVID-19 Pandemic on HIV, Tuberculosis, and Malaria in Low-Income and Middle-Income Countries: A Modelling Study. Lancet Glob. Health 2020, 8, e1132–e1141. [Google Scholar] [CrossRef]
- Asres, A.; Jerene, D.; Deressa, W. Delays to treatment initiation is associated with tuberculosis treatment outcomes among patients on directly observed treatment short course in southwest Ethiopia: A follow-up study. BMC Pulm. Med. 2018, 18, 64. [Google Scholar] [CrossRef]
- Jarovsky, D. Treatment of Tuberculosis in Brazil—Past, Present, and Future Challenges. Curr. Treat. Options Infect. Dis. 2019, 11, 58–72. [Google Scholar] [CrossRef]
- Yin, J.; Yuan, J.; Hu, Y.; Wei, X. Association between Directly Observed Therapy and Treatment Outcomes in Multidrug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150511. [Google Scholar] [CrossRef]
- Umeokonkwo, C.D.; Okedo-Alex, I.N.; Azuogu, B.N.; Utulu, R.; Adeke, A.S.; Disu, Y.O. Trend and Determinants of Tuberculosis Treatment Outcome in a Tertiary Hospital in Southeast Nigeria. J. Infect. Public Health 2020, 13, 1029–1033. [Google Scholar] [CrossRef]
- Thompson, R.R.; Kityamuwesi, A.; Kuan, A.; Oyuku, D.; Tucker, A.; Ferguson, O.; Kunihira Tinka, L.; Crowder, R.; Turyahabwe, S.; Cattamanchi, A.; et al. Cost and Cost-Effectiveness of a Digital Adherence Technology for Tuberculosis Treatment Support in Uganda. Value Health 2022, 25, 924–930. [Google Scholar] [CrossRef]
- Zegeye, A.; Dessie, G.; Wagnew, F.; Gebrie, A.; Islam, S.M.S.; Tesfaye, B.; Kiross, D. Prevalence and Determinants of Anti-Tuberculosis Treatment Non-Adherence in Ethiopia: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0210422. [Google Scholar] [CrossRef]
- Saukkonen, J.J.; Powell, K.; Jereb, J.A. Monitoring for Tuberculosis Drug Hepatotoxicity: Moving from Opinion to Evidence. Am. J. Respir. Crit. Care Med. 2012, 185, 598–599. [Google Scholar] [CrossRef]
- Saripalli, A.; Ramapuram, J. C-Reactive Protein as a Screening Test for Tuberculosis in People Living with HIV in Southern India: A Cross-Sectional, Observational Study. J. Clin. Med. 2022, 11, 3566. [Google Scholar] [CrossRef]
- Vidhya, R.; Rathnakumar, K.; Balu, V.; Pugalendi, K.V. Oxidative Stress, Antioxidant Status and Lipid Profile in Pulmonary Tuberculosis Patients before and after Anti-Tubercular Therapy. Indian J. Tuberc. 2019, 66, 375–381. [Google Scholar] [CrossRef]
- Ufoaroh, C.U.; Onwurah, C.A.; Mbanuzuru, V.A.; Mmaju, C.I.; Chukwurah, S.N.; Umenzekwe, C.C.; Aghanya, I.N.; Ushie, S.N.; Anyabolu, A.E.; Enemuo, E.H.; et al. Biochemical Changes in Tuberculosis. Pan Afr. Med. J. 2021, 38, 66. [Google Scholar] [CrossRef]
- Silva, D.R.; Rabahi, M.F.; Sant’Anna, C.C.; Da Silva-Junior, J.L.R.; Capone, D.; Bombarda, S.; De Miranda, S.S.; Da Rocha, J.L.; Dalcolmo, M.M.P.; Rick, M.F.; et al. Diagnosis of Tuberculosis: A Consensus Statement from the Brazilian Thoracic Association. J. Bras. Pneumol. 2021, 47, e20210054. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Shariq, M.; Sheikh, J.A.; Quadir, N.; Sharma, N.; Hasnain, S.E.; Ehtesham, N.Z. COVID-19 and Tuberculosis: The Double Whammy of Respiratory Pathogens. Eur. Respir. Rev. 2022, 31, 210264. [Google Scholar] [CrossRef]
- Tadolini, M.; Codecasa, L.R.; García-García, J.M.; Blanc, F.X.; Borisov, S.; Alffenaar, J.W.; Andréjak, C.; Bachez, P.; Bart, P.A.; Belilovski, E.; et al. Active Tuberculosis, Sequelae and COVID-19 Co-Infection: First Cohort of 49 Cases. Eur. Respir. J. 2020, 56, 2001398. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.F.P.S.; Santos, B.P.S.D.; Teixeira, C.S.S.; Nery, J.S.; Amorim, L.D.A.F.; Sanchez, M.N.; Barreto, M.L.; Pescarini, J.M. Performance Evaluation of Tuberculosis Control in Brazilian Municipalities. Rev. Saude Publica 2022, 56, 53. [Google Scholar] [CrossRef] [PubMed]
- Seara Muller, G.; Sasso Faccin, C.; Rossato Silva, D.; de Tarso Roth Dalcin, P. Association between the Radiological Presentation and Elapsed Time for the Diagnosis of Pulmonary Tuberculosis in the Emergency Department of a University Hospital. J. Bras. Pneumol. 2020, 46, e20180419. [Google Scholar] [CrossRef] [PubMed]
- Alshoabi, S.A.; Almas, K.M.; Aldofri, S.A.; Hamid, A.M.; Alhazmi, F.H.; Alsharif, W.M.; Abdulaal, O.M.; Qurashi, A.A.; Aloufi, K.M.; Alsultan, K.D.; et al. The Diagnostic Deceiver: Radiological Pictorial Review of Tuberculosis. Diagnostics 2022, 12, 306. [Google Scholar] [CrossRef]
- Costa Rabelo, J.V.; de Navarro, P.D.; da Silva Carvalho, W.; de Almeida, I.N.; Fonseca Oliveira, C.S.; Amaral Haddad, J.P.; de Miranda, S.S. Performance Assessment of Primary Healthcare Services in Tuberculosis Control in a City in Southeast Brazil. Cad. Saude Publica 2021, 37, e00112020. [Google Scholar] [CrossRef]
- Diallo, A.B.; Kollo, A.I.; Camara, M.; Lo, S.; Ossoga, G.W.; Mbow, M.; Karam, F.; Niang, M.Y.F.; Thiam, A.; Diawara, A.N.; et al. Performance of GeneXpert MTB/RIF® in the Diagnosis of Extrapulmonary Tuberculosis in Dakar: 2010–2015. Pan Afr. Med. J. 2016, 25, 129. [Google Scholar] [CrossRef]
- Ogata, T.; Nagasu, N.; Uehara, R.; Ito, K. Association of Low Sputum Smear Positivity among Tuberculosis Patients with Interferon-Gamma Release Assay Outcomes of Close Contacts in Japan. Int. J. Environ. Res. Public Health 2019, 16, 3713. [Google Scholar] [CrossRef]
- Ouédraogo, A.S.; Kabore, D.O.; Poda, A.; Sanogo, B.; Birba, E.; Sanou, I.; Godreuil, S.; Nacro, B. Evaluation of Stool Microscopy and Culture to Assist the Diagnosis of Pulmonary Tuberculosis in a Tuberculosis Endemic Country. Med. Sante Trop. 2016, 26, 97–100. [Google Scholar] [CrossRef]
- Duarte, R.; Lönnroth, K.; Carvalho, C.; Lima, F.; Carvalho, A.C.C.; Muñoz-Torrico, M.; Centis, R. Tuberculosis, Social Determinants and Co-Morbidities (Including HIV). Pulmonology 2018, 24, 115–119. [Google Scholar] [CrossRef]
- Ruhl, C.R.; Pasko, B.L.; Khan, H.S.; Kindt, L.M.; Stamm, C.E.; Franco, L.H.; Hsia, C.C.; Zhou, M.; Davis, C.R.; Qin, T.; et al. Mycobacterium Tuberculosis Sulfolipid-1 Activates Nociceptive Neurons and Induces Cough. Cell 2020, 181, 293–305.e11. [Google Scholar] [CrossRef]
- Turner, R.D. Cough in Pulmonary Tuberculosis: Existing Knowledge and General Insights. Pulm. Pharmacol. Ther. 2019, 55, 89–94. [Google Scholar] [CrossRef]
- Cox, V.; Wilkinson, L.; Grimsrud, A.; Hughes, J.; Reuter, A.; Conradie, F.; Nel, J.; Boyles, T. Critical Changes to Services for TB Patients during the COVID-19 Pandemic. Int. J. Tuberc. Lung Dis. 2020, 24, 542–544. [Google Scholar] [CrossRef]
- Machado Gomes, K.; Pereira Leite, S.; Helena Vieira, M.; Alfena de Souza, T.; de Cássia Batista, R.; de Claudio Motta, L.; Redner, P.; Pais Ramos, J.; Maria Gomes da Rocha, F.; de Oliveira, P.; et al. Anti-SARS-CoV-2 Antibodies Seroprevalence among Patients Submitted to Treatment for Tuberculosis in Rio de Janeiro, Brazil: A Cross-Sectional Study. medRxiv 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Aguiar, F.J.B.; Ferreira-Júnior, M.; Sales, M.M.; Cruz-Neto, L.M.; Fonseca, L.A.M.; Sumita, N.M.; Duarte, N.J.C.; Lichtenstein, A.; Duarte, A.J.S. C-Reactive Protein: Clinical Applications and Proposals for a Rational Use. Rev. Assoc. Méd. Bras. (Engl. Ed.) 2013, 59, 85–92. [Google Scholar] [CrossRef]
- Ciccacci, F.; Floridia, M.; Bernardini, R.; Sidumo, Z.; Mugunhe, R.J.; Andreotti, M.; Passanduca, A.; Magid, N.A.; Orlando, S.; Mattei, M.; et al. Plasma Levels of CRP, Neopterin and IP-10 in HIV-Infected Individuals with and without Pulmonary Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 16, 100107. [Google Scholar] [CrossRef]
- Yoon, C.; Semitala, F.C.; Atuhumuza, E.; Katende, J.; Mwebe, S.; Asege, L.; Armstrong, D.T.; Andama, A.O.; Dowdy, D.W.; Davis, J.L.; et al. Point-of-Care C-Reactive Protein-Based Tuberculosis Screening for People Living with HIV: A Diagnostic Accuracy Study. Lancet Infect. Dis. 2017, 17, 1285–1292. [Google Scholar] [CrossRef]
- Iwamura, A.P.D.; Tavares da Silva, M.R.; Hümmelgen, A.L.; Soeiro Pereira, P.V.; Falcai, A.; Grumach, A.S.; Goudouris, E.; Neto, A.C.; Prando, C. Immunity and Inflammatory Biomarkers in COVID-19: A Systematic Review. Rev. Med. Virol. 2021, 31, e2199. [Google Scholar] [CrossRef]
- Rohini, K.; Bhat, S.; Srikumar, P.S.; Mahesh Kumar, A. Assessment of Serum Calcium and Phosphorus in Pulmonary Tuberculosis Patients before, during and after Chemotherapy. Indian J. Clin. Biochem. 2014, 29, 377–381. [Google Scholar] [CrossRef]
- Wauthier, L.; Theunssens, X.; Durez, P.; Fillée, C.; Maisin, D.; Gruson, D. A Rare Case of Tuberculosis-Induced Hypercalcemia. Biochem. Med. 2020, 30, 471–474. [Google Scholar] [CrossRef]
- Lind, L.; Ljunghall, S. Hypercalcemia in Pulmonary Tuberculosis. Upsala J. Med. Sci. 1990, 95, 157–160. [Google Scholar] [CrossRef]
- Ijaz, A.; Saeed, W.; Javied Anwar, M.; Khan, F.; Mehmood, T.; Saeed, W.; Hussain Qureshi, A.; Dilawar, M.; Anwar, M.; Hussain, S.; et al. Calcium Abnormalities in Pulmonary Tuberculosis. Pak. J. Med. Res. 2004, 43, 1–4. [Google Scholar]
- Shirai, M.; Sato, A.; Suda, T.; Shichi, I.; Yasusa, K.; Iwata, M.; Okano, A.; Genma, H.; Chida, K. Calcium Metabolism in Tuberculosis. Kekkaku Tuberc. 1990, 65, 415–420. [Google Scholar] [CrossRef]
- Taslim, N.A.; Rasyid, H.; Atmanegara, M.K.; Angriavan, S.; Amelia, R. Effect of Chocolate Soybean Drink on Nutritional Status, Gamma Interferon, Vitamin D, and Calcium in Newly Lung Tuberculosis Patients. Open Access. Maced. J. Med. Sci. 2020, 8, 210–214. [Google Scholar] [CrossRef]
- Hoque, M.R.; Chakraborty, P.K.; Paul, U.K.; Sarkar, S.; Akhter, S.; Shahidullah, S.M.; Gautam, B.; Sultana, S.; Ferdous, N.; Samsunnahar, M. Serum Albumin and Creatinine Clearance Rate among Smear Positive Pulmonary Tuberculosis Patients in Bangladesh. Mymensingh Med. J. MMJ 2014, 23, 430–434. [Google Scholar] [PubMed]
- Yee, D.; Valiquette, C.; Pelletier, M.; Parisien, I.; Rocher, I.; Menzies, D. Incidence of Serious Side Effects from First-Line Antituberculosis Drugs among Patients Treated for Active Tuberculosis. Am. J. Respir. Crit. Care Med. 2012, 167, 1472–1477. [Google Scholar] [CrossRef]
- Bai, H.; Wu, T.; Jiao, L.; Wu, Q.; Zhao, Z.; Song, J.; Liu, T.; Lv, Y.; Lu, X.; Ying, B. Association of ABCC Gene Polymorphism With Susceptibility to Antituberculosis Drug-Induced Hepatotoxicity in Western Han Patients with Tuberculosis. J. Clin. Pharmacol. 2020, 60, 361–368. [Google Scholar] [CrossRef]
- Motswaledi, M.S.; Sekgwama, R.; Kasvosve, I. Tuberculosis Alters Pancreatic Enzymes in the Absence of Pancreatitis. Afr. J. Lab. Med. 2014, 3, 129. [Google Scholar] [CrossRef]
- Harling, G.; Lima Neto, A.S.; Sousa, G.S.; Machado, M.M.T.; Castro, M.C. Determinants of Tuberculosis Transmission and Treatment Abandonment in Fortaleza, Brazil. BMC Public. Health 2017, 17, 508. [Google Scholar] [CrossRef]
- De Seixas Maciel, E.M.G.; De Souza Amancio, J.; De Castro, D.B.; Braga, J.U. Social Determinants of Pulmonary Tuberculosis Treatment Non-Adherence in Rio de Janeiro, Brazil. PLoS ONE 2018, 13, e0190578. [Google Scholar] [CrossRef]
- Freitas de Andrade, K.V.; Silva Nery, J.; Santos de Araújo, G.; Lima Barreto, M.; Martins Pereira, S. Association between Treatment Outcome, Sociodemographic Characteristics and Social Benefits Received by Individuals with Tuberculosis in Salvador, Bahia, Brazil, 2014–2016. Epidemiol. Serv. Saude 2019, 28, e2018220. [Google Scholar] [CrossRef]

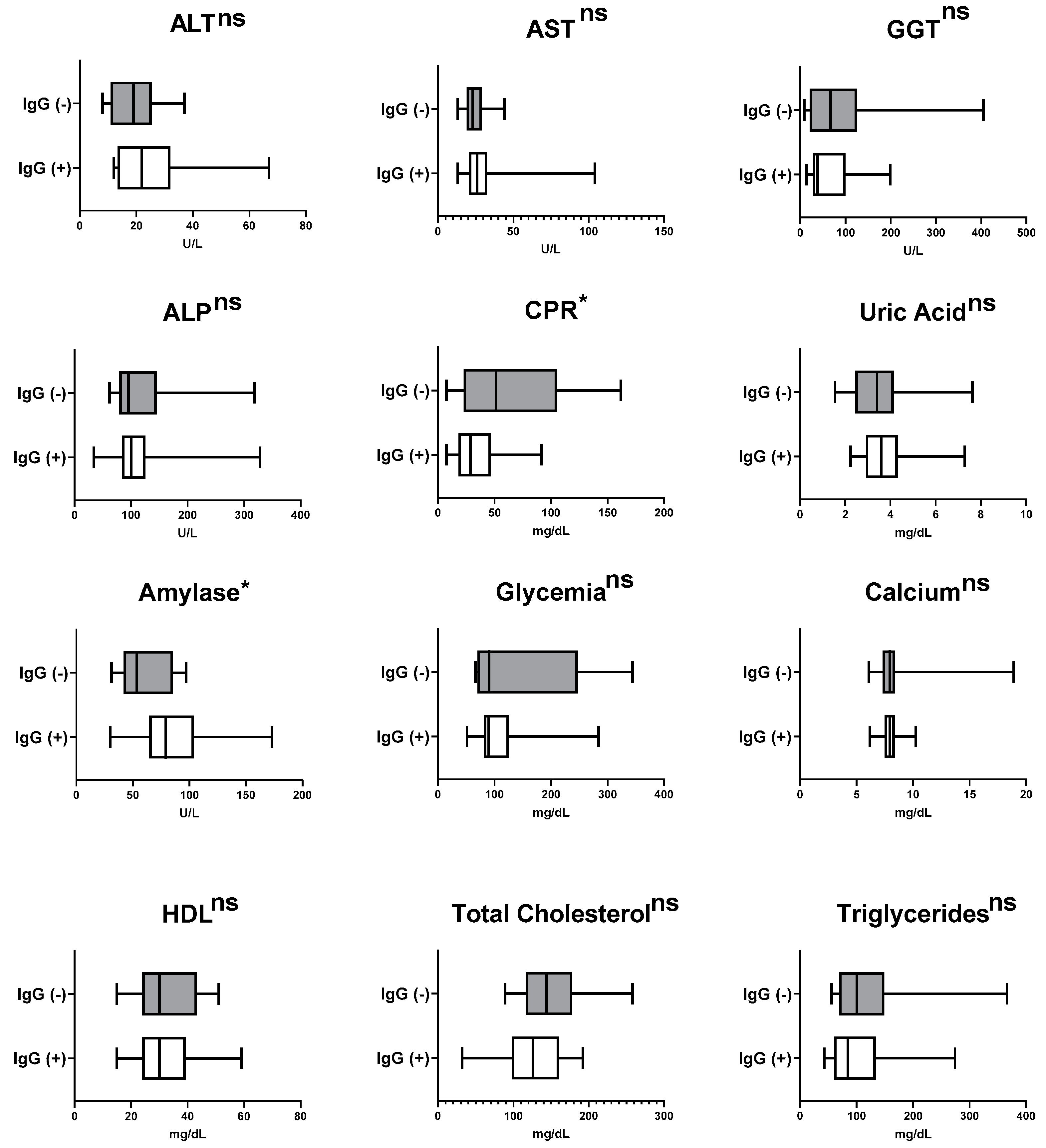
| Variables | TB Patients n (%) | Comparison Group n (%) |
|---|---|---|
| Gender | ||
| Male | 25 (69.4) | 24 (68.6) |
| Female | 11 (30.6) | 11 (31.4) |
| Age (years) | ||
| 18–24 | 08 (22.2) | 09 (25) |
| 25–34 | 11 (30.5) | 11 (30.5) |
| 35–44 | 07 (19.4) | 07 (19.4) |
| 45–54 | 06 (16.6) | 06 (16.6) |
| 55–64 | 03 (8.3) | 02 (5.5) |
| 65+ | 01 (3.0) | 01 (3.0) |
| Marital status | ||
| Single | 20 (55.6) | 24 (68.6) |
| Married | 16 (44.4) | 11 (31.4) |
| Education level | ||
| No formal education | 7 (19.4%) | 1 (2.9) |
| Elementary School | 6 (16.6%) | 2 (5.7) |
| High School | 20 (55.7%) | 21 (60.0) |
| Higher Education | 3 (8.3%) | 06 (17.1) |
| Postgraduate studies | - | 05 (14.3) |
| Ethnic-racial identity | ||
| White | 2 (5.6) | 09 (25.7) |
| Pardos (mixed) | 24 (66.7) | 21 (60.0) |
| Black | 10 (27.8) | 05 (14.3) |
| Family income | ||
| Up to 3 minimum wages * | 33 (91.7) | 22 (62.9) |
| More than 3 minimum wages * | 3 (8.3) | 13 (37.1) |
| Characteristics | TB Patients | Comparison Group |
|---|---|---|
| n (%) | n (%) | |
| Contact with people with Tuberculosis | 18 (50.0) | 7 (20.0) |
| Contact with individuals with Leprosy | 4 (11.1) | 4 (11.4) |
| Presence of the vaccine scar | 29 (80.6) | 31 (88.6) |
| Tuberculin test | ||
| Reactor | 7 (19.4) | 0 |
| No reactor | 0 | 1 (2.9) |
| Unrealized | 28 (77.8) | 34 (97.1) |
| Comorbidities | 6 (16.8) | 3 (8.6) |
| Other lung diseases | 3 (8.4) | 0 |
| Self-reported Smoking | 11 (30.6) | - |
| Frequent use of alcohol | 17 (47.2) | - |
| TB Patients | Comparison Group | ||
|---|---|---|---|
| IgG (−) n (%) | IgG (+) n (%) | IgG (+) n (%) | |
| Prevalence of anti-SARS-CoV-2 IgG | 12 (33.3) | 24 (66.7) | 35 (100) |
| Previous diagnosis for COVID-19 | 0 | 6 (25) | 14 (40) |
| Typical symptoms of COVID-19 | 3 (25) | 12 (50) | 16 (45.7) |
| Contact with subjects with COVID-19 | 4 (33.3) | 11 (45.8) | 22 (62.8) |
| Vaccination for COVID-19 | 2 (16.7) | 11 (45.8) | 35 (100) |
| Astrazeneca | 0 | 8 (33.3) | 19 (54.3) |
| Coronavac | 1 (8.3) | 0 | 13 (37.1) |
| Pfizer | 0 | 3 (12.5) | 9 (25) |
| Do not know | 1 (8.3) | 0 | 0 |
| Signal and Symptoms | TB Patients | Χ2 | p | ||
|---|---|---|---|---|---|
| Total | Anti-SARS-CoV-2 | ||||
| IgG (−) | IgG (+) | ||||
| Cough | 33 (91.7) | 11 (87.5) | 24 (100) | 1.636 | 0.2008 |
| Weight loss | 32 (88.9) | 10 (83.3) | 24 (100) | 2.250 | 0.1336 |
| Fever | 30 (83.3) | 9 (75) | 24 (100) | 3.600 | 0.0578 |
| Weakness | 21 (58.3) | 5 (45.8) | 20 (83.3) | 4.629 | 0.0314 |
| Inappetence | 10 (27) | 3 (25) | 8 (33.3) | 0.2769 | 0.5987 |
| Hemoptysis | 8 (22.2) | 2 (20.1) | 6 (25) | 0.2835 | 0.7768 |
| Abnormalities | n | % |
|---|---|---|
| Low HDL (M: <55 mg/dL; F: <65 mg/dL) | 36 | 100 |
| High CRP (>6 mg/dL) | 36 | 100 |
| Hypocalcemia (<8.8 mg/dL) | 32 | 88.9 |
| High ALP (>100 U/L) | 17 | 47.2 |
| High GGT (M: >55 U/L; F: >38 U/L) | 17 | 47.2 |
| High Triglycerides (>120 mg/dL) | 8 | 22.2 |
| High Amylase (>125 U/L) | 5 | 13.9 |
| High Uric acid (M: >7 mg/dL; F: >6 mg/dL) | 3 | 8.3 |
| High ALT (M: >39 U/L; F: >37 U/L) | 2 | 5.6 |
| High AST (M: >45 U/L; F: >37 U/L) | 2 | 5.6 |
| Hypercalcemia (>11 mg/dL) | 2 | 5.6 |
| High Total Cholesterol (>120 mg/dL) | 1 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, A.C.d.S.; Lopes, L.A.; Vale, M.C.d.S.; Costa, M.F.; Lima, J.V.d.S.; Silva, J.G.M.d.; Ferreira, B.S.d.C.; Nascimento, V.A.d.; Flor, S.E.d.S.; Sousa, E.L.C.d.; et al. Clinical Features, Biochemical Parameters, and Treatment Adherence of Individuals Who Started the Treatment for Active Pulmonary Tuberculosis during the Pandemic Period. J. Clin. Med. 2023, 12, 4843. https://doi.org/10.3390/jcm12144843
Sales ACdS, Lopes LA, Vale MCdS, Costa MF, Lima JVdS, Silva JGMd, Ferreira BSdC, Nascimento VAd, Flor SEdS, Sousa ELCd, et al. Clinical Features, Biochemical Parameters, and Treatment Adherence of Individuals Who Started the Treatment for Active Pulmonary Tuberculosis during the Pandemic Period. Journal of Clinical Medicine. 2023; 12(14):4843. https://doi.org/10.3390/jcm12144843
Chicago/Turabian StyleSales, Amanda Caroline de Souza, Larissa Araújo Lopes, Maria Caroliny dos Santos Vale, Mayara Ferreira Costa, João Victor de Souza Lima, João Gabriel Matos da Silva, Bruna Sthefanny da Cunha Ferreira, Victoria Alves do Nascimento, Saara Emanuele da Silva Flor, Elane Luiza Costa de Sousa, and et al. 2023. "Clinical Features, Biochemical Parameters, and Treatment Adherence of Individuals Who Started the Treatment for Active Pulmonary Tuberculosis during the Pandemic Period" Journal of Clinical Medicine 12, no. 14: 4843. https://doi.org/10.3390/jcm12144843
APA StyleSales, A. C. d. S., Lopes, L. A., Vale, M. C. d. S., Costa, M. F., Lima, J. V. d. S., Silva, J. G. M. d., Ferreira, B. S. d. C., Nascimento, V. A. d., Flor, S. E. d. S., Sousa, E. L. C. d., Paz, B. K. B., Garcia, R. A. d. S., Sousa, E. M. d., Santos, A. F. d., Silva, L. C. N. d., & Zagmignan, A. (2023). Clinical Features, Biochemical Parameters, and Treatment Adherence of Individuals Who Started the Treatment for Active Pulmonary Tuberculosis during the Pandemic Period. Journal of Clinical Medicine, 12(14), 4843. https://doi.org/10.3390/jcm12144843






